Abstract
Background
Preclinical models are needed to inform regulation of tobacco products by the Food and Drug Administration (FDA). Typically, animal models of tobacco addiction involve exposure to nicotine alone or nicotine combined with isolated tobacco constituents (e.g., minor alkaloids). The goal of this study was to develop a model using extracts derived from tobacco products that contain a range of tobacco constituents to more closely model product exposure in humans.
Methods
This study compared the addiction-related effects of nicotine alone and nicotine dose-equivalent concentrations of aqueous smokeless tobacco extracts on intracranial self-stimulation (ICSS) in rats. Extracts were prepared from Kodiak Wintergreen, a conventional product, or Camel Snus, a potential “modified risk tobacco product”. Binding affinities of nicotine alone and extracts at various nicotinic acetylcholine receptor (nAChR) subtypes were also compared.
Results
Kodiak and Camel Snus extracts contained levels of minor alkaloids within the range of those shown to enhance nicotine’s behavioral effects when studied in isolation. Nonetheless, acute injection of both extracts produced reinforcement-enhancing (ICSS threshold-decreasing) effects similar to those of nicotine alone at low to moderate nicotine doses, as well as similar reinforcement-attenuating/aversive (ICSS threshold-increasing) effects at high nicotine doses. Extracts and nicotine alone also had similar binding affinity at all nAChRs studied.
Conclusions
Relative nicotine content is the primary pharmacological determinant of the abuse liability of Kodiak and Camel Snus as measured using ICSS. These models may be useful to compare the relative abuse liability of other tobacco products and to model FDA-mandated changes in product performance standards.
Keywords: Nicotine, smokeless tobacco, non-nicotine tobacco constituents, extract, intracranial self-stimulation, policy
1. INTRODUCTION
The 2009 Family Smoking Prevention and Tobacco Control Act provides the Food and Drug Administration (FDA) regulatory authority over tobacco products (U.S. Congress, 2009; Deyton et al., 2010; Hatsukami et al., 2012, 2010; Zeller and Hatsukami, 2009). Among many other provisions under this law, the FDA has the authority to set performance standards for current tobacco products, including reductions in nicotine yields or levels of other constituents, if deemed appropriate for protection of public health. Part of this process includes evaluating the relative abuse liability of new tobacco products prior to marketing to determine if they are substantially equivalent to current products. That is, it must be determined whether they have the same characteristics (e.g., ingredients, design) as currently-marketed products; or have different characteristics, but do not pose a new or increased threat to public health (U.S. Congress, 2009). The tobacco industry has introduced several potential “modified risk tobacco products” (MRTPs) claimed to be safer than conventional tobacco products due to their lower levels of toxicants (e.g., tobacco-specific nitrosamines). However, they may not be safer in other respects, such as abuse liability (Hatsukami et al., 2012, 2007, 2010; Pederson and Nelson, 2007; Zeller and Hatsukami, 2009). Development of appropriate methodology for premarket evaluation of the relative abuse liability of potential MRTPs and other tobacco products is needed to inform FDA regulatory policy.
The Institute of Medicine has specifically recommended the use of animal models for the evaluation of tobacco products (Stratton et al., 2001), as they avoid limitations associated with human studies (e.g., inability to isolate the role of nicotine and other tobacco constituents from other factors). Animal models of tobacco addiction typically involve administration of nicotine alone or nicotine combined with other tobacco constituents (e.g., minor alkaloids, acetaldehyde) (Belluzzi et al., 2005; Clemens et al., 2009; Villegier et al., 2007). This approach is not sufficient to evaluate the abuse liability of tobacco products because as yet unidentified compounds may contribute (positively or negatively) to tobacco abuse. Moreover, it is the interaction of these compounds that ultimately determines the abuse liability of a product. Another limitation of many preclinical studies of isolated constituents is that the doses administered may not match the doses delivered during actual tobacco product use (Harris et al., 2012).
Animal models using extracts derived from tobacco or tobacco smoke and containing a comprehensive range of constituents would more accurately simulate tobacco product exposure in humans. Limited data address the feasibility and utility of this approach. Delivery of nicotine in extracts can enhance its addiction-related neurobiological and behavioral effects (Ambrose et al., 2007; Brennan et al., 2013a, 2014; Costello et al., 2014; Touiki et al., 2007), consistent with the ability of certain isolated non-nicotine constituents (e.g., minor alkaloids) to mimic or enhance nicotine’s effects in these assays (e.g., Bardo et al., 1999; Belluzzi et al., 2005; Dwoskin et al., 1999; Foddai et al., 2004; Guillem et al., 2005; Villegier et al., 2007). Additional behavioral and neurobiological evaluation of tobacco extracts is needed to further develop this approach for the evaluation of tobacco products by the FDA.
Intracranial self-stimulation (ICSS) has been used extensively to study the effects of nicotine and other addictive drugs on brain reinforcement systems. At low to moderate doses, nicotine lowers the minimal (threshold) stimulation intensity that maintains ICSS, reflecting its ability to enhance the function of brain reinforcement pathways and, thereby, enhance the reinforcing effects of other stimuli (Caggiula et al., 2009; Chaudhri et al., 2006; Harrison et al., 2002; Huston-Lyons and Kornetsky, 1992; Kornetsky et al., 1979; Paterson et al., 2008; Wise, 2002). This is a particularly sensitive predictor of abuse liability, as false positives are extremely rare and some addictive drugs that do not have abuse liability in i.v. self-administration models (e.g., hallucinogens) still reduce ICSS thresholds (Wise, 1996, 2002; Wise et al., 1992). At high doses, nicotine attenuates the reinforcing effects of brain stimulation and increases ICSS thresholds, a putative marker of nicotine’s acute aversive effects (Fowler et al., 2011; Kenny et al., 2003; Spiller et al., 2009). Nicotine’s reinforcement-enhancing and aversive effects are both thought to influence likelihood or rate of tobacco use (Donny et al., 2003; Laviolette and van der Kooy, 2003; Liu et al., 2007; Sellings et al., 2008; Wilmouth and Spear, 2004). Supporting the sensitivity of ICSS, we found that delivery of a high dose of nicotine in a smokeless tobacco extract produced less aversive effects in this assay compared to nicotine alone (Harris et al., 2012).
