Abstract
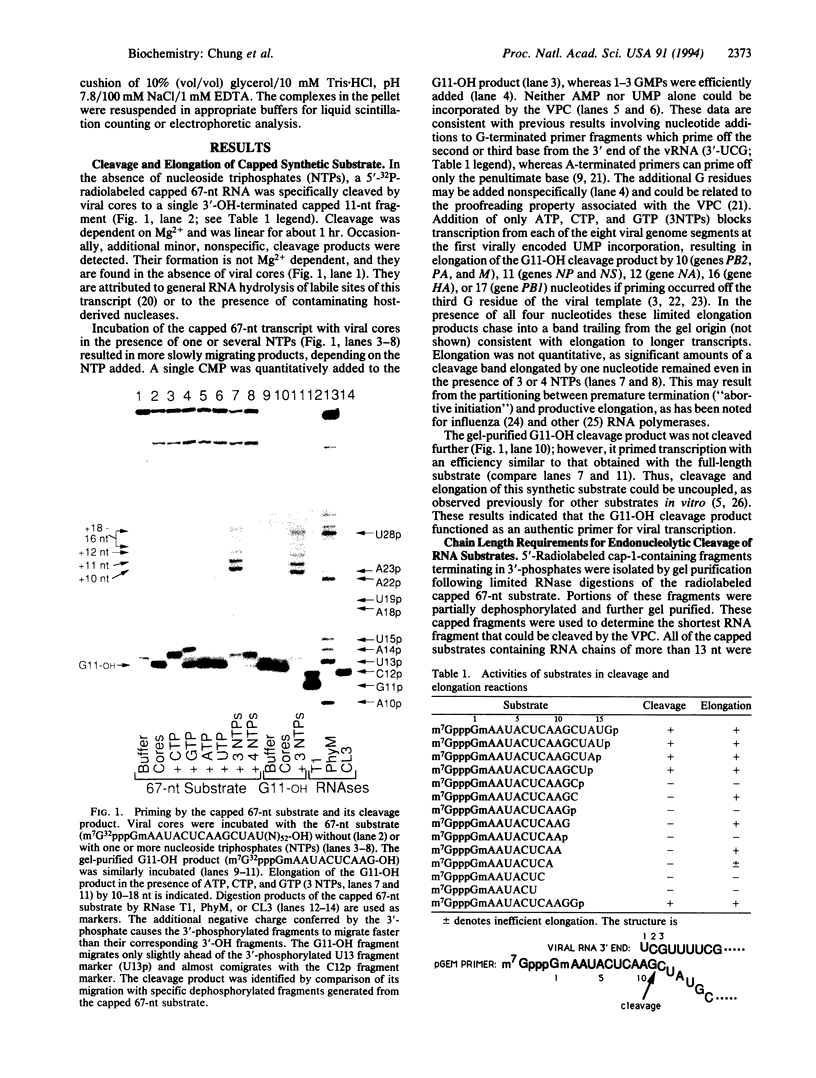
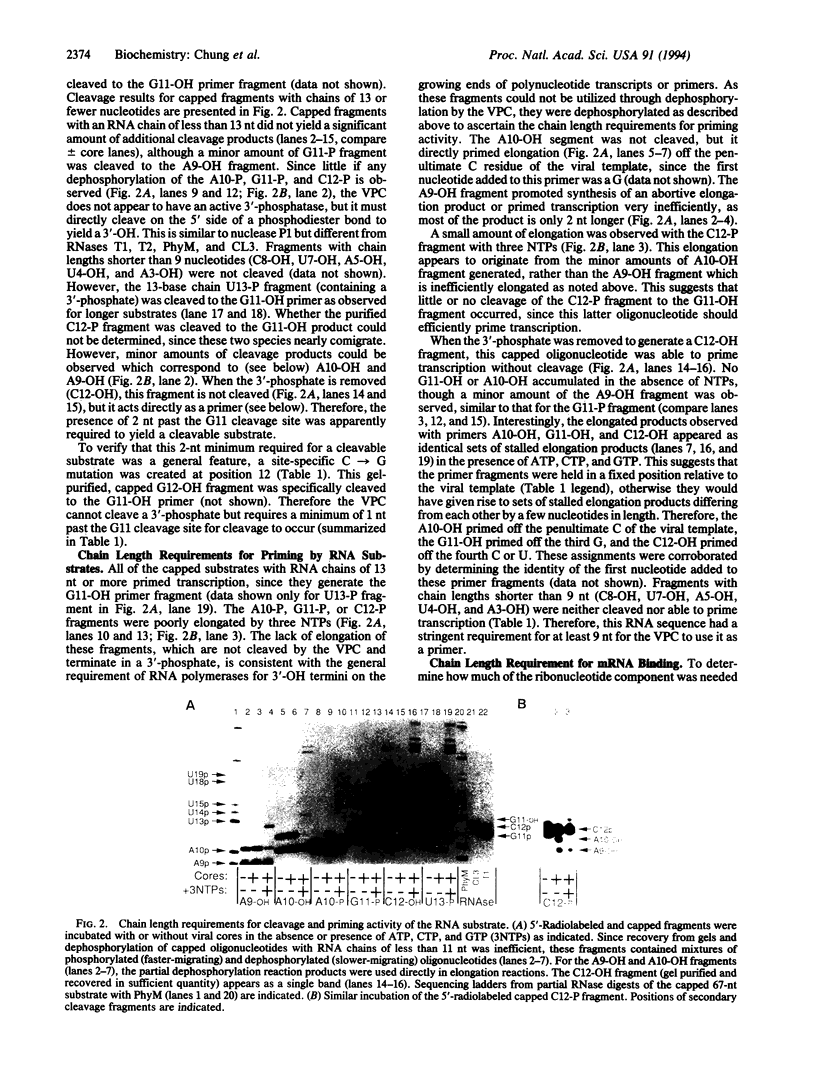
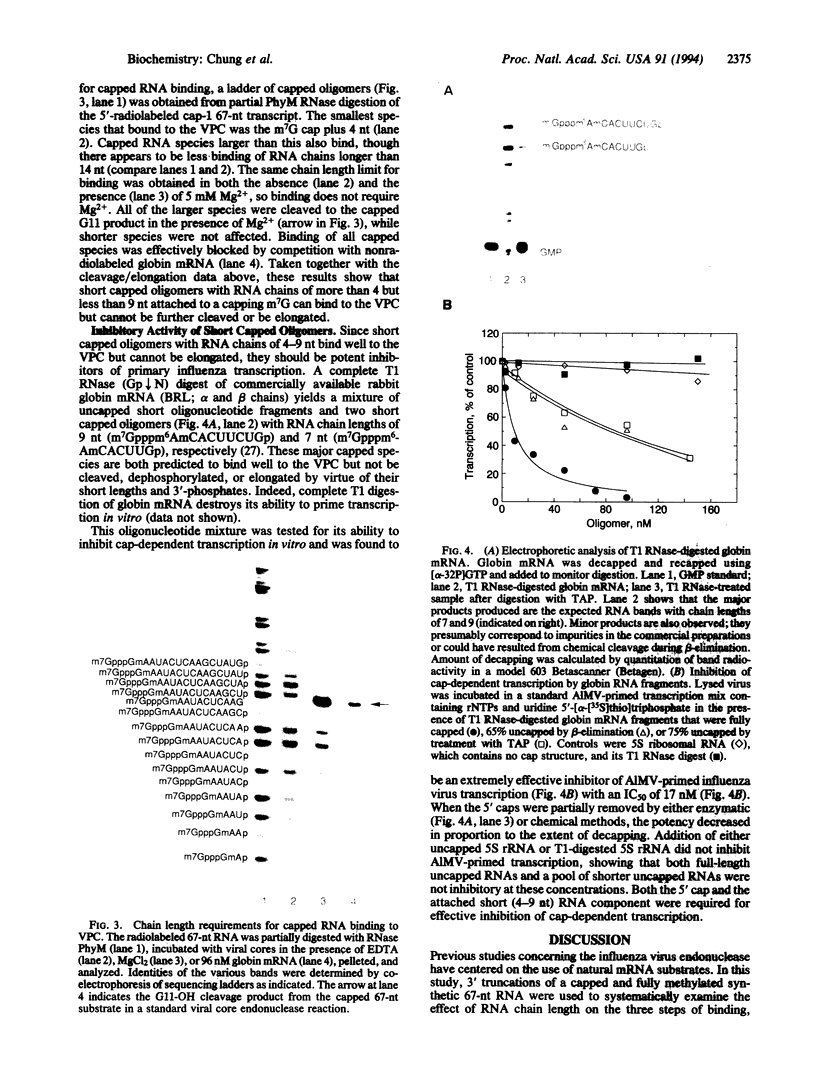
A synthetic 67-nt RNA substrate, containing a 32P-labeled cap-1 structure (m7G32pppGm) was specifically cleaved by the influenza virus RNA polymerase (EC 2.7.7.48) to yield a single capped 11-nt fragment capable of directly priming transcription. An analysis of systematic truncations of this RNA substrate demonstrated that an additional nucleotide beyond this cleavage site was required for cleavage. The minimal RNA chain length required for priming activity was found to be 9 nt, while in contrast an RNA chain length of at least 4 nt was required for efficient binding to the viral polymerase. On the basis of these chain length requirements we show that a pool of capped oligonucleotides too short to prime transcription, but long enough to bind with high affinity to the viral polymerase, are potent inhibitors of cap-dependent transcription in vitro.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa E., Moss B. mRNA(nucleoside-2'-)-methyltransferase from vaccinia virus. Characteristics and substrate specificity. J Biol Chem. 1978 Nov 10;253(21):7698–7702. [PubMed] [Google Scholar]
- Barbosa E., Moss B. mRNA(nucleoside-2'-)-methyltransferase from vaccinia virus. Purification and physical properties. J Biol Chem. 1978 Nov 10;253(21):7692–7697. [PubMed] [Google Scholar]
- Beaton A. R., Krug R. M. Selected host cell capped RNA fragments prime influenza viral RNA transcription in vivo. Nucleic Acids Res. 1981 Sep 11;9(17):4423–4436. doi: 10.1093/nar/9.17.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Both the 7-methyl and the 2'-O-methyl groups in the cap of mRNA strongly influence its ability to act as primer for influenza virus RNA transcription. