Abstract
Background
The orbit is a bony cavity within the skull that is composed of many structures which may undergo neoplastic transformation. Failure to diagnose the tumour and determine its extent may lead to high morbidity and mortality. The aim of this study was to evaluate the role of computed tomography in the diagnosis of orbital tumours in our centre.
Materials and methods
Computed Tomography images acquired from a multi-sliced CT machine, tissue diagnoses obtained from histopathology reports and patients’ clinical records were reviewed. The data were analyzed and presented using frequency tables, percentages and charts as appropriate.
Results
Sixty six patients made up of 34 (51.6%) males and 32 (48.4%) females were studied. The ages ranged between 1 and 80 years with a mean of 35 years. Majority (50%) of the patients were in their 4th – 6th decades of life. While secondary orbital tumours were seen in 42 (63.6%) patients, primary tumours occurred in 23 (34.8%) cases. Metastatic deposit was seen in 1 (1.6%) patient. Bone was the most commonly affected orbital tissue. The CT diagnoses of benign and malignant tumours were accurate when compared with histopathological diagnoses in 80.6% and 96.7 % of the cases respectively.
Conclusion
Computed Tomography is useful in characterizing the nature, precise location of a lesion within the orbit and to demonstrate the extension of the orbital lesion into contiguous structures. This study showed that Computed tomography is also a useful imaging technique in the diagnosis of orbital tumours with high concordance rate when compared with histological diagnoses.
Keywords: Computed tomography, Orbital tumours, High diagnostic accuracy, Ibadan, Nigeria
Introduction
The orbit is a pyramidal shaped cavity within the skull which is made up of seven bones and composed of structures such as the eyeball, the optic nerve, extraocular muscles, lacrimal gland, vessels and nerves, all of which are surrounded by orbital fat1. In addition to tumours arising from surrounding structures like paranasal sinuses and brain, any of these orbital contents may undergo neoplastic change. Metastasis from distant malignancies may also be deposited in the orbit2.
In children, most orbital tumours are developmental abnormalities such as haemangiomas, while malignancies are unusual with rhabdomyosarcoma being the commonest3,4. In the adult population, the most common benign tumour is haemangioma while the most common malignant tumours are lymphomas5. Imaging is important both in the diagnosis, treatment and monitoring of orbital lesions. Earlier modalities such as plain radiography, arteriography and venography had limited tumour detection rates and could not reliably differentiate between benign and malignant tumours6, 7. Magnetic Resonance Imaging (MRI) has been extensively explored, it provides critical anatomic information about ocular structures involved, perineural spread, and intracranial extension8, it is however not widely available. Ultrasonography (US) on the other hand is limited in the detection of these tumours and in determining intracranial extension9. Though CT has virtually been accepted as the radio-diagnostic tool of choice for the evaluation of orbital tumours, there are few studies in our environment correlating CT findings with histopathology. This study was undertaken to evaluate the role of this modality in orbital tumour diagnosis in our environment.
Reports
Materials and methods
This was a retrospective - prospective study that spanned a 12-month period between January and December 2010. The retrospective aspect of the study involved the use of existing data in the records of the Radiology Department of the hospital while the data for the prospective study were collected as patients presented in the department. Histological diagnosis of every case was obtained both from patients’ case notes and the records of the histopathology department. Cases without complete records were excluded from the study. The orbital CT images for the study were obtained using a GE helical multisliced CT machine. Two protocols i.e. axial and coronal were used. Intravenous contrast was given to patients after which the initial protocols were repeated. The acquired images were then viewed in both soft tissue and bone windows.
Tumours were classified as primary, secondary and metastatic. Tumours from orbital structures were termed primary, while those extending into the orbit from adjacent structures were termed secondary tumours. The primary and secondary tumours were categorized into benign and malignant. Orbital masses found in patients with known distant primary malignancy were termed metastatic tumours.
The data generated was analyzed and presented using frequency tables, percentages as appropriate. Chi square test was used to test association between qualitative variables at 5% level of significance. Ethical approval was obtained from the Ethics Review Board and informed consent obtained from all the prospective patients included in the study. All the findings were recorded in a standardized data collection form.
