Abstract
Clinical studies have shown that decreased tamoxifen effectiveness correlates with elevated levels of vascular endothelial growth factor (VEGF)-A165 in biopsy samples of breast cancers. To investigate the mechanisms underlying tamoxifen resistance and metastasis, we engineered the estrogen receptor (ER)–positive MCF-7 human breast cancer cell line to express VEGF to clinically relevant levels in a doxycycline-regulated manner. Induction of VEGF expression in orthotopically implanted xenografts that were initially tamoxifen responsive and noninvasive resulted in tamoxifen-resistant tumor growth and metastasis to the lungs. Lung metastases were also observed in a VEGF-dependent manner following tail vein injection of tumor cells. At both primary and metastatic sites, VEGF-overexpressing tumors exhibited extensive fibroblastic stromal content, a clinical feature called desmoplasia. VEGF-induced metastatic colonies were surrounded by densely packed stromal cells before detectable angiogenesis, suggesting that VEGF is involved in the initiation of desmoplasia. Because expression of VEGF receptors R1 and R2 was undetectable in these tumor cells, the observed VEGF effects on reduction of tamoxifen efficacy and metastatic colonization are most likely mediated by paracrine signaling that enhances tumor/stromal cell interactions and increases the level of desmoplasia. This study reveals new roles for VEGF in breast cancer progression and suggests that combination of antiestrogens and VEGF inhibitors may prolong tamoxifen sensitivity and prevent metastasis in patients with ER-positive tumors.
Introduction
Antiestrogen therapy with tamoxifen is effective for women with estrogen receptor (ER)–positive breast cancers in both metastatic and adjuvant settings (1). Unfortunately, in most cases, cancers that respond to tamoxifen in advanced disease settings will eventually relapse and become tamoxifen resistant. Recurrence in patients treated with tamoxifen in adjuvant settings is also a frequent event. Over the years, a number of molecular mechanisms have been proposed for tamoxifen resistance. Both ER-independent alternative signaling pathways that support tumor cell growth and ER-mediated mechanisms have been proposed (2). Although several possibilities have been found to account for or contribute to the resistant phenotype in patients, the precise mechanisms responsible for acquired or intrinsic antiestrogen resistance of breast tumors are still poorly understood.
Vascular endothelial growth factor (VEGF)-A occurs in a number of isoforms, with the 165-amino-acid isoform being the most common form secreted by tumor cells, which acts on endothelial cells, and is responsible for tumor angiogenesis (3). Two important VEGF receptors, VEGF-R1 (Flt-1) and VEGF-R2 (Flk-1/KDR) membrane tyrosine kinases, mediate VEGF signaling (4). A third VEGF receptor, neuropilin-1 (NP-1), which lacks an intracellular tyrosine kinase domain, presumably facilitates signal transduction as a coreceptor by associating with other membrane receptors (5). Clinical studies have shown that elevated levels of VEGF-A165 are associated with shorter progression-free survival and shorter survival following relapse in patients with advanced breast cancer receiving tamoxifen as a first line therapy (6). Elevated VEGF levels are also associated with shorter relapse and overall survival in patients with ER-positive, lymph node–positive disease receiving adjuvant tamoxifen (7, 8). Although a number of studies have shown the importance of VEGF as a prognostic indicator of the severity of breast cancer, direct evidence showing the ability of VEGF to enhance tamoxifen resistance is lacking.
As a stimulator of neoangiogenesis, VEGF plays an important role in the expansion of the metastatic tumor mass (9). The initial steps of metastasis (i.e., intravasation, accessing distant organs through hematogenous circulation, and vascular bed adhesion in distant organs) can be done by tumor cells with high efficiency. However, the subsequent steps, extravasation at the distant site and proliferation in the parenchyma of the target organ to develop macroscopic metastatic lesions, are inefficient and limiting steps in the metastatic process (10). Recent results have shown a role for VEGF in tumor cell extravasation (11). Mobilization of VEGF-R1–expressing hematopoietic progenitor cells is also needed to establish a “premetastatic niche” for the arriving tumor cells (12). Thus, VEGF might have significant roles not only in the vascularization of the metastasis but also in “preangiogenic” stages at these sites.
In human solid tumors, a high level of desmoplasia is also commonly observed at both primary and metastatic sites, wherein stroma composes the majority of the tumor mass, in some cases accounting for more than 90% (13–15). The importance of the stromal response in supporting tumor growth has now been substantiated by a number of studies, although induction of desmoplasia is still poorly understood (13, 16). Several mechanisms that result in stromal fibroblast activation and collagen synthesis have been proposed, including paracrine activation of fibroblast proliferation by growth factors released by tumor cells (17). Various growth factors secreted from cancer cells have been identified that stimulate stromal cells including platelet-derived growth factor (PDGF), transforming growth factor (TGF)-α, TGF-β, and insulin-like growth factors (IGF; refs. 18, 19). However, the role of VEGF in the desmoplastic response has not been studied. In this report, we show that VEGF overexpression was also able to increase the level of desmoplasia in the primary tumors. Collectively, our findings suggest that VEGF overexpression in ER-positive breast cancer cells increases tumor-stromal interactions and facilitates a suitable stromal environment at the metastatic site, where the cancer cells thrive and form large nodules even in mice treated with tamoxifen.
Materials and Methods
Vector constructions
The construction of the pBTE bicistronic expression vector for the reverse tet transactivator rtTA2S-M2 has previously been described (20). The tet-responsive VEGF expression vector pTRE-hVEGF was constructed by subcloning VEGF cDNA, including the NH2-terminal secretory signal peptide sequences, into the pTRE vector (Clontech) at the EcoRI site.
C9V cell lines and VEGF expression
Isolation of the MCF-7–derived subline C9, which stably expresses the reverse tet transactivator rtTA2S-M2, has been described before (20). C9 cells were retransfected with the pTRE-hVEGF and pZeo-SV (Invitrogen) plasmids. Zeocin-resistant cell clones, designated as C9V cell lines, were isolated and expanded for evaluation of doxycycline-induced VEGF induction by ELISA and Western blot analysis and also for evaluation of their growth characteristics, which needed to be similar to the parental MCF-7 clone ML20 under different stimulations, including 17β-estradiol (E2), 4-hydroxytamoxifen, and 5% fetal bovine serum (FBS).
Animal experiments
All studies were done according to the guidelines of the Institutional Animal Care and Use Committee of Southern Research Institute. Ovariectomized athymic nude mice, ~8 wk old, were obtained from the National Cancer Institute-Frederick Cancer Research and Development Center. For xenograft experiments, tumor volume (Vt) was determined by Vt (mm3) = L × W2/2, where L and W refer to the larger and smaller dimensions of each tumor measurement, respectively. At the end of the experiment, the tumor was excised and cut into two halves, one was stored in liquid nitrogen for biochemical analyses and the other was fixed in 10% formalin and paraffin embedded for histologic analysis.
