Abstract
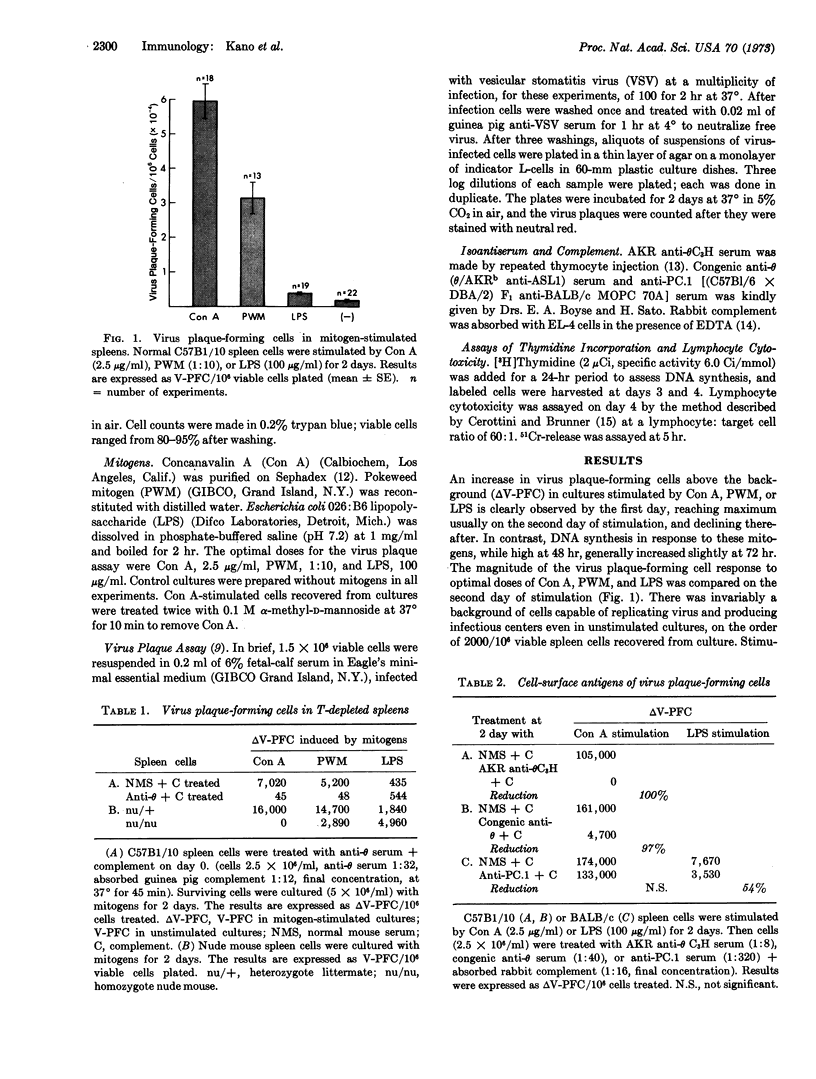
Lymphocytes activated by antigens or mitogens acquire the capacity to replicate viruses, and the number of activated lymphocytes can be estimated by the virus plaque assay. Concanavalin A and pokeweed mitogen produced 33-fold and 17-fold increases in virus plaqueforming cells (V-PFC), respectively, above background, while lipopolysaccharide produced only a 2- to 3-fold increase. T (thymus-derived lymphocyte)-depleted lymphocyte populations, derived from anti-θ-treated or nude (arthymic) mouse spleens, failed to produce V-PFC after culture with concanavalin A or pokeweed mitogen. The present studies thus demonstrate that the virus plaque assay measures activated T-lymphocytes.
A dissociation between the V-PFC response and cell proliferation was previously observed in antigen-stimulated cells cultured in the presence of mitotic inhibitors. In the present studies, while stimulation of CBA (H2k) lymphocytes by DBA/2 (H2d) cells produced high levels of thymidine incorporation, lymphocyte target-cell cytotoxicity, and V-PFC, stimulation of BALB/c (H2d) lymphocytes against DBA/2 (H2d) cells resulted in even higher levels of thymidine incorporation with a virtual absence of cytotoxic lymphocytes or V-PFC. These results indicate that proliferation is not a sufficient condition for permitting lymphocytes either to exert cytotoxicity on target cells or to replicate viruses, and suggest that there may be a correlation between the development of V-PFC and cytotoxic lymphocytes. They are consistent with the view that there are at least two functional subpopulations of T-lymphocytes.