The primary goal of this study was to compare the acute effects of nicotine alone and nicotine dose-equivalent concentrations of smokeless tobacco extracts on ICSS. In our previous study (Harris et al., 2012), extracts were prepared from Kodiak Wintergreen, a popular conventional product. An important limitation of that study was that animals were not experimentally naïve. It is also well established that constituent levels within the same tobacco product can vary substantially across time (Stepanov et al., 2014, 2012). Therefore, we first evaluated the acute effects of Kodiak extract on ICSS in an attempt to replicate our previous findings. In a separate experiment, extracts were prepared from Camel Snus, a widely-marketed potential MRTP that has not been studied in a preclinical behavioral model. Levels of the behaviorally relevant minor alkaloids nornicotine, anabasine, and anatabine in Kodiak and Camel Snus extracts were also measured. Finally, binding affinities of extracts and nicotine alone at a panel of nicotinic acetylcholine receptor (nAChR) subtypes were compared. Affinities of formulations at α4ß2, α3ß4, and α7 nAChRs were of particular interest because of the important role of these nAChRs in tobacco addiction (Changeux, 2010; De Biasi and Salas, 2008; Fowler et al., 2008).
2. METHODS
2.1. Animals
Experimentally-naive male Holtzman Sprague Dawley rats (Harlan, Indianapolis, IN) weighing 250-300 g at arrival were housed individually in a temperature- and humidity-controlled colony room with unlimited access to water. Rats were housed under a reversed 12-hr light/dark cycle and tested during the dark (active) phase. Beginning one week after arrival, rats were food-restricted to ≈18 g/day rat chow to facilitate operant performance and avoid detrimental effects of long-term ad libitum feeding on health. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the 2011 NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
2.2. Drugs
Nicotine-alone solutions consisted of (−)-Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) dissolved in sterile saline. Aqueous tobacco extract was prepared from Kodiak Wintergreen or Camel Snus Winterchill smokeless tobacco products (purchased in the Minneapolis area between January, 2013 and January, 2014) using general procedures described elsewhere (Harris et al., 2012). Briefly, tobacco product was mixed with saline vehicle at a concentration of 400 mg/ml (Kodiak extract) or 200 mg/ml (Camel Snus extract) for 18 hours using a tube tipper. The different concentrations of the extracts reflects the higher volume of saline required for preparation of extract from Camel Snus, which is considerably more absorbant than Kodiak. A saline extraction produces a similar alkaloid extraction profile as artificial saliva and simplifies extract preparation while avoiding toxicity (Harris et al., 2012). The resulting solution was filtered through gauze, centrifuged, and the supernate was filtered. The nicotine concentration was determined, and extract was diluted to the nicotine concentrations required for the current studies. The pH of all solutions was adjusted to 7.4 using dilute NaOH. Nicotine doses are expressed as the base. All injections were administered s.c. in a volume of 1 ml/kg.
2.3. Experiment 1: Alkaloid analyses
Nicotine and minor alkaloid levels in Kodiak and Camel Snus extract stock solutions were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) by modification of a previously described method (Rangiah et al., 2011). Briefly, the extracts were mixed with stable isotope-labeled nicotine and nornicotine, anatabine, and anabasine (internal standards), diluted with 10 mM ammonium acetate containing 5% methanol, and analyzed by LC-MS/MS on a Hypercarb column (Thermo Scientific), using 10mM ammonium acetate (with 0.001% formic acid) and methanol as mobile phase.
2.4. Experiment 2: Effects of nicotine alone and Kodiak extract on ICSS
2.4.1. Intracranial self-stimulation
Surgery, apparatus, and training procedure used here are described in detail elsewhere (Harris et al., 2010, 2011; Roiko et al., 2009). Briefly, animals were anesthetized with i.m. ketamine (75 mg/kg)/xylazine (7.5 mg/kg) and implanted with a bipolar stainless steel electrode in the medial forebrain bundle at the level of the lateral hypothalamus. Rats were later trained to respond for electrical brain stimulation using a modified version of the Kornetsky and Esposito (1979) discrete-trial current-threshold procedure (Markou and Koob, 1992). Each session was approximately 45 minutes and provided two dependent variables: ICSS thresholds (a measure of brain reinforcement function) and response latencies (a measure of non-specific, e.g., motor, effects).
2.4.2. Experiment 2a: First assessment
Animals (N = 12) were tested in daily ICSS sessions conducted Mon-Fri until thresholds were stable (i.e., less than 10% coefficient of variation over a 5-day period and no apparent trend). To habituate animals to the injection procedure, saline was administered 10 minutes prior to ICSS testing twice per week (Tuesdays and Fridays) for at least 1 session and until thresholds were stable. Effects of 10-minute pretreatment with nicotine alone (half of the animals) or Kodiak extract (the other half) were subsequently determined at nicotine doses of 0, 0.06, 0.125, 0.25, 0.50, 0.75, 1.0, or 1.25 mg/kg. These doses bracket the range of nicotine doses that reduce or increase ICSS thresholds when administered acutely (Bauco and Wise, 1994; Harris et al., 2012; Harrison et al., 2002; Huston-Lyons and Kornetsky, 1992; Spiller et al., 2009). Nicotine and extract injections typically occurred on Tuesdays and Fridays, provided that thresholds were within baseline range on intervening days. Doses were administered in a counterbalanced order. Following completion of dose-response testing, animals were tested for ICSS under drug-free conditions for at least 2 weeks and until ICSS thresholds were stable. All rats then underwent the same procedure as described above with the exception that formulation (i.e., nicotine alone versus extract) was crossed-over within each subject.