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside staining
Mouse tissues were fixed in 10% formalin for 4 to 5 h, stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) staining solution overnight in the dark at room temperature, and then rinsed with PBS. After examining the blue-stained metastatic lesions under a dissecting microscope, photographs were taken and the tissue samples were further fixed in 10% formalin overnight and then paraffin embedded for histologic analysis. In some experiments, lung and tumor samples were directly fixed and paraffin embedded for immunohistologic analysis. The X-gal staining solution contained 5 mmol/L potassium ferricyanide, 5 mmol/L potassium ferrocyanide, 2 mmol/L MgCl2, 0.02% NP40, 0.01% sodium deoxycholate, and 1 mg/mL X-gal.
Determination of tumor VEGF and fibroblast growth factor-2 levels
Tumor samples stored in liquid nitrogen were thawed, dissected free of surrounding fat tissue, and minced into small pieces. Cytosolic preparation buffer [10 mmol/L Tris (pH 7.4), 1.5 mmol/L EDTA, 10 mmol/L sodium molybdate, 1.0 mmol/L 1-thioglycerol] was added at 0.5 mL to every 100-mg tissue sample. The tissue was homogenized on ice with a Polytron homogenizer and disintegrated by sonication. The samples were spun at 12,000 rpm for 30 min at 4°C. The cytosol samples collected from the supernatant were used for VEGF and fibroblast growth factor (FGF)-2 content determination by ELISA (R&D Systems). Protein concentration was also determined by the modified Lowry protein assay (Pierce).
Real-time quantitative reverse transcription-PCR analysis
For quantitative reverse transcription-PCR (RT-PCR), mRNA was isolated using mRNACatcher (Invitrogen) and first-strand cDNA was synthesized using the SuperScript III kit (Invitrogen) according to the manufacturer’s protocols. Aliquots of the cDNA combined in a 20-µL reaction mix containing 300 nmol/L 5′ forward and 3′ reverse primers of each receptors and 100 nmol/L double fluorescently labeled MGB (minor groove binding) probe were subjected to quantitative PCR analysis on 384-plate (Applied Biosystems). The PCR primers for VEGF-R1, VEGF-R2, and NP-1 are described in ref. 21.
Statistical methods
Tumor volumes (in cubic millimeters) were calculated for each treatment group and are represented as mean ± SE. The tumor growth pattern was characterized over time. These patterns were analyzed using the growth curve modeling method (22). One-way ANOVA, t test, and tests of repeated measures were used to compare the mean tumor volumes between groups. All of the analyses were done using SAS software (version 6.12; SAS Institute, Inc.). P < 0.05 was considered statistically significant in all of the analyses.
Results
Induction of VEGF expression to clinically relevant levels accelerates xenograft growth in a dose-dependent manner
Doxycycline-inducible gene expression systems are useful for controlling expression of targeted genes and for determining functions of gene products both in vitro and in vivo. We generated a bicistronic expression vector using a modified reverse tet-transactivator (rtTA2S-M2), which showed homogeneous inducible gene expression over long periods, high doxycycline sensitivity, and minimal basal activity in the absence of doxycycline (20). Using this system, we engineered the ER-positive MCF-7 breast cancer line to overexpress the 165-amino-acid form of VEGF-A in a doxycycline-regulated manner. The resulting cell lines were designated as C9V lines. Western blotting of conditioned media collected from two C9V clones, C9V4 and C9V18, showed tightly controlled VEGF secretion by addition of doxycycline (Fig. 1). Noninduced basal VEGF levels are undetectable in this assay, which is similar to untransfected parental MCF-7 cells and to the parental C9 cells that are only transfected with the tet-transactivator expression vector. The upper band of VEGF in Fig. 1 with a higher molecular weight is most likely due to posttranslational modifications, consistent with other studies. More sensitive ELISA assays done with conditioned media showed that doxycycline at 0.2 µg/mL induced an ~100-fold increase in VEGF secretion from both C9V4 and C9V18 cell cultures in 48 hours (data not shown).
Figure 1.
Doxycycline-inducible VEGF expression. Conditioned media were collected from cell cultures of parental MCF-7, pBTE-transfected C9, C9V4, and C9V18, treated with or without 0.2 µg/mL doxycycline (Dox) for 48 h and Western blotted with a specific antibody against human VEGF (R&D Systems). The upper band of VEGF with higher molecular weight is most likely due to the posttranslational modifications. E. coli–expressed human recombinant VEGF lacking posttranslational modifications was used as standard in the first two lanes. Each lane contains the equivalent VEGF secreted by ~500 cells over a 48-h period.
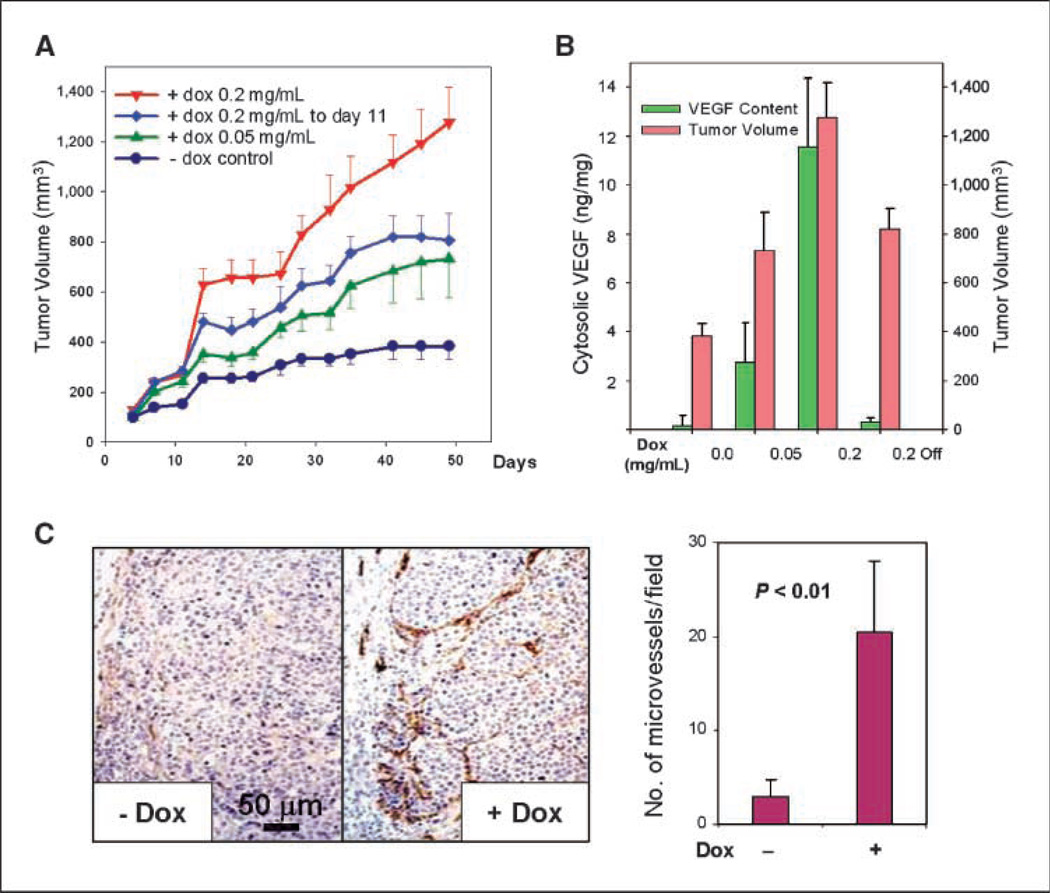
C9V4 cells were then inoculated into the mammary fat pad of ovariectomized nude mice on day 0. One day before the cell inoculation, all mice were implanted with slow-release E2 pellet and divided into four groups receiving different doses of doxycycline in their drinking water. By day 50, tumors in control group grew to a mean size of 400 mm3 (Fig. 2A). In contrast, the average tumor volumes were about 730 and 1,200 mm3 in groups that were supplied with a low (0.05 mg/mL) and high (0.2 mg/mL) dose of doxycycline, respectively. Thus, doxycycline given in drinking water significantly increased tumor growth in a dose-dependent manner (P < 0.001). In another mouse group, when the high dose of doxycycline was withdrawn at day 11, the tumor growth rate was reduced significantly in comparison with the tumor growth observed in the group that continued to receive the same dose of doxycycline throughout the experiment (P < 0.01).