Keywords: cell-mediated immunity, mitogen stimulation, mixed lymphocyte culture
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Mitogens as probes for immunocyte activation and cellular cooperation. Transplant Rev. 1972;11:131–177. doi: 10.1111/j.1600-065x.1972.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Bach F. H., Bach M. L., Sondel P. M., Sundharadas G. Genetic control of mixed leukocyte culture reactivity. Transplant Rev. 1972;12:30–56. doi: 10.1111/j.1600-065x.1972.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Gaffney J., Jimenez L. Dissociation of MIF production and cell proliferation. J Immunol. 1972 Dec;109(6):1395–1398. [PubMed] [Google Scholar]
- Bloom B. R. In vitro approaches to the mechanism of cell-mediated immune reactions. Adv Immunol. 1971;13:101–208. doi: 10.1016/s0065-2776(08)60184-4. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Jimenez L., Marcus P. I. A plaque assay for enumerating antigen-sensitive cells in delayed-type hypersensitivity. J Exp Med. 1970 Jul 1;132(1):16–30. doi: 10.1084/jem.132.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyse E. A., Hubbard L., Stockert E., Lamm M. E. Improved complementation in the cytotoxic test. Transplantation. 1970 Nov;10(5):446–449. doi: 10.1097/00007890-197011000-00019. [DOI] [PubMed] [Google Scholar]
- Cerottini J. C., Nordin A. A., Brunner K. T. Specific in vitro cytotoxicity of thymus-derived lymphocytes sensitized to alloantigens. Nature. 1970 Dec 26;228(5278):1308–1309. doi: 10.1038/2281308a0. [DOI] [PubMed] [Google Scholar]
- David J. R., David R. R. Cellular hypersensitivity and immunity. Inhibition of macrophage migration and the lymphocyte mediators. Prog Allergy. 1972;16:300–449. [PubMed] [Google Scholar]
- Dutton R. W. Further studies of the stimulation of DNA synthesis in cultures of spleen cell suspensions by homologous cells in inbred strains of mice and rats. J Exp Med. 1965 Oct 1;122(4):759–770. doi: 10.1084/jem.122.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton R. W. Spleen cell proliferation in response to homologous antigens studied in congenic resistant strains of mice. J Exp Med. 1966 Apr 1;123(4):665–671. doi: 10.1084/jem.123.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festenstein H., Abbasi K., Sachs J. A., Oliver R. T. Serologically undetectable immune responses in transplantation. Transplant Proc. 1972 Jun;4(2):219–222. [PubMed] [Google Scholar]
- Gery I., Krüger J., Spiesel S. Z. Stimulation of B-lymphocytes by endotoxin. Reactions of thymus-deprived mice and karyotypic analysis of dividing cells in mice bearing T 6 T 6 thymus grafts. J Immunol. 1972 Apr;108(4):1088–1091. [PubMed] [Google Scholar]
- Greaves M., Janossy G. Elicitation of selective T and B lymphocyte responses by cell surface binding ligands. Transplant Rev. 1972;11:87–130. doi: 10.1111/j.1600-065x.1972.tb00047.x. [DOI] [PubMed] [Google Scholar]
- Jimenez L., Bloom B. R., Blume M. R., Oettgen H. F. On the number and nature of antigen-sensitive lymphocytes in the blood of delayed-hypersensitive human donors. J Exp Med. 1971 Apr 1;133(4):740–751. doi: 10.1084/jem.133.4.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Lohmann-Matthes M. L., Fischer H. Specific cytotoxicity of a mouse thymocyte population sensitized in vitro against H-2 alloantigens. Eur J Immunol. 1972 Jun;2(3):290–292. doi: 10.1002/eji.1830020320. [DOI] [PubMed] [Google Scholar]
- Mauel J., Rudolf H., Chapuis B., Brunner K. T. Studies of allograft immunity in mice. II. Mechanism of target cell inactivation in vitro by sensitized lymphocytes. Immunology. 1970 Apr;18(4):517–535. [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Thymus and antigen-reactive cells. Transplant Rev. 1969;1:3–42. doi: 10.1111/j.1600-065x.1969.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Nowakowski M., Feldman J. D., Kano S., Bloom B. R. The production of vesicular stomatitis virus by antigen- or mitogen-stimulated lymphocytes and continuous lymphoblastoid lines. J Exp Med. 1973 Apr 1;137(4):1042–1059. doi: 10.1084/jem.137.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E. Production of migration inhibitory factor by non-dividing lymphocytes. J Immunol. 1973 Mar;110(3):674–678. [PubMed] [Google Scholar]
- Sprent J., Miller J. F. Activation of thymus cells by histocompatibility antigens. Nat New Biol. 1971 Sep 15;234(50):195–198. doi: 10.1038/newbio234195a0. [DOI] [PubMed] [Google Scholar]
- Stobo J. D. Phytohemagglutin and concanavalin A: probes for murine 'T' cell activivation and differentiation. Transplant Rev. 1972;11:60–86. doi: 10.1111/j.1600-065x.1972.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Old L. J., Boyse E. A. Surface alloantigens of plasma cells. J Exp Med. 1970 Jun 1;131(6):1325–1341. doi: 10.1084/jem.131.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H. The correlation between the proliferative and the cytotoxic responses of mouse lymphocytes to allogeneic cells in vitro. J Immunol. 1972 Sep;109(3):630–637. [PubMed] [Google Scholar]
- Williamson A. R., Askonas B. A. Senescence of an antibody-forming cell clone. Nature. 1972 Aug 11;238(5363):337–339. doi: 10.1038/238337a0. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Howard J. C., Nowell P. C. Some biological aspects of lymphocytes reactive to strong histocompatibility alloantigens. Transplant Rev. 1972;12:3–29. doi: 10.1111/j.1600-065x.1972.tb00051.x. [DOI] [PubMed] [Google Scholar]