2.4.3. Experiment 2b: Second assessment
Although we previously found that the ICSS threshold-elevating (i.e., aversive) effects of 0.75 mg/kg nicotine were attenuated when delivered in Kodiak extract (Harris et al., 2012), this effect was not observed in Experiment 2a (see Results). Given that age can influence the aversive effects of nicotine (O'Dell, 2009; Shram et al., 2006), this discrepancy could reflect the younger age of animals in Experiment 2a compared to those in our previous study (postnatal day (PND) at onset of dosing protocol = 132.3 +/− 6.2 versus 235.9 +/− 20.5, respectively). To evaluate this possibility, rats from Experiment 2a were tested for ICSS under drug-free conditions for at least one month and until ICSS thresholds were stable, at which point their age (PND = 274.4 +/− 6.8) did not differ from that of animals in our previous study (Harris et al., 2012). Rats were then tested as described in Experiment 2a.
2.5. Experiment 3: Effects of nicotine alone and Camel Snus extract on ICSS
A separate group of 10 rats was tested in Experiments 3a and 3b exactly as described in Experiments 2a and 2b, respectively, except that rats were administered extract prepared from Camel Snus rather than Kodiak smokeless tobacco.
2.6. Experiment 4: Binding affinities of nicotine alone and extracts at nAChRs
Radioligand ([3H]-epibatidine) binding assays were conducted using solutions of nicotine alone, Kodiak extract, and Camel Snus extract (nicotine content for all solutions = 0.6 mg/ml) by the National Institute of Mental Health-Psychoactive Drug Screening Program. Detailed descriptions of these assays, which are based on procedures reported in (Xiao et al., 2006; Xiao et al., 1998), are available at http://pdsp.med.unc.edu. Briefly, assays were conducted using human embryonic kidney (HEK) 293 cell lines stably expressing human α4ß2, α3ß4, α2ß2, α2ß4, α3ß2, or α4ß4 nAChR subtypes, or the rat α7 nAChR subtype Assays were also conducted using α4ß2 or α7 nAChRs expressed in rat forebrain or cortex, respectively. Primary binding assays were performed in quadruplicate. Test compounds with a minimum of 25% inhibition of radioligand-specific binding in primary binding assays were subjected to secondary binding assays to determine binding affinity.
2.7. Statistical analyses
In Experiments 2 and 3, paired baseline ICSS threshold and latency values (in µA and sec, respectively) were compared between formulations (i.e., nicotine alone versus extract) using mixed effects linear regression with fixed effects for formulation and formulation testing order and a random effect for rat. ICSS threshold and latency values during testing, expressed as a percent of baseline (i.e., mean during last 5 sessions prior to each dose-response determination), were analyzed to account for the crossover repeated measures study design using a mixed effects linear regression model with fixed effects for dose, formulation, formulation testing order, and a formulation by treatment interaction, and a random effect for rat. Among the subset of rats that completed Experiments 2b and 3b, ICSS threshold and latency data from the first and second assessments were compared within-subjects to confirm that these measures did not differ. Means ± standard error of the mean (SEM) are reported unless otherwise noted. P-values reported for multiple comparisons within each outcome were adjusted to reduce the false discovery rate using the Benjamini and Hochberg method (Benjamini and Hochberg, 1995). In all experiments, p-values ≤ 0.05 were considered statistically significant.
3. RESULTS
3.1. Experiment 1: Alkaloid Levels in extracts
Levels of nicotine, nornicotine, and anabasine in the current Kodiak extract were similar to those in the Kodiak extract used in our previous study (2012; Table 1). However, anatabine levels (expressed as absolute levels or as % of nicotine) were higher in the current Kodiak extract.
Table 1.
Nicotine and minor alkaloid levels (μg/mL) in Kodiak extract used in our previous study (Harris et al., 2012), as well as Kodiak and Camel Snus extracts in Experiments 2 and 3. Data in parentheses indicate relative levels of each minor alkaloid (expressed as % of nicotine) in that extract. Total Minor = Nornicotine + Anabasine + Anatabine.
| Nicotine | Nornicotine | Anabasine | Anatabine | Total Minor | |
|---|---|---|---|---|---|
| Kodiak (2012) | 3110 | 28.7 (0.9%) | 14.8 (0.5%) | 10.8 (0.3%) | 54.3 (1.7%) |
| Kodiak | 3430 | 21.7 (0.6%) | 14.2 (0.4%) | 41.8 (1.2%) | 77.7 (2.3%) |
| Camel Snus | 2110 | 25.9 (1.2%) | 12.8 (0.6%) | 18.9 (0.9%) | 57.6 (2.7%) |
Comparison of alkaloid levels in the current Kodiak and Camel Snus extracts indicated that absolute levels of nicotine and minor alkaloids were lower in Camel Snus extract. However, minor alkaloid levels were similar across products when expressed as % of nicotine (Table 1).
Table 2 shows the range of nicotine and minor alkaloid exposure levels (µg/kg/session) in rats following experimenter-administered Kodiak or Camel Snus extracts in Experiments 2 and 3, as well as during i.v. self-administration of a tobacco constituent cocktail that had greater reinforcing effects than nicotine alone (data derived from Fig 4 in Clemens et al., 2009). There was considerable overlap in exposure levels (see Table 2), suggesting that the current extract doses contained behaviorally relevant levels of minor alkaloids.
Table 2.