Figure 2.
VEGF expression increases tumor growth and vascularization. A, tumor growth in athymic nude mice. C9V4 cells (107), suspended in 200 µL of 50% phenol red–free Matrigel (BD Biosciences), were inoculated into the inguinal mammary fat pad of four groups of ovariectomized athymic nude mice on day 0. A slow-release 0.72-mg E2 pellet (Innovative Research) was implanted into each mouse the day before the cell inoculation. The control group was provided with drinking water containing 2.5% sucrose only; three other groups were provided with 2.5% sucrose and 0.05 or 0.2 mg/mL of doxycycline (dox) starting the day before the cell inoculation. Doxycycline was removed from one of 0.2 mg/mL groups on day 11. There were six mice for each group (n = 6). Tumor sizes were measured twice a week. Symbols, mean tumor volume; bars, SE. B, relationship between tumor growth and VEGF levels. Cytosolic extracts were prepared from xenograft tumors as described in ref. 6, and the amount of VEGF present was determined by ELISA (R&D Systems) and normalized to total protein concentration. The mean tumor volume at the end of the study is indicated by the red columns and the average tumor VEGF level of each mouse group (n = 6) is indicated by the green columns. C, VEGF overexpression is associated with increased tumor microvessel density. Paraffin-embedded tumor samples prepared from the animal study were sectioned and immunostained with a rat monoclonal antibody against mouse PECAM-1 (BD Biosciences). Representative images of tumor microvessels from two tumors, one with 0.2 mg/mL doxycycline induction of VEGF expression and the other without. Quantitative analysis, as shown in the histogram, was done by counting the number of PECAM-1–positive blood vessels per microscopic field with five tumor samples for each condition and three microscopic fields per sample (×10 magnification).
Tumor VEGF content was determined by ELISA of lysates prepared from individual tumor samples collected at the end of the animal study. The continuous low and high doses of doxycycline increased average tumor VEGF content by 14- and 58-fold, respectively. The mean tumor volumes in these groups correlated with the VEGF levels and dosage of doxycycline (Fig. 2B). Most importantly, the doxycycline-regulated tumor VEGF levels in these groups fall within the range of cytosolic VEGF levels found in biopsy samples from breast cancer patients with significantly shorter progression-free survival and post-relapse survival times after first-line tamoxifen treatment for advanced disease (6). In this study of 618 breast cancer patients, tumors from the group of 78 patients that were the least responsive to tamoxifen treatment had a high level of VEGF ranging from 1.73 to 542 ng/mg of cytosolic protein (6). The mean VEGF levels in our experimental system were 3 and 12 ng/mg, induced by the low-dose and high-dose doxycycline, respectively. The basal level of tumor VEGF was 0.17 ng/mg in the control group that did not receive doxycycline. Thus, the VEGF levels in our model system are clinically relevant and correspond to the range that is associated with tamoxifen resistance in breast cancer patients with advanced disease.
Tumor microvessel density was characterized by immunohistochemical staining with an antibody against the mouse endothelial cell–specific marker platelet/endothelial cell adhesion molecule 1 (PECAM-1). As expected, an increased microvessel density was readily observed in xenografts where VEGF expression was induced by doxycycline (Fig. 2C). This result is consistent with clinical studies showing that human breast tumors with elevated VEGF levels are associated with increased microvessel density (23) and is also consistent with other reported in vivo studies (24–26).
Induction of VEGF expression is associated with reduced tamoxifen efficacy in vivo and acquisition of metastatic capability
We next studied C9V xenograft growth in nude mice that were treated with tamoxifen. Four groups of ovariectomized mice were supplemented with slow-release E2 pellets and then inoculated with C9V18 cells on day 0. Two of four groups were given doxycycline in their drinking water to induce VEGF expression in the tumors starting one day before the inoculation. Two groups of mice, one with doxycycline and the other without, received tamoxifen treatment by replacing the E2 pellet with a tamoxifen pellet on day 9 when the tumors were ~200 mm3 in size. Tumors in tamoxifen-treated mice that did not receive doxycycline became static and eventually regressed (Fig. 3A). In contrast, tumors in tamoxifen-treated mice that received doxycycline continued to grow. Induction of VEGF expression by doxycycline increased tumor growth significantly in both tamoxifen-treated mice (group 3 versus group 1; P < 0.01) and E2-suplemented mice (group 4 versus group 2; P < 0.01). Xenografts established with another C9V cell line, C9V4, also showed similar results (data not shown).
Figure 3.
Induction of VEGF expression results in tamoxifen-resistant tumor growth and spontaneous lung metastases. A, tumor growth in tamoxifen-treated mice. Four groups of E2-supplemented mice were inoculated with 107 C9V18 cells on day 0. The day before inoculation, doxycycline was supplied at 0.2 mg/mL in the drinking water for groups 3 and 4. On day 11, when the average tumor size in all groups reached ~200 mm3, the animals in groups 1 and 3 had their E2 pellet replaced with a tamoxifen pellet (5-mg 60-day release; Innovative Research). B, Ki-67 immunostaining. Paraffin-embedded tumor samples collected from tamoxifen (Tam)-treated xenograft mice were sectioned and immunostained with antibody against Ki-67 (Santa Cruz), a cell proliferation marker. The number of Ki-67–positive cells per microscopic view is approximately doubled when VEGF was induced by doxycycline. Quantitative analysis, as shown in the histogram, was done by counting the number of Ki-67–positive cells per microscopic field. Three microscopic fields (×20 magnification) per tumor and six tumors per group were analyzed. C, macroscopic metastases. X-gal staining of the lungs (top) showed metastatic deposits of LacZ-tagged tumor cells in the tamoxifen-treated tumor-bearing mice that also received doxycycline (group 3). Top left, a mouse lung with a number of metastatic deposits. Top right, an image with higher magnification. Bottom, H&E staining of lung samples confirming the presence of metastatic lesions.
