Abstract
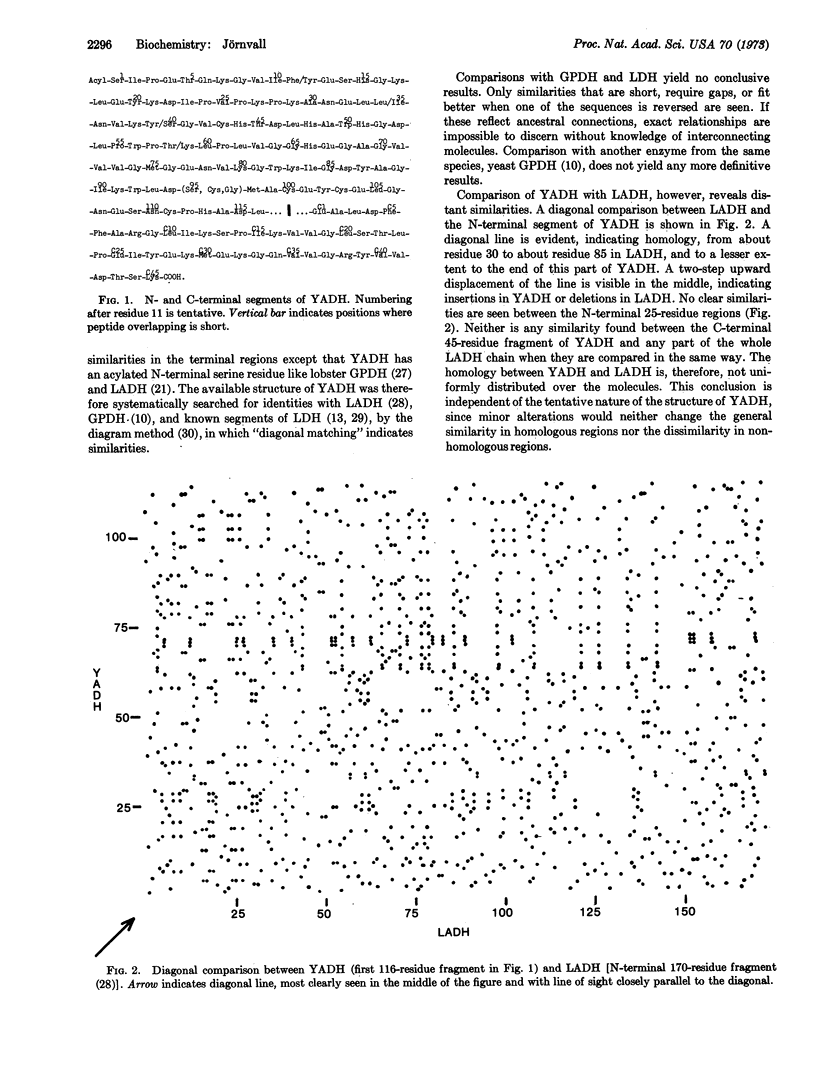
The primary structure of about half of the protein chain of yeast alcohol dehydrogenase has been determined and compared with the amino-acid sequences of other dehydrogenases. The enzyme is found to be distantly related to horse-liver alcohol dehydrogenase, although these two proteins have different quaternary structures and subunit sizes. Some regions show no significant similarities, but long segments within the N-terminal parts of the molecules are homologous, suggesting a common and important function for these segments. Ancestral connections between some different dehydrogenases can be concluded and the degree of evolutionary changes may be estimated.
Keywords: primary structure, diagram comparison, conserved region, amino-acid exchanges
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison W. S., Admiraal J., Kaplan N. O. The subunits of dogfish M4 lactic dehydrogenase. J Biol Chem. 1969 Sep 10;244(17):4743–4749. [PubMed] [Google Scholar]
- Barkman D. E., Sandstead H. H., Park J. H. An examination of the zinc requirement for the catalytic activity of 3-phosphoglyceraldehyde dehydrogenase. J Biol Chem. 1970 Mar 10;245(5):1036–1040. [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Leberman R. Reversible blocking of peptide amino groups by maleic anhydride. Biochem J. 1967 Jun;103(3):78P–79P. [PMC free article] [PubMed] [Google Scholar]
- Davidson B. E., Sajgò M., Noller H. F., Harris J. I. Amino-acid sequence of glyceraldehyde 3-phosphate dehydrogenase from lobster muscle. Nature. 1967 Dec 23;216(5121):1181–1185. doi: 10.1038/2161181a0. [DOI] [PubMed] [Google Scholar]
- Drum D. E., Harrison J. H., 4th, Li T. K., Bethune J. L., Vallee B. L. Structural and functional zinc in horse liver alcohol dehydrogenase. Proc Natl Acad Sci U S A. 1967 May;57(5):1434–1440. doi: 10.1073/pnas.57.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drum D. E., Li T. K., Vallee B. L. Zinc isotope exchange in horse liver alcohol dehydrogenase. Biochemistry. 1969 Sep;8(9):3792–3797. doi: 10.1021/bi00837a045. [DOI] [PubMed] [Google Scholar]
- Engel P. C. Evolution of enzyme regulator sites: evidence for partial gene duplication from amino-acid sequence of bovine glutamate dehydrogenase. Nature. 1973 Jan 12;241(5385):118–120. doi: 10.1038/241118a0. [DOI] [PubMed] [Google Scholar]
- Fondy T. P., Everse J., Driscoll G. A., Castillo F., Stolzenbach F. E., Kaplan N. O. The comparative enzymology of lactic dehydrogenases. IV. Function of sulfhydryl groups in lactic dehydrogenases and the sequence around the essential group. J Biol Chem. 1965 Nov;240(11):4219–4234. [PubMed] [Google Scholar]
- Fondy T. P., Ross C. R., Sollohub S. J. Structural studies on rabbit muscle glycerol 3-phosphate dehydrogenase and a comparison of chemical and physical determinations of its molecular weight. J Biol Chem. 1969 Mar 25;244(6):1631–1644. [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- Gibbs A. J., McIntyre G. A. The diagram, a method for comparing sequences. Its use with amino acid and nucleotide sequences. Eur J Biochem. 1970 Sep;16(1):1–11. doi: 10.1111/j.1432-1033.1970.tb01046.x. [DOI] [PubMed] [Google Scholar]
- HARRIS I., MERIWETHER B. P., PARK J. H. Chemical nature of the catalytic sites in glyceraldehyde-3-phosphate dehydrogenase. Nature. 1963 Apr 13;198:154–157. doi: 10.1038/198154a0. [DOI] [PubMed] [Google Scholar]
- HARRIS I. STRUCTURE AND CATALYTIC ACTIVITY OF ALCOHOL DEHYDROGENASES. Nature. 1964 Jul 4;203:30–34. doi: 10.1038/203030a0. [DOI] [PubMed] [Google Scholar]