Range of nicotine and minor alkaloid exposure levels (μg/kg/session) in rats following experimenter-administered Kodiak or Camel Snus extracts in Experiments 2 and 3, or self-administration of a tobacco constituent cocktail during a within-session dose-response test in Clemens et al. (2009), (estimated from Figure 4 of that paper; training doses = 30, 0.9, 0.9, and 0.09 ug/kg for nicotine, nornicotine, anabasine, and anatabine, respectively). Total Minor = Nornicotine + Anabasine + Anatabine.
| Nicotine | Nornicotine | Anabasine | Anatabine | Total Minor | |
|---|---|---|---|---|---|
| Kodiak Extract | 60-1250 | 0.4-7.9 | 0.3-5.2 | 0.7-15.2 | 1.4-28.3 |
| Camel Snus Extract | 60-1250 | 0.7-15.4 | 0.4-7.6 | 0.5-11.2 | 1.6-34.3 |
| Clemens et al (2009) | 139-312 | 4.2-9.4 | 4.2-9.4 | 0.4-0.9 | 8.8-19.7 |
3.2. Experiment 2: Effects of nicotine alone and Kodiak extract on ICSS
3.2.1. Experiment 2a: First assessment
The nicotine alone and Kodiak extract dose-response determinations did not differ in terms of baseline thresholds (109.6 ± 8.2 µA versus 106.2 ± 6.6 µA) or response latencies (2.93 ± 0.2 seconds versus 2.92 ± 0.2 seconds).
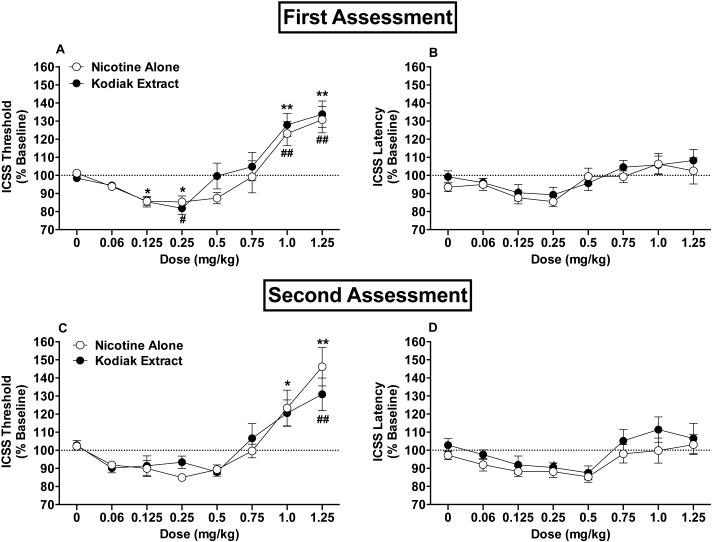
Kodiak extract and nicotine alone produced similar effects on ICSS thresholds (Fig 1A). There was a statistically significant main effect of dose (F(7,165)=27.7, p <0.0001), but the formulation and dose x formulation interaction effects were not significant. Thresholds did not differ between formulations at any dose (Fig 1A). For the nicotine alone condition, ICSS thresholds were significantly reduced compared to saline at 0.125 (p = 0.045) and 0.25 (p = 0.045) mg/kg and elevated compared to saline at 1.0 (p = 0.005) and 1.25 (p = 0.0004) mg/kg (Fig 1A). For Kodiak extract, ICSS thresholds were significantly reduced compared to saline at 0.25 (p = 0.041) mg/kg and elevated compared to saline at 1.0 (p = 0.004) and 1.25 (p = 0.004) mg/kg.
Figure 1.
ICSS thresholds (A) and response latencies (B) (expressed as percent of baseline, mean ± SEM) following injection of nicotine alone or Kodiak extract (0 - 1.25 mg/kg) in Experiment 2a. Fig (C) and (D) show ICSS threshold and latency data from Experiment 2b (second assessment). *,** Significantly different from saline (0 mg/kg) for nicotine alone, p < 0.05 or 0.01. #,## Significantly different from saline (0 mg/kg) for Kodiak extract, p < 0.05 or 0.01.
There was a significant main effect of dose on response latencies (F(7,165)=6.0, p <0.0001), but the effects for formulation and dose x formulation interaction were not significant (Fig 1B). Latencies did not significantly differ from saline at any dose for either formulation.
3.2.2. Experiment 2b: Second assessment
Four animals were lost to attrition prior to completion of this experiment due to removal of their ICSS headcap or loss of stability of ICSS thresholds. Only data for the remaining 8 animals that completed the protocol are reported below. In this subset of animals, ICSS threshold and latency data did not differ across the first and second assessments for either the nicotine alone or Kodiak extract condition (p > 0.05).
Data were similar to those in Experiment 2a, indicating that advanced age did not influence effects of nicotine alone or extract in this model. Baseline thresholds (100.1 ± 9.7 µA versus 103.1 ± 9.6 µA) and response latencies (2.7 ± 0.1 seconds versus 2.6 ± 0.1 seconds) did not significantly differ between the nicotine alone and Kodiak extract dose-response determinations. There was a significant main effect of dose on ICSS thresholds (F(7,105)=24.3, p <0.0001; Fig 1C), but the formulation and dose x formulation interaction effects were not significant. Compared to saline, ICSS thresholds were significantly elevated at the 1.0 (p = 0.024) and 1.25 (p = 0.001) mg/kg doses of nicotine alone and at the 1.25 (p = 0.001) mg/kg dose of extract. Neither formulation significantly reduced ICSS thresholds compared to saline.
There were significant main effects of dose (F(7,105)=7.1, p <0.0001) and formulation (F(7,105)=6.9, p = 0.010) on response latencies, although the dose x formulation interaction effect was not significant (Fig 1D). Latencies for Kodiak extract were somewhat higher than for nicotine alone, but formulations did not differ significantly at any dose after adjustment for multiple comparisons. Latencies also did not differ from saline at any dose for either formulation.