Results
A total of 66 orbital CT images of histologically diagnosed patients with orbital tumours were reviewed during the study period. Their ages ranged between 1 and 80 years with a mean age of 35 years. The patients were made up of 34 (51.6%) males and 32 (48.4%) females. The age range with the highest disease frequency was 30 – 59 with a total of 33(50%) patients while the lowest frequency was found in the ≥60 age range as shown in Table 1. Primary orbital tumours were found in 23 (34.8%) of the patients with 47.8% in patients less than 30 years. Secondary tumour was found in 42(63.6%) with the majority in patients less than 60yrs. Metastatic orbital disease was found in 1 (1.6%) female patient. Secondary tumours predominated in both males and females with a percentage of 58.8 and 68.8 respectively. Primary tumours however showed a higher male preponderance as indicated in Table2
TABLE 1: THE AGE AND GENDER DISTRIBUTION OF THE PATIENTS
| AGE RANGE | Males | Female | Total (%) |
| <20 | 7(50%) | 7(50%) | 14(100%) |
| 20-29 | 7(70%) | 3(30%) | 10(100%) |
| 30-39 | 6(37.5%) | 10(62.5%) | 16(100%) |
| 40-49 | 5(62.5%) | 3(37.5%) | 8(100%) |
| 50-59 | 4(44.4%) | 5(55.6%) | 9(100%) |
| ≥60 | 5(55.5%) | 4(44.4%) | 9(100%) |
| Total | 34(51.5%) | 32(48.5%) | 66(100%) |
TABLE 2: DISTRIBUTION OF TUMOURS ACCORDING TO THE ORIGIN
| Age group (years) | Primary | Secondary | Metastasis | Total |
| < 30 | 11 (47.8%) | 13 (31%) | 0 | 24(36.4%) |
| 30-59 | 12 (52.2%) | 20 (47.6%) | 1 (100%) | 33(50.0%) |
| ≥ 60 | 0 | 9 (21.4%) | 0 | 9 (13.6%) |
| Total | 23 (100%) | 42 (100%) | 1 (100%) | 66 (100%) |
| Sex | ||||
| Male | 14 (41.2%) | 20 (58.8%) | 0 | 34 (100%) |
| Female | 9 (28.1%) | 22 (68.8%) | 1 (3.1%) | 32 (100%) |
| Total | 23 (34.8%) | 42 (63.6%) | 1 (1.6%) | 66 (100%) |
Malignant tumours were more frequent in females (20, 62.5%); however both benign and malignant tumours had equal frequency in the males. Overall, there were 29(44%) benign cases and 37(56%) malignant cases in this study . Six of the nine patients ≥60 years in this study had malignant diseases, however majority of malignancies were seen in those less than 60 years as shown in Table 3. Bone (79.2%) was the most affected orbital structure and this was seen more with malignant tumours in 65.3% cases than benign which was seen in 34.7% of cases. The muscles were the structures most affected after the bony orbit with a frequency of 51.5% and this was also associated more with malignant tumours. Lacrimal gland was the least structure affected involved in 3 cases (Table 4).
TABLE 3: DISTRIBUTION OF TUMOURS INTO BENIGN AND MALIGNANT TUMOURS
| Age group (years) | Benign | Malignant | Total |
| < 30 | 9 (37.5%) | 15 (62.5%) | 24 (36.4%) |
| 30 -59 | 17 (51.5%) | 16 (48.5%) | 33 (50.0%) |
| ≥ 60 | 3 (33.3%) | 6 (66.7%) | 9 (13.6%) |
| Total | 29 (43.9%) | 37 (56.1%) | 66 (100%) |
| Sex | |||
| Male | 17(50.0%) | 17 (50.0%) | 34 (100%) |
| Female | 12 (37.5%) | 20 (62.5%) | 32 (100%) |
| Total | 29 (43.9%) | 37 (56.1%) | 66 (100%) |
TABLE 4: ORBITAL STRUCTURE INVOLVEMENT
| Structure involved | Benign (%) | Malignant (%) | Total (%) |
| Bone | 17 (34.7) | 32 (65.3) | 49 (100.0) |
| Muscle | 11 (32.4) | 23 (67.6) | 34 (100.0) |
| Optic nerve | 9 (36.0) | 16 (64.0) | 25 (100.0) |
| Vessels | 2 (28.6) | 5 (71.4) | 7 (100.0) |
| Globe | 0 (0.0) | 4 (100.0) | 4 (100.0) |
| Lacrimal gland | 2 (66.7) | 1 (33.3) | 3 (100.0) |
The diagnosis of benign tumours using CT agreed with histology in 80.6% of cases. One case each of pleomorphic adenoma, ossifying fibroma, haemangioma, and ameloblastoma was misdiagnosed as malignant. One case each of inverted papilloma and fibromyxoma were classified as malignant. The CT diagnoses of malignant tumours matched the histopathological diagnosis in 96.7% of cases. Only one case of lymphoma was misdiagnosed as benign. The concordance of CT for orbital tumour diagnosis was 80.6% for benign tumours and 96.7 for malignancies as indicated in Tables 5.
