Abstract
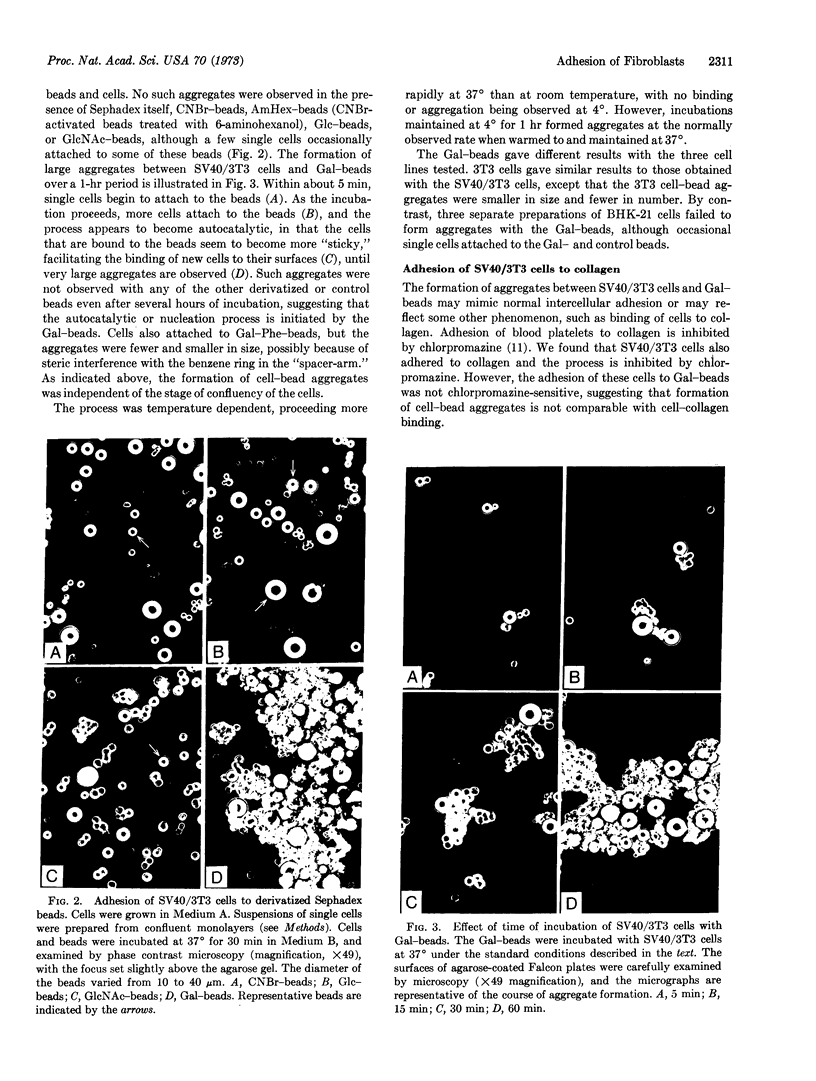
Animal cells are coated with complex carbohydrates. Insoluble analogues of these substances were prepared by coupling monosaccharides to Sephadex beads (crosslinked dextran), and the interactions between these derivatives and three established cell lines were studied. A virally transformed fibroblast, simian virus 40-transformed 3T3 cells, adhered to the beads derivatized with D-galactose, but did not adhere to the corresponding beads derivatized with D-glucose or N-acetyl-D-glucosamine. Cells that adhered to the galactose-beads appeared to initiate a nucleation process in that they became more adhesive towards the cells in suspension, leading to the formation of large aggregates containing both cells and galactose-beads. The results suggest that specific carbohydrates are involved in the processes of cell recognition or cell adhesion, or both.
Keywords: SV40-transformed 3T3 cells, sugar-derivatized Sephadex beads
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Ernback S. Chemical fixation of enzymes to cyanogen halide activated polysaccharide carriers. Eur J Biochem. 1971 Feb 1;18(3):351–360. doi: 10.1111/j.1432-1033.1971.tb01250.x. [DOI] [PubMed] [Google Scholar]
- Barker R., Olsen K. W., Shaper J. H., Hill R. L. Agarose derivatives of uridine diphosphate and N-acetylglucosamine for the purification of a galactosyltransferase. J Biol Chem. 1972 Nov 25;247(22):7135–7147. [PubMed] [Google Scholar]
- Bosmann H. B. Platelet adhesiveness and aggregation: the collagen:glycosyl, polypeptide:N-acetylgalactosaminyl and glycoprotein:galactosyl transferases of human platelets. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1118–1124. doi: 10.1016/0006-291x(71)90578-x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Inman J. K., Dintzis H. M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemical adsorbents. Biochemistry. 1969 Oct;8(10):4074–4082. doi: 10.1021/bi00838a026. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Urban C. L., Barber A. J. Enzymatic basis for platelet: collagen adhesion as the primary step in haemostasis. Nat New Biol. 1971 Nov 3;234(44):5–7. doi: 10.1038/newbio234005a0. [DOI] [PubMed] [Google Scholar]
- LEVVY G. A., MCALLAN A. The N-acetylation and estimation of hexosamines. Biochem J. 1959 Sep;73:127–132. doi: 10.1042/bj0730127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- McKelvy J. F., Lee Y. C. Microheterogeneity of the carbohydrate group of aspergillus oryzae alpha-amylase. Arch Biochem Biophys. 1969 Jun;132(1):99–110. doi: 10.1016/0003-9861(69)90341-5. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970 Jun;22(2):97–187. [PubMed] [Google Scholar]
- Oppenheimer S. B., Edidin M., Orr C. W., Roseman S. An L-glutamine requirement for intercellular adhesion. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1395–1402. doi: 10.1073/pnas.63.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pricer W. E., Jr, Ashwell G. The binding of desialylated glycoproteins by plasma membranes of rat liver. J Biol Chem. 1971 Aug 10;246(15):4825–4833. [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. An assay for intercellular adhesive specificity. J Cell Biol. 1971 Nov;51(21):525–535. doi: 10.1083/jcb.51.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971 Nov;51(21):536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., White D. Intercellular contact and cell-surface galactosyl transferase activity (cell culture-mouse-radioautography-contact inhibition-cis-and trans-galactosylation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):485–489. doi: 10.1073/pnas.69.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Habel K., Green H. Antigenic and cultural properties of cells doubly transformed by polyoma virus and SV40. Virology. 1965 Oct;27(2):179–185. doi: 10.1016/0042-6822(65)90157-1. [DOI] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. II. Glycosyltransferases and endogenous acceptors of the undifferentiated cell surface membrane. J Biol Chem. 1973 Apr 10;248(7):2542–2548. [PubMed] [Google Scholar]