3.3. Experiment 3: Effects of nicotine alone and Camel Snus extract on ICSS
3.3.1. Experiment 3a: First assessment
Baseline thresholds were slightly higher for the Camel Snus dose-response determination compared to the nicotine alone dose-response determination (117.1 ± 14.7 µA versus 106.7 ± 14.4 µA, respectively; F(1,9)= 6.21, p = 0.034). This small (<%10) difference in baseline thresholds between the two conditions is controlled for in the analysis by expressing data as percentage of baseline. Baseline response latencies did not differ significantly (2.7 ± 0.11 sec versus 2.6 ± 0.09 sec).
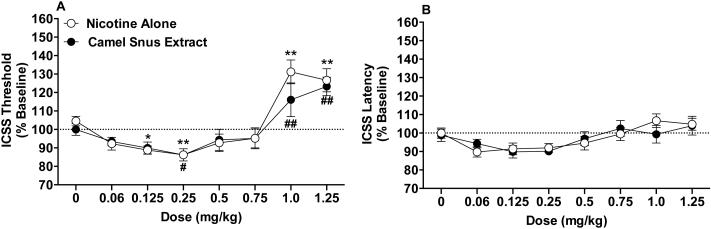
Nicotine alone and Camel Snus extract produced similar effects on ICSS thresholds (Fig 2A). There was a significant main effect of dose on threshold (F(7,135)=24.4, p <0.0001), but the formulation or dose x formulation interaction effects were not significant. The apparent difference between formulations at the 1.0 mg/kg dose was not significant following adjustment for multiple comparisons (p = 0.098). For nicotine alone, thresholds were significantly reduced compared to saline at the 0.125 (p = 0.028) and 0.25 (p = 0.008) mg/kg doses and elevated compared to saline at the 1.0 (p = 0.001) and 1.25 (p = 0.002) mg/kg doses. For Camel Snus extract, ICSS thresholds were reduced compared to saline at the 0.25 mg/kg dose (p = 0.050) and elevated compared to saline at the 1.0 (p = 0.004) and 1.25 (p = 0.001) mg/kg doses.
Figure 2.
ICSS thresholds (A) and response latencies (B) (expressed as percent of baseline, mean ± SEM) following injection of nicotine alone or Camel Snus extract (0 - 1.25 mg/kg) in Experiment 3a. *,** Significantly different from saline (0 mg/kg) for nicotine alone, p < 0.05 or 0.01. #,## Significantly different from saline (0 mg/kg) for Camel Snus, p ≤ 0.05 or 0.01.
There was a significant main effect of dose on response latencies (F(7,135)=5.7, p <0.0001), but the formulation or dose x formulation interaction effects were not significant (Fig 2B). Latencies did not differ significantly from saline at any dose for either formulation.
3.3.2. Experiment 3b: Second assessment
Five animals were lost to attrition prior to completion of this experiment due to removal of ICSS headcap or loss of stability of ICSS thresholds. Analysis of data for the 5 animals that completed the protocol indicated a similar pattern of results as in Experiment 3a (data not shown).
3.4. Experiment 4: Binding affinities of nicotine alone and extracts at nAChRs
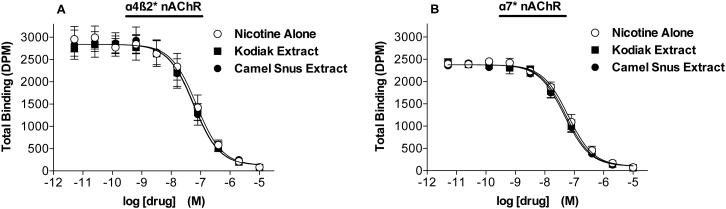
Nicotine alone, Kodiak extract, and Camel Snus extract produced similar, marked (>90%) inhibition of labeled epibatadine binding to all nAChRs in primary binding assays (data not shown). Results of secondary binding assays indicated that the three formulations had similar binding affinities at all nAChR subtypes studied (Table 3; see Fig 3A and 3B for competition binding curves for α4ß2 and α7 nAChRs expressed in rat brain).
Table 3.
Binding affinities for Kodiak extract, Camel Snus extract, and nicotine alone at nAChRs expressed in HEK 393 cells or rat brain (denoted by *) and labeled by [3H]-epibatidine. Values represent mean Ki (nM) and 95% CI (in parentheses) from 2-3 independent determinations.
| Kodiak Extract | Camel Extract | Nicotine Alone | |
|---|---|---|---|
| α4β2 | 3.9 (2.8-5.0) | 3.8 (2.3-5.3) | 4.4 (1.5-7.3) |
| α4li2* | 6.3 (3.7-8.9) | 6.6 (2.9-10.3) | 7.9 (6.9-8.9) |
| α7 | 202.1 (-409.8-813.9) | 195.3 (108.7-281.8) | 323.5 (189.5-457.4) |
| α7* | 4.4 (2.8-6.0) | 4.9 (4.2-5.6) | 5.9 (2.6-9.2) |
| α3β4 | 168.4 (81.3-255.6) | 177.5 (109.1-245.8) | 230.6 (148.5-312.7) |
| α2β2 | 2.9 (0.3-5.4) | 2.9 (1.4-4.4) | 3.1 (2.3-3.8) |
| α2β4 | 39.7 (24.3-55.1) | 44.5 (16.6-72.5) | 48.3 (13.4-83.1) |
| α3β2 | 4.9 (3.1-6.7) | 5.7 (5.0-6.3) | 5.3 (4.3-6.3) |
| α4β4 | 17.9 (4.9-31.0) | 17.6 (10.3-24.9) | 22.2 (12.4-32.1) |
Figure 3.
Competition by nicotine alone, Kodiak extract, and Camel Snus extract for α4ß2 (A) or α7 (B) nAChR binding sites expressed in rat brain and labeled by [3H]-epibatidine. Binding data (mean DPM ± SEM) are pooled across 2-3 experiments. Ki values for formulations at these and other nAChRs are shown in Table 3.