TABLE 5: CONCORDANCE BETWEEN HISTOLOGY AND CT DIAGNOSIS
| Radiologic diagnosis | |||
| Histologic diagnosis | Benign | Malignant | Total |
| Benign | 29 (80.6%) | 7 (19.4%) | 36 (54.5%) |
| Malignant | 1 (3.3%) | 29 (97.6%) | 30 (45.5%) |
| Total | 30 (100%) | 36 (100%) | 66 (100%) |
Discussion
Orbital tumours are classified based on their origins into primary orbital tumours, secondary orbital tumours, and metastatic tumours10. Age at presentation, associated ophthalmological findings and radiological features however provide invaluable information as to the possible histological type of tumour whether benign or malignant11. In a study by Dunarinbu et al12, primary orbital tumours were predominant, occurring in 114cases (75.5%), while secondary orbital tumours were found in 33 cases (22 %) with 4 (2.6%) cases of metastasis to the orbit. This is at variance with the findings from this study where secondary tumours predominated, accounting for 64% of cases. This may be partly due to the fact that patients were more likely to have a CT for extensive craniofacial tumour with orbital extension. Also cases of orbital tumours without histological diagnosis were excluded. There was however concurrence with regards to metastatic tumours.
The incidence of neoplastic lesions has been found to show a bimodal age distribution with peaks in the first and seventh decades of life3,4, which is not the case in this report. These studies also reported that secondary orbital tumours occurred more in the adults with peak incidence in the middle age thus agreeing with findings from this study.
The orbit is said to be an unusual location for metastatic disease accounting for 1 – 3% of lesions in a large series of orbital tumours14. Several studies reported that breast cancer is considered to be the most prevalent metastatic tumour to the orbit and it is estimated at 29 – 70 % of all orbital metastasis15,16. The only metastasis to the orbit in this study was from breast cancer.
Sabhawal et al 16observed a CT and histologic correlation in 78.26% of their cases, while the CT-histological correlation was 83.3% in a study by Hu et al17. In this study, based on whether a tumour was benign or malignant, the concordance for malignant tumour was 96.7%, while it was 80.6% for benign tumour. Some benign tumours such as ameloblastoma and giant cell tumour, which were craniofacial tumours common in our environment, could display characteristics of malignancy thus leading to a misdiagnosis.
Conclusions
Computed Tomography is useful in characterizing the nature, precise location of a lesion within the orbit and to demonstrate the extension of the orbital lesion into contiguous structures. This study showed that Computed tomography is also a useful imaging technique in the diagnosis of orbital tumours with high concordance rate when compared with histological diagnoses.
Figure 1 .

Axial and coronal CT images of a 36-year-old man with histologic diagnosis of optic nerve sheath meningioma. This shows an enhancing hyperdense mass (arrows) in the left orbit, posterior and superior to the globe. The mass is seen to displace the globe anterio- inferiorly.
Figure 2:
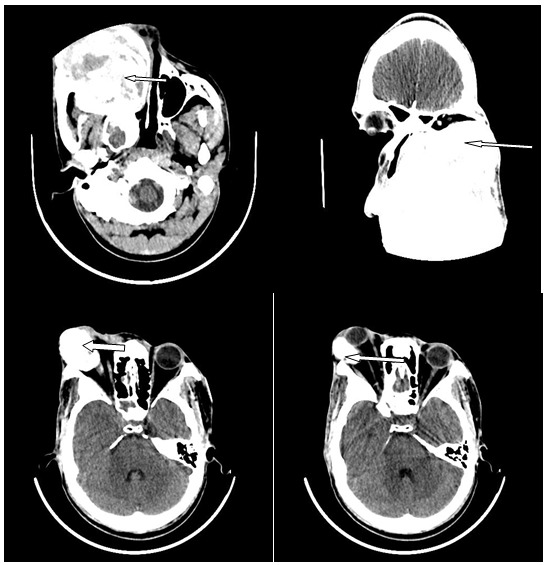
Enhanced axial and coronal images of the craniofacial bone in a 31-year-old male showing an expansile and destructive mass of the right maxillary bone (arrows) with compression of the orbit, nasal cavity and oral cavity. The histology diagnosis was fibromyxoma, which is a benign tumour.
Figure 3 .

Axial and coronal images of the craniofacial bone of a 45-year-old female with histology diagnosis of mucocoele. These show an expansile non enhancing hypodense mass (arrows) involving the left frontal and ethmoidal sinuses with associated anterior and inferior displacement of the ipsilateral orbit.