Tumor samples collected from this study were subjected to immunostaining with a specific antibody against a cell proliferation marker, Ki-67. VEGF expression increased the number of Ki-67–positive cells in tamoxifen-treated tumors (Fig. 3B). On an average, the number of Ki-67–positive cells was increased ~2-fold when VEGF expression was induced. Together, the results depicted in Fig. 3A and B seem to be the first direct in vivo evidence that VEGF expression drives ER-positive, tamoxifen-responsive breast cancer to become less sensitive to tamoxifen treatment. Tumors in the group where VEGF was induced and also received tamoxifen treatment (group 3) seemed to grow even larger than those in the group that received only continued E2 supplement (group 2), although this trend did not quite meet statistical significance (P = 0.1). Comparing tumor growth in two groups that continued to receive E2 also indicates that the effect of VEGF expression on tumor growth might be additive to that of estrogen on ER-positive breast tumors as has previously been reported by others (24, 25, 27).
Although the MCF-7 cell line was originally isolated from a metastatic breast cancer, it lacks the ability to form either macroscopic or microscopic metastasis in vivo. We therefore examined the effect of VEGF overexpression on the metastatic potential of C9 cells, which are a derivative of a MCF-7 subline, ML20, which was stably transfected with the bacterial lacZ gene encoding β-galactosidase (28). This allows X-gal staining of mouse tissues to detect metastatic lesions in different organs. X-gal staining of mouse lungs showed that the induction of tumor VEGF overexpression resulted in spontaneous lung metastases in the orthotopically implanted xenograft-bearing mice (Fig. 3C). Lung tissue H&E staining confirmed metastasis indicated by X-gal staining. VEGF induced lung metastases were observed in both E2-supplemented mice and mice that received tamoxifen treatment. Metastases in lymph nodes, liver, kidney, and femur were not observed. The incidence of lung metastases detected by X-gal staining in tumor-bearing mice is summarized in Table 1. When doxycycline was given to induce VEGF expression, lung metastases were observed in six of nine E2-supplemented mice and four of nine tamoxifen-treated mice (including both C9V4 and C9V18 xenografts). No lung metastases were observed in 18 mice that did not receive doxycycline in their drinking water. There were also no metastases detected in the group of six mice where VEGF overexpression was down-regulated by removal of doxycycline on day 11, although most of these tumors were of the same size or larger than some of the primary tumors in the doxycycline- and tamoxifen-treated groups that did give rise to metastases. Therefore, the exclusive presence of metastases in mice that underwent continued induction of VEGF expression is not simply a function of the larger mean tumor size observed in these groups. These results show that VEGF overexpression is sufficient to cause a nonmetastatic breast tumor to become metastatic.
Table 1.
Continued VEGF overexpression in primary tumors results in frequent spontaneous metastases to the lung in either estrogen- or tamoxifen-treated mice
| Treatment | Cell line |
No. of mice |
No. of mice with lung metastasis |
|---|---|---|---|
| E2 | |||
| −Dox | C9V4 | 5 | 0 |
| C9V18 | 5 | 0 | |
| +Dox (0.2 mg/mL) | C9V4 | 5 | 3 |
| C9V18 | 4 | 3 | |
| +Dox to day 11 (0.2 mg/mL) | C9V4 | 6 | 0 |
| Tamoxifen | |||
| −Dox | C9V4 | 3 | 0 |
| C9V18 | 5 | 0 | |
| +Dox (0.2 mg/mL) | C9V4 | 4 | 1 |
| C9V18 | 5 | 3 |
NOTE: Incidence of mouse lung metastases was summarized for several animal studies, including the studies of Figs. 2A and 3A. Mouse lung tissues were collected from C9V4 and C9V18 xenograft–bearing mice at the end of each animal study, which typically occurred ~50 d after cell inoculation. Lung metastases were examined by X-gal staining as described in ref. 33.
Abbreviation: Dox, doxycycline.
VEGF promotes in vivo growth through paracrine signaling mechanisms associated with the induction of a desmoplastic response
To determine if the reduced tamoxifen efficacy observed in vivo with the VEGF-inducible transfected cell lines was related to either a possible reduction of ERα expression levels or reduction of in vitro sensitivity to antiestrogens, we examined protein expression of ERα by Western blotting and the in vitro growth characteristics of these lines. Protein expression levels of ERα in C9, C9V4, C9V18, and the parental lacZ-transfected MCF-7 cell lines were all similar (data not shown). In addition, the in vitro growth characteristics of the C9V cell lines were similar to their parental MCF-7 cell line in either estrogen- or antiestrogen-containing conditions, and addition of doxycycline to the cultures had no effect on estrogen responsiveness or antiestrogen sensitivity (data not shown). These results indicate that an alteration in ER status or function is not related to the reduction of tamoxifen efficacy observed in vivo in this experimental system.