4. DISCUSSION
The present study examined the utility of a preclinical ICSS model for evaluating the abuse liability of widely-available conventional and potential modified-risk smokeless tobacco products. Acute injection of Kodiak or Camel Snus extracts produced reinforcement-enhancing (ICSS threshold-decreasing) effects similar to nicotine alone at low to moderate nicotine doses, as well as similar reinforcement-attenuating/aversive (ICSS threshold-increasing) effects at high nicotine doses. Nicotine alone and dose-matched concentrations of Kodiak or Camel Snus extracts also had similar binding affinity at a range of nAChR subtypes. These findings indicate that nicotine content is the primary pharmacological determinant of the abuse liability of Kodiak and Camel Snus smokeless tobacco as measured using this model.
Our findings complement reports of similar effects of nicotine alone and nicotine dose-equivalent concentrations of tobacco or tobacco smoke extracts in other behavioral models (e.g., cue-induced reinstatement of i.v. self-administration, locomotor sensitization; Brennan et al., 2014, 2013c; Costello et al., 2014; Harris et al., 2012). To the extent that these findings predict tobacco use in humans and generalize to other products, they suggest that levels of non-nicotine constituents found in Kodiak and Camel Snus do not significantly contribute to the abuse liability of these products in terms of their direct CNS effects. However, further research is needed to determine if higher levels of non-nicotine constituents modulate the abuse-related CNS effects of smokeless tobacco and/or contribute to abuse liability in other respects (e.g., taste or other peripheral sensory effects). Such work will help determine whether and to what extent substantial equivalence claims regarding the abuse liability of new smokeless tobacco products need to account for levels of non-nicotine constituents.
Our findings contrast with reports of greater reinforcing effects of tobacco or tobacco smoke extracts compared to nicotine alone on certain measures of i.v. self-administration (e.g., acquisition; Brennan et al., 2013a, 2014; Costello et al., 2014). Factors that could account for this discrepancy include differences in dependent measure, nicotine dose, and rat strain. Differences in route of administration may also be significant given that the effects of nicotine and other drugs can differ when delivered via i.v. infusion versus s.c. injection (e.g., Bardo et al., 1995; Matta et al., 2007). Differences in source of extraction (i.e., tobacco versus cigarette smoke) and profile of behaviorally-relevant non-nicotine constituents between extracts may also account for the discrepancy. The latter possibility is difficult to evaluate, however, as characterization of non-nicotine constituents in extracts in other studies has been limited to minor alkaloids (Harris et al., 2012) and the beta-carbolines harman and norharman (Brennan et al., 2013a), or not conducted at all. Implementation of standardized chemical profiling of extracts (i.e., measuring levels of the same comprehensive list of constituents) will help to reconcile inconsistencies across studies.
The extracts used in the present study contained levels of nornicotine, anabasine, and anatabine that were within the range of those shown to enhance the effects of nicotine in an i.v. self-administration assay when administered as a cocktail in isolation from tobacco smoke (Clemens et al., 2009). As such, our extracts contained levels of minor alkaloids previously shown to be behaviorally relevant. The lack of behavioral effects of these minor alkaloids in the current study could reflect a variety of methodological differences across studies (e.g., dependent measure, route of administration, etc.). In addition, while the cocktail used in Clemens et al. (2009) contained only nicotine and five minor alkaloids, the current extracts contained a range of other tobacco constituents to more closely model actual tobacco product exposure in humans. It is possible that one or more unidentified constituents in the extracts opposed effects of minor alkaloids that would otherwise have been expressed if studied in isolation. This account would support the notion that studying the relationship between nicotine and non-nicotine constituents in a product-derived mixture can provide information that is unique from that provided by the study of isolated tobacco constituents (Brennan et al., 2014, 2013b, 2013c; Costello et al., 2014; Harris et al., 2012).
Our evaluation of Camel Snus provides the first characterization of a potential MRTP in a preclinical model of addiction. Extending our ICSS model to this product is important because Camel Snus or other potential MTRPs can differ from conventional products in terms of their levels of behaviorally relevant non-nicotine constituents (e.g., acetaldehyde; Stepanov et al., 2008), subjective effects in humans (Cobb et al., 2009; Gray et al., 2008; Kotlyar et al., 2007). and relative market share (Delnevo et al., 2014). Among potential MRTPs, Camel Snus is of particular interest given that this product is showing significant growth in sales (Delnevo et al., 2014) and is being evaluated as a tool for reducing carcinogen exposure and facilitating cessation in smokers (Burris et al., 2014; Kotlyar et al., 2011).
The fact that Kodiak and Camel Snus extracts were tested in separate experiments precludes a direct comparison of their relative abuse liability and represents a limitation of this study. Nonetheless, the very similar pattern of results across experiments suggests similar abuse liability of both products compared to nicotine alone, at least as measured using ICSS. As such, differences in the use of Kodiak and Camel Snus in humans may reflect differences in their nicotine delivery or factors not related to direct CNS effects (e.g., marketing, peripheral sensory effects such as taste, etc.). Nonetheless, direct comparison of the relative abuse liability of these and other tobacco products using ICSS and other behavioral measures (e.g., i.v. self-administration) is needed to provide a comprehensive assessment of this issue. This approach may also be useful for substantial equivalence testing as required by the FDA.