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3952–3956. doi: 10.1073/pnas.77.7.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braam J., Ulmanen I., Krug R. M. Molecular model of a eucaryotic transcription complex: functions and movements of influenza P proteins during capped RNA-primed transcription. Cell. 1983 Sep;34(2):609–618. doi: 10.1016/0092-8674(83)90393-8. [DOI] [PubMed] [Google Scholar]
- Caton A. J., Robertson J. S. Structure of the host-derived sequences present at the 5' ends of influenza virus mRNA. Nucleic Acids Res. 1980 Jun 25;8(12):2591–2603. doi: 10.1093/nar/8.12.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J. Characterization of cap structures. Methods Enzymol. 1989;180:164–176. doi: 10.1016/0076-6879(89)80100-4. [DOI] [PubMed] [Google Scholar]
- Hagler J., Shuman S. Stability of ternary transcription complexes of vaccinia virus RNA polymerase at promoter-proximal positions. J Biol Chem. 1992 Apr 15;267(11):7644–7654. [PubMed] [Google Scholar]
- Honda A., Mizumoto K., Ishihama A. RNA polymerase of influenza virus. Dinucleotide-primed initiation of transcription at specific positions on viral RNA. J Biol Chem. 1986 May 5;261(13):5987–5991. [PubMed] [Google Scholar]
- Ishihama A., Mizumoto K., Kawakami K., Kato A., Honda A. Proofreading function associated with the RNA-dependent RNA polymerase from influenza virus. J Biol Chem. 1986 Aug 5;261(22):10417–10421. [PubMed] [Google Scholar]
- Kato A., Mizumoto K., Ishihama A. Purification and enzymatic properties of an RNA polymerase-RNA complex from influenza virus. Virus Res. 1985 Sep;3(2):115–127. doi: 10.1016/0168-1702(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Ishihama A., Ohtsuka E., Tanaka T., Takashima H., Ikehara M. RNA polymerase of influenza virus. II. Influence of oligonucleotide chain length on the priming activity of RNA synthesis by virion-associated RNA polymerase. J Biochem. 