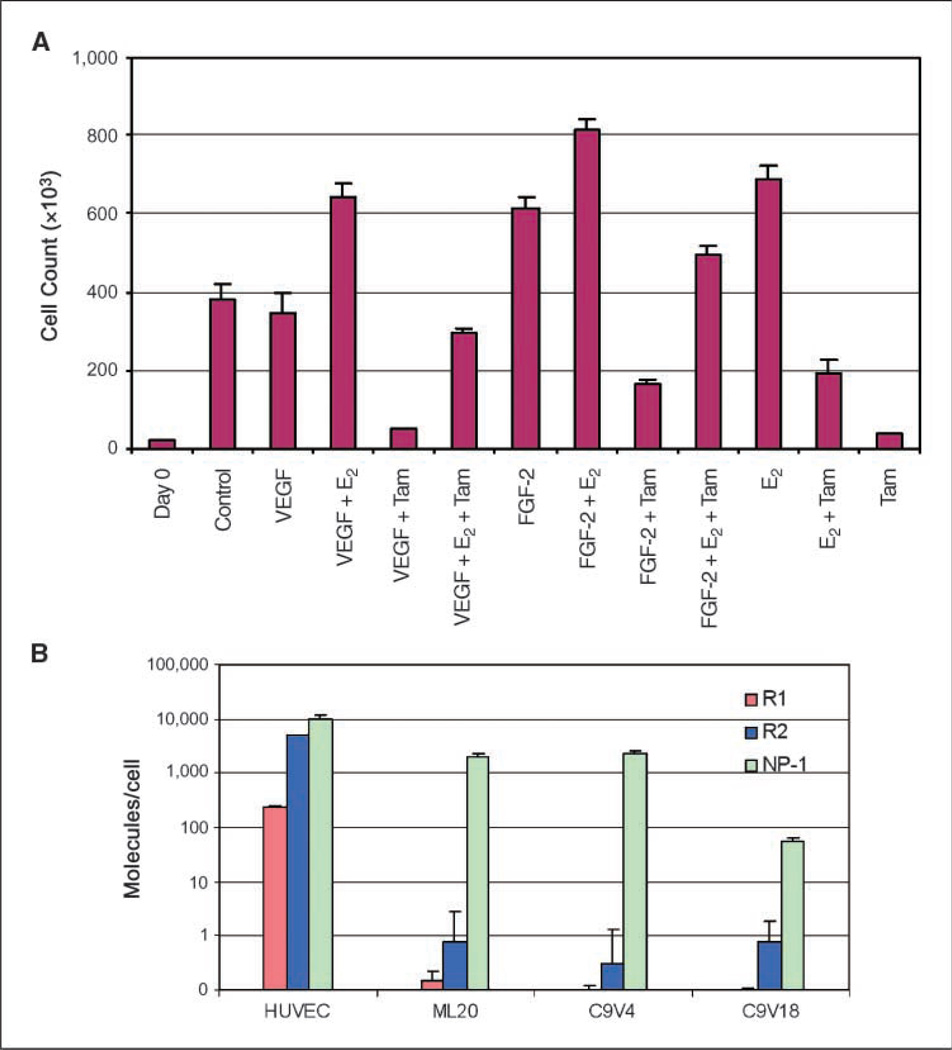
Direct effects of VEGF on C9V cell proliferation and sensitivity to tamoxifen were examined by an in vitro cell proliferation assay. The results showed that in the presence and absence of recombinant human VEGF, C9V18 and the parental ML20 cell line grew at a similar rate and remained equally sensitive to 4-hydroxytamoxifen, an active metabolite of tamoxifen used for in vitro studies (Fig. 4A). These experiments showed that VEGF has no direct effect on C9V18 cells with respect to proliferation and sensitivity to tamoxifen, consistent with our finding that the MCF-7 derivative cell line ML20, C9V4, or C9V18 had non-detectable expression of VEGF receptor R1 and R2 proteins by Western blotting and ELISA (data not shown) or quantitative RT-PCR (Fig. 4B). The third VEGF receptor, NP-1, was found to be expressed in both C9V cell lines and ML20 cell line. Because NP-1 lacks intracellular tyrosine kinase domain, it is likely that NP-1 by itself is not a functional receptor for VEGF. Because VEGF was able to stimulate C9V18 cell proliferation in vivo resulting in tamoxifen-resistant tumor growth, and because VEGF had no effect on the same cell line in vitro on the cell growth or tamoxifen sensitivity, it can be concluded that the VEGF effect observed in this experimental system is mediated by a paracrine, rather than autocrine, signaling mechanism.
Figure 4.
Exogenous VEGF does not affect the sensitivity of C9V18 cells to estrogen or tamoxifen in vitro presumably due to the lack of VEGF tyrosine kinase receptor expression. A, in vitro cell proliferation assay. C9V18 cells were plated at a density of 104 per well in 24-well plates in 10% FBS IMEM. After 24 h, the culture media were exchanged with phenol red–free IMEM containing 0.1% charcoal-stripped calf serum to remove the estrogens overnight. Then, three wells were counted on day 0, and the indicated reagents in 1% charcoal-stripped calf serum, phenol red–free IMEM were added to the cultures in triplicate. The concentrations used were VEGF, 20 ng/mL; FGF-2, 20 ng/mL; 4-hydroxytamoxifen, 10−7 mol/L; and E2, 10−8 mol/L. The media were exchanged with the same conditions on day 4 and the cell numbers were counted with a Coulter counter on day 7. B, real-time quantitative RT-PCR analysis. Expression of VEGF receptors R1, R2, and NP-1 was examined with quantitative RT-PCR as described in Materials and Methods. The number of cells in each culture used for mRNA isolation was determined to allow calculation of the specific number of mRNA molecules per cell. mRNAs of the receptors R1 and R2 were determined to be <1 molecule per cell in MCF-7 and C9V cells, whereas about 200 R1 mRNAs and 5,000 R2 mRNAs are expressed per cell in human umbilical vein endothelial cells (HUVEC).
It is well known that VEGF activates endothelial cells in the tumor environment. To study whether other cell types possibly mediate the paracrine signaling of VEGF involved in tamoxifen resistance and metastasis, the stromal content was delineated from the epithelial component by immunohistologic staining with a mixture of specific antibodies against the epithelial cell marker keratin, which was able to specifically label the C9V cells present within the tumor mass. The immunostaining results showed that VEGF expression significantly increased the stromal content in the tamoxifen-treated tumors. The stromal content associated with VEGF expression encompassed ~40% of tumor section areas as compared with only 10% of tumor areas when VEGF was not induced (Fig. 5A). Based on morphology, fibroblasts accounted for most of the stroma, which resembled desmoplasia, a clinical feature of human breast cancer. Activated stromal cells in the tumor environment can provide additional mitogenic stimuli to drive tumor growth.
Figure 5.
VEGF expression increased the extent of the desmoplastic response observed at both primary and metastatic sites and facilitates metastatic colonization in an experimental metastasis assay. A, cytokeratin immunostaining. Tumor samples collected from tamoxifen-treated mice were immunostained for an epithelial specific cell marker using mixed antibodies against cytokeratin (Keratin Pan Ab-1, NeoMarkers) and counterstained with hematoxylin. Because C9V tumor cells were cytokeratin positive, the cytokeratin-negative areas were considered to be the stromal-enriched compartment within the tumors. The fraction of the cytokeratin-negative stromal compartment within the total area of the microscopic view was analyzed microscopically with the aid of a counting grid and determining the stromal areas over the total areas for 5 primary tumors in each group using three sections per tumor separated by 100 µm. The results are plotted as percentage of stromal area over total counted areas. Increased desmoplasia correlated with doxycycline induction of VEGF expression. B, FGF-2 ELISA assay. Cytosolic tumor FGF-2 was determined by ELISA and normalized by protein concentration. Tumor FGF-2 levels correlated with the induction of tumor VEGF expression. C, experimental metastasis assay. C9V18 cells (0.5 × 106) were directly introduced into the blood circulation by tail vein injection. Doxycycline was provided in the drinking water at 0.2 mg/mL to one of two E2-supplemented mouse groups starting the day before cell injection. Mouse lung tissues were harvested at 2 h, 4 wk, and 10 wk after the tail vein injection. Paraffin-embedded lung samples were sectioned and immunostained with the cytokeratin antibodies to identify C9V18 cells. Red arrows, disseminated tumor cells; black arrows, fibroblastic stromal cells that surrounded the cytokeratin-positive C9V18 colonies.