We previously found that the ICSS threshold-elevating effects of 0.75 mg/kg nicotine were attenuated when delivered in Kodiak extract (Harris et al., 2012). In the current study, higher doses of nicotine alone (1.0 and 1.25 mg/kg) were required to elicit ICSS threshold elevations, and these effects were not attenuated in the Kodiak extract condition. The cause of this discrepancy is unclear, but is apparently unrelated to differences in the age of the animals across the two studies (see Experiment 2b). Another potentially important methodological difference is that animals in our current study were experimentally naïve while those in our previous study had been exposed to a chronic infusion of saline or nicotine (3.2 mg/kg/day, s.c.). Prior infusion condition (nicotine versus saline) did not influence the pattern of results, suggesting that data were not influenced by a history of nicotine exposure per se (see Harris et al., 2012). Nonetheless, other aspects of the experimental history of all animals in our previous study (e.g., surgical implantation/removal of osmotic pumps) may limit the generalizability of these findings.
Differences in the chemical composition of the Kodiak extract itself may also account for the contrasting results. Despite being prepared under identical conditions as in our previous study, the current Kodiak extract contained somewhat higher levels of anatabine (see Table 2). While these findings could reflect a variety of factors (e.g., variability in the extraction procedure, etc.), they could reflect a true change in the chemical composition of Kodiak smokeless tobacco over time as has been observed for other products (Stepanov et al., 2014, 2012). Any changes in levels of minor alkaloids or other unmeasured, behaviorally active non-nicotine constituents (e.g., acetaldehyde) in this product over time complicate comparison of the current findings with our previous study.
Our data do not rule out a potential involvement of non-nicotine constituents in the acute effects of Kodiak and Camel Snus extract on ICSS. For example, differences could emerge between extracts and nicotine alone following a pharmacological challenge (see Brennan et al., 2013b). Given that some non-nicotine constituents require repeated dosing to produce an enhancement of nicotine’s effects (Clemens et al., 2009; Villegier et al., 2007), comparing the effects of chronic exposure to nicotine alone and extracts could also reveal differences between formulations. This approach would also better simulate the chronic nature of tobacco use in humans than the current acute exposure models. It is also possible that changes in individual constituent concentrations in these products might reveal other interactions in the ICSS model.
Nicotine alone and extracts had similar binding affinity at several nAChR subtypes, including the α4ß2, α3ß4, and α7 nAChR subtypes that contribute to tobacco addiction (Changeux, 2010; De Biasi and Salas, 2008; Fowler et al., 2008). These findings, which provide the first characterization of nAChR binding affinity for nicotine delivered in a smokeless tobacco extract, complement the behavioral data and are consistent with a report that tobacco smoke extract and nicotine alone had similar binding affinity at several neuronal nAChRs including α4ß2 and α3ß4 nAChRs (Costello et al., 2014). It remains to be determined whether tobacco extracts and nicotine alone differentially alter nAChR function or exert important effects at non-nACh receptors.
In conclusion, the current studies found that the abuse liability of nicotine alone and Kodiak or Camel Snus extracts were similar as measured using ICSS. The use of tobacco extracts is feasible and improves the face validity of animal models by incorporating a wide range of non-nicotine constituents and allowing study of the sum of their interactions as occurs with actual tobacco product use. To the extent that this approach also improves the predictive validity of animal models, the present model may be useful for comparing the relative abuse liability of other tobacco products to conduct premarket testing of new products and to model FDA-mandated changes in product performance standards.
Highlights.
We tested effects of tobacco extracts on intracranial self-stimulation in rats.
Effects of nicotine alone and dose-equivalent extract concentrations did not differ.
Nicotine alone and extracts also had similar binding affinity at nicotinic receptors.
Relative nicotine content determines the abuse liability of extracts in this model.
Acknowledgements
The authors would like to thank Danielle Burroughs for technical assistance. Ki determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA. For experimental details please refer to the PDSP web site http://pdsp.med.unc.edu/ and click on "Binding Assay" on the menu bar.
Role of Funding Source. This work was supported by NCI U19-CA157345 (Hatsukami, DK and Shields, PG, Co-PI; LeSage, PL), the Minneapolis Medical Research Foundation (MMRF) Translational Addiction Research Program (Harris, PI), and the University of Minnesota Undergraduate Research Opportunity Program (UROP, Schmidt, Muelken, PI). The NIH, Minneapolis Medical Research Foundation, and University of Minnesota had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. All authors declare that they have no conflict of interest.
REFERENCES
- Ambrose V, Miller JH, Dickson SJ, Hampton S, Truman P, Lea RA, Fowles J. Tobacco particulate matter is more potent than nicotine at upregulating nicotinic receptors on SH-SY5Y cells. Nicotine Tob. Res. 2007;9:793–799. doi: 10.1080/14622200701485117. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology. 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci. Biobehav. Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J. Pharmacol. Exp. Ther. 1994;271:294–301. [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a powerful and practical approach to multiple testing. in behavior genetics research. J. R. Stat. Soc. Series B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- Brennan KA, Crowther A, Putt F, Roper V, Waterhouse U, Truman P. Tobacco particulate matter self-administration in rats: differential effects of tobacco type. Addict. Biol. epub. 2013a doi: 10.1111/adb.12099. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Laugesen M, Truman P. Whole tobacco smoke extracts to model tobacco dependence in animals. Neurosci. Biobehav. Rev. 2014;47C:53–69. doi: 10.1016/j.neubiorev.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Putt F, Roper V, Waterhouse U, Truman P. Nicotine and tobacco particulate self-administration: effects of mecamylamine, SCH23390 and ketanserin pretreatment. Curr. Psychopharmacol. 2013b;2:229–240. [Google Scholar]
- Brennan KA, Putt F, Truman P. Nicotine-, tobacco particulate matter- and methamphetamine-produced locomotor sensitisation in rats. Psychopharmacology. 2013c;228:659–672. doi: 10.1007/s00213-013-3071-3. [DOI] [PubMed] [Google Scholar]