1981 Jun;89(6):1759–1768. doi: 10.1093/oxfordjournals.jbchem.a133375. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Mizumoto K., Ishihama A. RNA polymerase of influenza virus. IV. Catalytic properties of the capped RNA endonuclease associated with the RNA polymerase. Nucleic Acids Res. 1983 Jun 11;11(11):3637–3649. doi: 10.1093/nar/11.11.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., LaFiandra A. J., Morgan M. A., Shatkin A. J. Priming and inhibitory activities of RNAs for the influenza viral transcriptase do not require base pairing with the virion template RNA. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5874–5878. doi: 10.1073/pnas.77.10.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhard R. E., Rajbhandary U. L. Nucleotide sequences at the 5'termini of rabbit alpha and beta globin mRNA. Cell. 1976 Dec;9(4 Pt 2):747–760. doi: 10.1016/0092-8674(76)90138-0. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Parvin J. D., Palese P., Honda A., Ishihama A., Krystal M. Promoter analysis of influenza virus RNA polymerase. J Virol. 1989 Dec;63(12):5142–5152. doi: 10.1128/jvi.63.12.5142-5152.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Krug R. M. Transfer of 5'-terminal cap of globin mRNA to influenza viral complementary RNA during transcription in vitro. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1618–1622. doi: 10.1073/pnas.76.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Ulmanen I., Krug R. M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981 Mar;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Plotch S. J., Krug R. M. Identification of the RNA region transferred from a representative primer, beta-globin mRNA, to influenza mRNA during in vitro transcription. Nucleic Acids Res. 1980 Mar 11;8(5):925–942. doi: 10.1093/nar/8.