Figure 4 .

Axial CT images of the craniofacial bone of a 56-year-old female with histological diagnosis of antral squamous cell carcinoma. These show an expansile and destructive hypodense mass involving the left maxillary sinus and extending into the ipsilateral nasal cavity and orbit.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Grant support: None
References
- 1.Novitskaya E, Rene C. enophthalmos as a sign of metastatic breast cancer. CMAJ. 2013;185(13):1159. doi: 10.1503/cmaj.120726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weisman RA. Surgical anatomy of the orbit. Otolaryngol Clin North Am. 1999;12(2):271–291. [PubMed] [Google Scholar]
- 3.De CONCILIS C. Epidemiology of orbital disease. Ophthalmology. 2003;110:1288. doi: 10.1016/S0161-6420(03)00706-1. [DOI] [PubMed] [Google Scholar]
- 4.Bilaniuk LT, Farber M. Imaging of developmental anomalies of the eye and orbit. Am J Neuroradiol. 1992;13:793–803. [PMC free article] [PubMed] [Google Scholar]
- 5.Fafowora OF, Cookey-gam AI, Obajimi MO. Radiological evaluation of orbital tumours in Ibadan, Nigeria. Afr J Med Med Sci. 1996;25(4):361–364. [PubMed] [Google Scholar]
- 6.Dallow RL, Momose KJ, Weber AL. Comparison of Ultrasonography, Computerized tomography (EMI) and radiographic techniques in evaluation of Exophthalmos. Trans Am Acad Ophthalmol otolaryngol. 1976;81:305–322. [PubMed] [Google Scholar]
- 7.Khan SN, Sepahdari AR. Orbital masses: CT and MRI of common vascular lesions, benign tumours and malignancies. Saudi J Ophthalmol. 2012;26(4):373–383. doi: 10.1016/j.sjopt.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tailor TD, Gupta D, Dalley RW, Keene CD, Anzai Y. Orbital neoplasms in adults: clinical, radiologic, and pathologic review. Radiographics. 2013;33:1739–1758. doi: 10.1148/rg.336135502. [DOI] [PubMed] [Google Scholar]
- 9.Coleman DJ, Dallows RL. Clin Ophthalmol. CH. 27. Vol. 4. Philadelphia: Harper and Row.; 1987. Orbital Ultrasonography in: Duane T D (ed). [Google Scholar]
- 10.Lang DA, Neil-Dwyer G, Evans BT. A multidisciplinary approach to tumours involving the orbit: orbital re-construction, a 3 –dimensional concept. Acta Neurochiurgical. 1994;128:101–108. doi: 10.1007/BF01400658. [DOI] [PubMed] [Google Scholar]
- 11.Tim E, Dar Saut, Giuseppe Lanzino, Betriz Lopes. An introductory overview of orbital tumours. . Neurosurg focus. 2001;10(5):1. doi: 10.3171/foc.2001.10.5.2. [DOI] [PubMed] [Google Scholar]
- 12.Dunarintu S, Birsasteanu F, Onet D, Magda P, Costea D, Mogoeanu M. Radio-imaging diagnosis of the ocular and orbital tumours. Journal of experimental medical & surgical research. 2008;1(2):5–12. [Google Scholar]
- 13.Shields JA, Shields CL, Brotman HK, Carvalho C, Perez N, Eagle RC, Cancer metastatic to the orbit: the 2000 Robert M .Curts lecture. Ophtal Plast Reconst Surg. 2000;17:346–354. doi: 10.1097/00002341-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg RA, Rootman J, Cline RA. Tumours metastatic to the orbit: a changing picture. Surv Ophthalmol. 1990;35:1–24. doi: 10.1016/0039-6257(90)90045-w. [DOI] [PubMed] [Google Scholar]
- 15.Cheung YK, Chan FL, Cheung KM, Linian LY, Leong CT evaluation of orbital conditions: experience at two regional hospitals. Jour HKMA. 1989;41(2):169–172. [Google Scholar]
- 16.Sabarwal KK, Chonhan AL, Jain S. CT evaluation of Proptosis. Indian J Radiol Imaging. 2006;16:683–688. [Google Scholar]
- 17.Hu Yanhua, Wang J, Xiao S. Clinical and histological diagnosis of orbital tumours. Jour Tongji Med Univ. 2000;20(1):82–85. doi: 10.1007/BF02887686. [DOI] [PubMed] [Google Scholar]