To begin to identify which stromal derived factors may be involved in promoting tamoxifen-resistant growth and metastasis, we examined FGF-2 levels in tumors from tamoxifen-treated mice by ELISA, as previous results from our laboratory linked FGF signaling to paracrine mechanisms of tamoxifen resistance (29, 30). The results showed that the FGF-2 level was also increased by ~40% when tumor VEGF expression was induced by doxycycline (Fig. 5B), suggesting that the increase in FGF-2 levels possibly attributes to the increased fibroblastic stromal content within the xenografts. Because it has been reported that stromal cell–derived factor 1 (SDF-1) is produced by carcinoma-associated fibroblasts (16), which also mimics the mitogenic effect of estrogen when added to MCF-7 cells in vitro (31), we also examined SDF-1 levels in tumor lysates by ELISA. However, unlike FGF-2, the levels of SDF-1 did not change when tumor VEGF expression was induced by doxycycline (data not shown), suggesting that SDF-1 levels were most likely not correlated with the observed reduction of tamoxifen efficacy in this experimental system.
Overexpression of FGF family members as well as their receptors has been reported in a significant percentage of human breast tumors. FGF-1 and FGF-4 are both able to stimulate MCF-7 cell proliferation in the absence of the mitogenic stimulus provided by estrogen (28, 30, 32). The effect of FGF-2 on C9V18 cells was examined in the same experiment shown in Fig. 4A, which showed that VEGF had no effect on the in vitro growth of C9V18 cells. In contrast to VEGF, exogenous FGF-2 increased the proliferation of both tamoxifen-treated (FGF-2 + tamoxifen versus tamoxifen alone; P < 0.01) and estrogen-depleted (FGF-2 versus control; P < 0.01) C9V18 cells. These results indicate that the stromal-derived FGF-2 possibly reduces tamoxifen sensitivity of C9V18 cells in vivo similar to that previously reported for FGF-1 and FGF-4. Tumor stroma is a rich resource of mitogenic growth factors and cytokines, which can stimulate tumor growth and confer drug resistance. The increased stroma content within VEGF-overexpressing tumors could be responsible for the observed reduction in tamoxifen efficacy.
VEGF promotes metastatic colonization and desmoplasia in an experimental metastasis assay
The metastatic process consists of series of steps, all of which must be successfully completed to give rise to a metastatic lesion. A limiting step in this process is one wherein a cell disseminated from the primary tumor colonizes at a distant organ site. To investigate whether VEGF influences the step of colonization of disseminated tumor cells at a distant organ, C9V18 tumor cells were directly introduced into the mouse circulation by tail vein injection in an experimental metastasis assay. The presence of disseminated tumor cells in mouse lungs at various time points after the injection was visualized by immunostaining with antibodies against keratin. Two hours after the tail vein injection, many single tumor cells were present in the lungs taken from mice supplied either with or without doxycycline, confirming the success of the tail vein injection (Fig. 5C). Four weeks later, no tumor cells were detected in the lungs from the mice that did not receive doxycycline (n = 6), whereas keratin-positive C9V18 cell colonies were found in two of the three mice that were given doxycycline. Most interestingly, the tumor cell colonies were found to be densely surrounded by stromal cells. The small C9V18 cell colonies and their surrounding stroma were negative by immunostaining for the mouse endothelial cell marker PECAM-1, consistent with the idea that metastatic colonization occurred before angiogenesis. Histologic examination revealed that these stromal cells were fibroblasts, indicating that initiation of desmoplasia may precede angiogenesis.
At 10 week s after tail vein injection, there were still no disseminated tumor cells or metastatic lesions in six mice that did not receive doxycycline, whereas two of the six mice treated with doxycycline developed macroscopic lung metastases. A mixed pattern of single tumor cells, tumor cell colonies, and metastatic lesions with a high level of desmoplasia was observed in the mouse lungs (Fig. 5C). Blood vessels were easily observed within the desmoplastic zone of metastatic lesions. Immunostaining with keratin antibodies showed that the majority of the area in the metastatic lesion was found to be occupied by desmoplastic stroma. Desmoplasia accounted for 50% to 90% of the total area of the lung metastatic lesions, similar to that found in the spontaneous metastatic lesions from orthotopically implanted tumor-bearing mice. These observations indicate that disseminated tumor cells that do not overexpress VEGF either undergo apoptosis or are cleared by macrophages and suggest that VEGF-overexpressing tumor cells are able to activate fibroblastic stroma, which then increases their potential for survival and colonization in a secondary organ before angiogenesis.
Discussion
MCF-7 is an ER-positive breast cancer cell line that is unable to grow as a xenograft in ovariectomized mice without E2 supplementation. MCF-7 tumors also do not grow in tamoxifen-treated mice, are poorly invasive, and are unable to consistently disseminate to distant sites. Many attempts to increase its metastatic capabilities and to impart antiestrogen resistance have been made to develop models for the progression of human breast cancer. For instance, FGF-1 or FGF-4 overexpression in MCF-7 cells confers these cells with the ability to form highly vascularized tumors in tamoxifen-treated mice and also enhances their metastatic capabilities in the mice bearing these xenografts, exhibiting micrometastases in the lungs and lymph nodes (26, 28, 32, 33). Overexpression of heregulin in MCF-7 induces antiestrogen resistance, promotes metastasis, and also induces preneoplastic transformation of the adjacent mouse mammary tissue (34). Whereas these cells exhibit lymphatic invasion and axillary lymph node metastases, no lung metastases were reported. It is suggested that heregulin imparts this aggressive phenotype on MCF-7, in part, via an increase in the expression of VEGF (35). Recently, angiopoietin-2 overexpression in MCF-7 tumors was also shown to stimulate angiogenesis and confer on these cells the ability to form lung metastases (36). As in the case of heregulin, angiopoietin-2 has also been suggested to stimulate angiogenesis via a VEGF-dependent mechanism (36).
To directly address the role of VEGF in metastatic and antiestrogen resistance phenotypes, we developed a model system where VEGF expression in MCF-7 xenografts can be induced to clinically relevant levels for extended periods of time. This allowed us to study the biological effect of VEGF on tamoxifen resistance and metastasis in vivo. The results showed that overexpression of VEGF caused ER-positive breast tumors to grow in tamoxifen-treated mice, promoted metastatic colonization, and allowed the establishment of macroscopic lung metastases. VEGF expression in tumors also resulted in increased desmoplasia.