- Hill E., Tsernoglou D., Webb L., Banaszak L. J. Polypeptide conformation of cytoplasmic malate dehydrogenase from an electron density map at 3.0 angstrom resolution. J Mol Biol. 1972 Dec 30;72(3):577–589. doi: 10.1016/0022-2836(72)90176-3. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Pfleiderer G., Mella K., Volz M., Leskowac W., Jeckel R. The importance of SH-groups for enzymic activity. 7. The amino acid sequence around the essential SH-group of pig heart lactate dehydrogenase, isoenzyme I. Eur J Biochem. 1967 Jun;1(4):476–481. doi: 10.1111/j.1432-1033.1967.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Jones G. M.T., Harris J. I. Glyceraldehyde 3-phosphate dehydrogenase: Amino acid sequence of enzyme from baker's yeast. FEBS Lett. 1972 May 1;22(2):185–189. doi: 10.1016/0014-5793(72)80040-1. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Harris J. I. Horse liver alcohol dehydrogenase. On the primary structure of the ethanol-active isoenzyme. Eur J Biochem. 1970 Apr;13(3):565–576. doi: 10.1111/j.1432-1033.1970.tb00962.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. Horse liver alcohol dehydrogenase. On the primary structures of the isoenzymes. Eur J Biochem. 1970 Sep;16(1):41–49. doi: 10.1111/j.1432-1033.1970.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. Horse liver alcohol dehydrogenase. The primary structure of an N-terminal part of the protein chain of the ethanol-active isoenzyme. Eur J Biochem. 1970 Jul;14(3):521–534. doi: 10.1111/j.1432-1033.1970.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H. Horse liver alcohol dehydrogenase. The primary structure of the protein chain of the ethanol-active isoenzyme. Eur J Biochem. 1970 Sep;16(1):25–40. doi: 10.1111/j.1432-1033.1970.tb01049.x. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Pietruszko R. Structural studies of alcohol dehydrogenase from human liver. Eur J Biochem. 1972 Feb 15;25(2):283–290. doi: 10.1111/j.1432-1033.1972.tb01695.x. [DOI] [PubMed] [Google Scholar]
- LI T. K., VALLEE B. L. ACTIVE-CENTER PEPTIDES OF LIVER-ALCOHOL DEHYDROGENASE. I. THE SEQUENCE SURROUNDING THE ACTIVE CYSTEINYL RESIDUES. Biochemistry. 1964 Jun;3:869–873. doi: 10.1021/bi00894a025. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Adams M. J., Buehner M., Ford G. C., Hackert M. L., Lentz P. J., Jr, McPherson A., Jr, Schevitz R. W., Smiley I. E. Structural constraints of possible mechanisms of lactate dehydrogenase as shown by high resolution studies of the apoenzyme and a variety of enzyme complexes. Cold Spring Harb Symp Quant Biol. 1972;36:179–191. doi: 10.1101/sqb.1972.036.01.025. [DOI] [PubMed] [Google Scholar]
- SCHELLENBERG K. A. EVIDENCE FOR THE PARTICIPATION OF TRYPTOPHAN AS INTERMEDIATE IN TRANSFER OF HYDROGEN BETWEEN DIPHOSPHOPYRIDINE NUCLEOTIDE AND SUBSTRATE IN YEAST ALCOHOL DEHYDROGENASE. J Biol Chem. 1965 Mar;240:1165–1169. [PubMed] [Google Scholar]
- SMITH E. L., MARGOLIASH E. EVOLUTION OF CYTOCHROME C. Fed Proc. 1964 Nov-Dec;23:1243–1247. [PubMed] [Google Scholar]
- Smith E. L., Landon M., Piszkiewicz D., Brattin W. J., Langley T. J., Melamed M. D. Bovine liver glutamate dehydrogenase: tentative amino acid sequence; identification of a reactive lysine; nitration of a specific tyrosine and loss of allosteric inhibition by guanosine triphosphate. Proc Natl Acad Sci U S A. 1970 Oct;67(2):724–730. doi: 10.1073/pnas.67.2.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLEE B. L., HOCH F. L. Zinc in horse liver alcohol dehvdrogenase. J Biol Chem. 1957 Mar;225(1):185–195. [PubMed] [Google Scholar]
- Vallee B. L., Hoch F. L. ZINC, A COMPONENT OF YEAST ALCOHOL DEHYDROGENASE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]