- Burris JL, Carpenter MJ, Wahlquist AE, Cummings KM, Gray KM. Brief, instructional smokeless tobacco use among cigarette smokers who do not intend to quit: a pilot randomized clinical trial. Nicotine Tob. Res. 2014;16:397–405. doi: 10.1093/ntr/ntt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr. Symp. Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat. Rev. Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int. J. Neuropsychopharmacol. 2009;12:1355–1366. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Cobb CO, Weaver MF, Eissenberg T. Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tob. Control. 2009;19:367–373. doi: 10.1136/tc.2008.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39:1843–1851. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M, Salas R. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp. Biol. Med. 2008;233:917–929. doi: 10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- Delnevo CD, Wackowski OA, Giovenco DP, Manderski MT, Hrywna M, Ling PM. Examining market trends in the United States smokeless tobacco use: 2005-2011. Tob. Control. 2014;23:107–112. doi: 10.1136/tobaccocontrol-2012-050739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyton L, Sharfstein J, Hamburg M. Tobacco product regulation--a public health approach. N. Engl. J. Med. 2010;362:1753–1756. doi: 10.1056/NEJMp1004152. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology. 1999;145:442–451. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav. Pharmacol. 2008;19:461–484. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JN, Breland AB, Weaver M, Eissenberg T. Potential reduced exposure products (PREPs) for smokeless tobacco users: clinical evaluation methodology. Nicotine Tob. Res. 2008;10:1441–1448. doi: 10.1080/14622200802323258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J. Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Mattson C, Lesage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol. Biochem. Behav. 2010;96:217–227. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology (Berl.) 2011;217:153–166. doi: 10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Stepanov I, Pentel PR, Lesage MG. Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats. Psychopharmacology (Berl.) 2012;220:565–576. doi: 10.1007/s00213-011-2514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl.) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller M. Nicotine reduction: strategic research plan. Nicotine Tob. Res. 2012;15:1003–1013. doi: 10.1093/ntr/nts214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products new tobacco-delivery systems. Am. J. Prev. Med. 2007;33:S368–378. doi: 10.1016/j.amepre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger CL, Zeller M. Nicotine reduction revisited: science and future directions. Tob. Control. 2010;19:e1–10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol. Biochem. Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur. J. Neurosci. 2003;17:191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed. Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch. Gen. Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Hertsgaard LA, Lindgren BR, Jensen JA, Carmella SG, Stepanov I, Murphy SE, Hecht SS, Hatsukami DK. Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study. Cancer Epidemiol. Biomarkers Prev. 2011;20:91–100. doi: 10.1158/1055-9965.EPI-10-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar M, Mendoza-Baumgart MI, Li ZZ, Pentel PR, Barnett BC, Feuer RM, Smith EA, Hatsukami DK. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tob. Control. 2007;16:138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Mol. Psychiatry. 2003;8:50–59. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- Liu X, Palmatier MI, Caggiula AR, Donny EC, Sved AF. Reinforcement enhancing effect of nicotine and its attenuation by nicotinic antagonists in rats. Psychopharmacology. 2007;194:463–473. doi: 10.1007/s00213-007-0863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol. Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- O’Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56(Suppl. 1):263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob. Res. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Pederson LL, Nelson DE. Literature review and summary of perceptions, attitudes, beliefs, and marketing of potentially reduced exposure products: communication implications. Nicotine Tob. Res. 2007;9:525–534. doi: 10.1080/14622200701239548. [DOI] [PubMed] [Google Scholar]
- Rangiah K, Hwang WT, Mesaros C, Vachani A, Blair IA. Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LC-MS. Bioanalysis. 2011;3:745–761. doi: 10.4155/bio.11.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, LeSage MG, Keyler DE, Pentel PR. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacol. Biochem. Behav. 2009;93:105–111. doi: 10.1016/j.pbb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LH, Baharnouri G, McQuade LE, Clarke PB. Rewarding and aversive effects of nicotine are segregated within the nucleus accumbens. Eur. J. Neurosci. 2008;28:342–352. doi: 10.1111/j.1460-9568.2008.06341.x. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology. 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Li X, Ashby CR, Jr., Callahan PM, Tehim A, Gardner EL. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology. 2009;57:60–66. doi: 10.1016/j.neuropharm.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Biener L, Yershova K, Nyman AL, Bliss R, Parascandola M, Hatsukami DK. Monitoring tobacco-specific n-nitrosamines and nicotine in novel smokeless tobacco products: findings from round ii of the new product watch. Nicotine Tob. Res. 2014;16:1070–1078. doi: 10.1093/ntr/ntu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Biener L, Bliss RL, Hecht SS, Hatsukami DK. Increased pouch sizes and resulting changes in the amounts of nicotine and tobacco-specific N-nitrosamines in single pouches of Camel Snus and Marlboro Snus. Nicotine Tob. Res. 2012;14:1241–1245. doi: 10.1093/ntr/ntr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob. Res. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tob. Control. 2001;10:189–195. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touiki K, Rat P, Molimard R, Chait A, de Beaurepaire R. Effects of tobacco and cigarette smoke extracts on serotonergic raphe neurons in the rat. Neuroreport. 2007;18:925–929. doi: 10.1097/WNR.0b013e32811d6d21. [DOI] [PubMed] [Google Scholar]
- U.S. Congress . Family Smoking Prevention and Tobacco Control Act. US Government Printing Office; 2009. Retrieved from http://wwwgpogov/ [Google Scholar]
- Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52:1415–1425. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Adolescent and adult rats' aversion to flavors previously paired with nicotine. Ann. N. Y. Acad. Sci. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu. Rev. Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr., Trojniar W. Self-stimulation and drug reward mechanisms. Ann. N. Y. Acad. Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol. Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol. Pharmacol. 1998;54:322–333. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]
- Zeller M, Hatsukami D. The Strategic Dialogue on Tobacco Harm Reduction: a vision and blueprint for action in the US. Tob. Control. 2009;18:324–332. doi: 10.1136/tc.2008.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]