A highly interesting and intriguing aspect of these findings is that VEGF imparts a more pronounced metastatic phenotype to these cells than FGF-1, another angiogenic growth factor frequently expressed in human breast cancer, which is as potent as VEGF in stimulating endothelial cell proliferation. Whereas both FGF-1– and VEGF-overexpressing MCF-7 xenografts are highly vascularized and both are quite capable of growing in tamoxifen-treated nude mice, the FGF-1–overexpressing cells are incapable of establishing distant macrometastases owing to their inability to extravasate from blood vessels and proliferate in the parenchyma of organs such as the lungs (31, 37). One reason for such a difference could be related to the critical role of VEGF receptor signaling in the establishment of a “premetastatic niche” and subsequent neovascularization of these sites following tumor cell arrival (12). It has been shown that VEGF-R1–expressing, bone marrow–derived hematopoietic precursor cells home to sites in the target organ and precondition these sites before the arrival of tumor cells (38). VEGF-R2–expressing circulating endothelial precursor cells are recruited to the nascent metastatic site as well (39). Preconditioning of these sites also involves VEGF-R1 signaling–mediated production of matrix metalloproteinase-9 by activated macrophages and endothelial cells in the lung thereby facilitating tumor cell invasion on their arrival (40). Tumor-produced VEGF is also involved in the induction of expression of the chemokines S100A8 and S100A9 by myeloid and endothelial cells in the lung, which also facilitate the homing of tumor cells to this tissue and the establishment of a permissive environment for their docking and invasion (41). VEGF is also unique when compared with other angiogenic factors in that it also disrupts the endothelial barrier function, facilitating tumor cell extravasation (11).
Although VEGF stimulates neovascularization, it is not clear how adequate oxygen and nutrient supply and waste product removal by blood circulation can be mechanistically linked directly with tamoxifen resistance. Previously reported studies indicate that mitogenic factors that can act in an autocrine as well as a paracrine manner, such as FGFs, are able to confer a tamoxifen-resistant phenotype to MCF-7 cells (28, 30, 32). In the present study, we have shown the presence of high levels of fibroblastic desmoplasia in the tumors from tamoxifen-treated mice only when VEGF expression is induced. Desmoplasia is rarely observed in xenograft models possibly due to the rapid overgrowth of epithelial tumor cells. Previous studies with H-ras–transfected MCF-7 cells indicated that the desmoplasia observed in that system was only seen when estrogen was not provided and was related to PDGF production by the transfected cells (19). Whereas the effect of VEGF expression on PDGF production in MCF-7 cells remains to be determined, we have shown herein an increased FGF-2 level in xenograft tumors when VEGF expression was induced, concomitant with increased stromal content. We have also shown that exogenous FGF-2 can increase the growth of MCF-7 cells in vitro even when 4-hydroxytamoxifen is present. Therefore, the tamoxifen-resistant phenotype in this system could be, in part, a result of the direct effects of FGF-2 on MCF-7 cells. In addition to FGF-2, other growth factors, cytokines, or direct cell-cell interactions stimulated by VEGF paracrine signaling may also play a role in conferring tamoxifen resistance to breast tumor cells. Of particular interest is the recent finding that fibroblast-induced tamoxifen resistance of MCF-7 cells in a three-dimensional in vitro culture system that mimics the epithelial-fibroblast interactions that can occur in vivo is independent of IGF-I receptor and epidermal growth factor receptor signaling (42).
Our investigation is the latest of a series of transfection studies that have explored the role of expression of various VEGF isoforms in ER-positive breast cancer cell lines (24–27). A consistent finding throughout all the studies reported thus far is the enhanced in vivo growth of VEGF-overexpressing xenografts in estrogen-supplemented animals. However, the effects on hormone dependence and antiestrogen sensitivity in vitro and in vivo have been variable depending on the particular study. Zhang and colleagues (24) found that VEGF-A121 overexpression in MCF-7 tumors did not affect estrogen dependence or tamoxifen sensitivity. We have also previously shown that constitutive overexpression of VEGF in the same parental MCF-7 line that was also used herein failed to affect estrogen dependence in vitro or in vivo but did affect tamoxifen sensitivity in vivo (26). In contrast, Guo and colleagues (25) recently reported that overexpression of either VEGF-A165 or VEGF-A121 in MCF-7 cells did promote estrogen-independent tumor growth in vitro or in vivo. Because additional transfected cell lines that produced less VEGF failed to exhibit an estrogen-independent phenotype, these authors attributed the phenotypic differences of their transfectants from those reported in other studies to differences in expression levels of VEGF, although VEGF levels in tumors were not measured. Owing to the lack of estrogen independence that we previously observed with our MCF-7 cells overexpressing VEGF in a constitutive manner (26), we did not directly address the question of estrogen independence in vivo in our inducible model. In addition, a direct comparison between our model and that described by Guo and colleagues cannot be made because the MCF-7 line used in their study expresses VEGF-R1 and VEGF-R2 mRNAs whereas our parental and transfected cells, MCF-7 derivative cell line, do not express detectable levels of these mRNAs (25). Autocrine loops involving VEGF and both VEGF-R1 and VEGF-R2 have been shown to promote growth as well as survival of breast cancer cell lines (43). Our cells do express mRNA for a NP-1 that has been proposed to facilitate metastasis and allow VEGF to act as a survival but not as a proliferation factor in other breast cancer cell lines (44). It is therefore possible that signaling through this receptor may be at least partially involved in the in vivo phenotypes we observe when VEGF is induced.
Our study provides the first direct in vivo evidence that VEGF plays an important role in metastatic colonization of ER-positive breast cancer cells and suggests a new function of VEGF in mediating the desmoplastic response. Based on the results, we hypothesize that VEGF is involved in a “vicious cycle” of tumor/stromal cell interactions, in which tumor-released VEGF interacts with and recruits stromal cells, and stromal cells, in turn, provide mitogenic and angiogenic growth factors that stimulate both tumor cell and additional stromal cell growth. We anticipate that blockade of VEGF signaling may restore tamoxifen sensitivity and reduce metastatic potential. Combination of tamoxifen with an anti-VEGF signaling agent may inhibit both ER-mediated signaling and VEGF-stimulated stromal activation and angiogenesis and may be a more effective therapy for ER-positive breast cancer.