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Aug 24;6(12):3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochovansky O. M. RNA synthesis by ribonucleoprotein-polymerase complexes isolated from influenza virus. Virology. 1976 Sep;73(2):327–338. doi: 10.1016/0042-6822(76)90394-9. [DOI] [PubMed] [Google Scholar]
- Seong B. L., Brownlee G. G. A new method for reconstituting influenza polymerase and RNA in vitro: a study of the promoter elements for cRNA and vRNA synthesis in vitro and viral rescue in vivo. Virology. 1992 Jan;186(1):247–260. doi: 10.1016/0042-6822(92)90079-5. [DOI] [PubMed] [Google Scholar]
- Seong B. L., Brownlee G. G. Nucleotides 9 to 11 of the influenza A virion RNA promoter are crucial for activity in vitro. J Gen Virol. 1992 Dec;73(Pt 12):3115–3124. doi: 10.1099/0022-1317-73-12-3115. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Lamb R. A. A specific sub-set of host-cell mRNAs prime influenza virus mRNA synthesis. Virus Res. 1984 Sep;1(6):455–467. doi: 10.1016/0168-1702(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Surratt C. K., Milan S. C., Chamberlin M. J. Spontaneous cleavage of RNA in ternary complexes of Escherichia coli RNA polymerase and its significance for the mechanism of transcription. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7983–7987. doi: 10.1073/pnas.88.18.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Broni B. A., Krug R. M. Role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7355–7359. doi: 10.1073/pnas.78.12.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]