Acknowledgments
Grant support: P50 CA089019 (F. Kern), Komen Foundation Grant 00-0456 (J. Thottassery), and a NCI Breast SPORE Developmental Project (J. Thottassery).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 2.Schiff R, Massarweh S, Shou J, Osborne CK. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9:447–54S. [PubMed] [Google Scholar]
- 3.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 5.Mamluk R, Gechtman Z, Kutcher ME, et al. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem. 2002;277:24818–24825. doi: 10.1074/jbc.M200730200. [DOI] [PubMed] [Google Scholar]
- 6.Foekens JA, Peters HA, Grebenchtchikov N, et al. High tumor levels of vascular endothelial growth factor predict poor response to systemic therapy in advanced breast cancer. Cancer Res. 2001;61:5407–5414. [PubMed] [Google Scholar]
- 7.Gasparini G, Toi M, Miceli R, et al. Clinical relevance of vascular endothelial growth factor and thymidine phosphophorylase in patients with node positive breast cancer treated with either adjuvant chemotherapy or homone therapy. Cancer J Sci Am. 1999;5:101–111. [PubMed] [Google Scholar]
- 8.Linderholm B, Grankvist K, Wilking N, et al. Correlation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatment. J Clin Oncol. 2000;18:1423–1431. doi: 10.1200/JCO.2000.18.7.1423. [DOI] [PubMed] [Google Scholar]
- 9.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 10.Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am. 2001;10:243–255. vii. [PubMed] [Google Scholar]
- 11.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 14.Iacobuzio-Donahue CA, Argani P, Hempen PM, Jones J, Kern SE. The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res. 2002;62:5351–5357. [PubMed] [Google Scholar]
- 15.Seemayer TA, Lagace R, Schurch W, Tremblay G. Myofibroblasts in the stroma of invasive and metastatic carcinoma: a possible host response to neoplasia. Am J Surg Pathol. 1979;3:525–533. doi: 10.1097/00000478-197912000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Walker RA. The complexities of breast cancer desmoplasia. Breast Cancer Res. 2001;3:143–145. doi: 10.1186/bcr287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 19.Shao ZM, Nguyen M, Barsky SH. Human breast carcinoma desmoplasia is PDGF initiated. Oncogene. 2000;19:4337–4345. doi: 10.1038/sj.onc.1203785. [DOI] [PubMed] [Google Scholar]
- 20.Qu Z, Thottassery JV, Van Ginkel S, et al. Homogeneity and long-term stability of tetracycline-regulated gene expression with low basal activity by using the rtTA2S-M2 transactivator and insulator-flanked reporter vectors. Gene. 2004;327:61–73. doi: 10.1016/j.gene.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Lacal PM, Failla CM, Pagani E, et al. Human melanoma cells secrete and respond to placenta growth factor and vascular endothelial growth factor. J Invest Dermatol. 2000;115:1000–1007. doi: 10.1046/j.1523-1747.2000.00199.x. [DOI] [PubMed] [Google Scholar]
- 22.Littell RC, Henry PR, Ammerman CB. Statistical analysis of repeated measures data using SAS procedures. J Anim Sci. 1998;76:1216–1231. doi: 10.2527/1998.7641216x. [DOI] [PubMed] [Google Scholar]
- 23.Toi M, Bando H, Kuroi K. The predictive value of angiogenesis for adjuvant therapy in breast cancer. Breast Cancer. 2000;7:311–314. doi: 10.1007/BF02966396. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H-T, Craft P, Scott PAE, et al. Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J Natl Cancer Inst. 1995;87:213–219. doi: 10.1093/jnci/87.3.213. [DOI] [PubMed] [Google Scholar]
- 25.Guo P, Fang Q, Tao HQ, et al. Overexpression of vascular endothelial growth factor by MCF-7 breast cancer cells promotes estrogen-independent tumor growth in vivo. Cancer Res. 2003;63:4684–4691. [PubMed] [Google Scholar]
- 26.McLeskey SW, Tobias CA, Vezza PR, et al. Tumor growth of FGF or VEGF transfected MCF-7 breast carcinoma cells correlates with density of specific microvessels independent of the transfected angiogenic factor. Am J Pathol. 1998;153:1993–2006. doi: 10.1016/S0002-9440(10)65713-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshiji H, Harris SR, Thorgeirsson UP. Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res. 1997;57:3924–3928. [PubMed] [Google Scholar]
- 28.McLeskey SW, Kurebayashi J, Honig SF, et al. Fibroblast growth factor 4 transfection of MCF-7 cells produces cell lines that are tumorigenic and metastatic in ovariectomized or tamoxifen-treated athymic nude mice. Cancer Res. 1993;53:2168–2177. [PubMed] [Google Scholar]
- 29.Zhang L, Kharbanda S, Hanfelt J, Kern FG. Both autocrine and paracrine effects of transfected acidic fibroblast growth factor are involved in the estrogen-independent and antiestrogen-resistant growth of MCF-7 breast cancer cells. Cancer Res. 1998;58:352–361. [PubMed] [Google Scholar]
- 30.McLeskey SW, Zhang L, El-Ashry D, et al. Tamoxifen-resistant fibroblast growth factor-transfected MCF-7 cells are cross-resistant in vivo to the antiestrogen ICI 182,780 and two aromatase inhibitors. Clin Cancer Res. 1998;4:697–711. [PubMed] [Google Scholar]
- 31.Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17:792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Kharbanda S, Chen D, et al. MCF-7 breast carcinoma cells overexpressing FGF-1 form vascularized, metastatic tumors in ovariectomized or tamoxifen-treated nude mice. Oncogene. 1997;15:2093–2108. doi: 10.1038/sj.onc.1201386. [DOI] [PubMed] [Google Scholar]
- 33.Kurebayashi J, McLeskey SW, Johnson MD, et al. Quantitative demonstration of spontaneous metastasis by MCF-7 human breast cancer cells cotransfected with fibroblast growth factor 4 and lacZ. Cancer Res. 1993;53:2178–2187. [PubMed] [Google Scholar]
- 34.Atlas E, Cardillo M, Mehmi I, et al. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol Cancer Res. 2003;1:165–175. [PubMed] [Google Scholar]
- 35.Xiong S, Grijalva R, Zhang L, et al. Up-regulation of vascular endothelial growth factor in breast cancer cells by the heregulin-β1-activated p38 signaling pathway enhances endothelial cell migration. Cancer Res. 2001;61:1727–1732. [PubMed] [Google Scholar]
- 36.Imanishi Y, Hu B, Jarzynka MJ, et al. Angiopoietin-2 stimulates breast cancer metastasis through the α5β1 integrin-mediated pathway. Cancer Res. 2007;67:4254–4263. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Kharbanda S, McLeskey SW, Kern FG. Overexpression of fibroblast growth factor 1 in MCF-7 breast cancer cells facilitates tumor cell dissemination but does not support the development of macro-metastases in the lungs or lymph nodes. Cancer Res. 1999;59:5023–5029. [PubMed] [Google Scholar]
- 38.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 39.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175–181. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hiratsuka S, Nakamura K, Iwai S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 41.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated up-regulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 42.Shekhar MP, Santner S, Carolin KA, Tait L. Direct involvement of breast tumor fibroblasts in the modulation of tamoxifen sensitivity. Am J Pathol. 2007;170:1546–1560. doi: 10.2353/ajpath.2007.061004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Hooper AT, Zhong Z, et al. The vascular endothelial growth factor receptor (VEGFR-1) supports growth and survival of human breast carcinoma. Int J Cancer. 2006;119:1519–1529. doi: 10.1002/ijc.21865. [DOI] [PubMed] [Google Scholar]
- 44.Bachelder RE, Crago A, Chung J, et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]