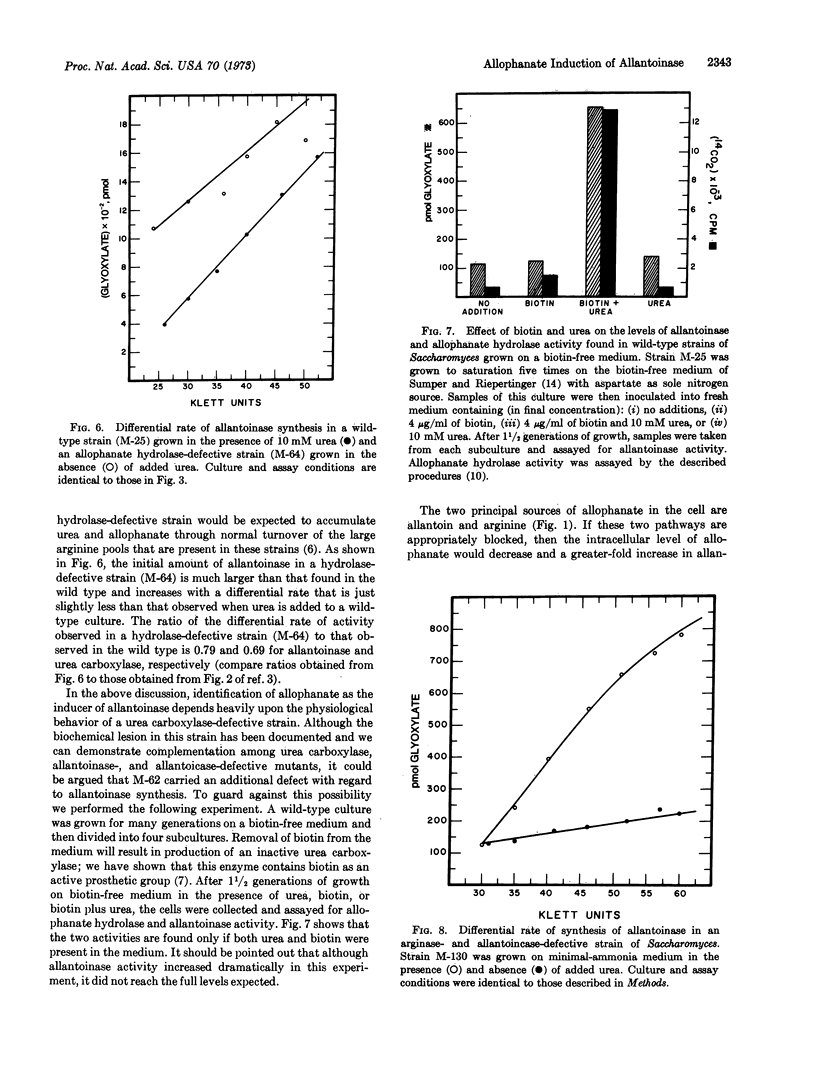
Abstract
Saccharomyces cerevisiae can degrade allantoin in five steps to glyoxylate, ammonia, and “CO2.” We previously demonstrated that synthesis of the urea carboxylase-allophanate hydrolase multienzyme complex is contingent upon the presence of allophanic acid, the product of the urea carboxylase reaction. Since these enzymes catalyze the last two reactions of allantoin degradation, experiments were performed to establish whether or not the presence of allophanic acid was required for synthesis of any other enzymes participating in this degradative pathway. The data presented here indicate that allophanic acid is required for synthesis of all enzymes participating in allantoin degradation. This conclusion is based upon the observation that: (i) wild-type strains produced a large amount of allantoinase upon addition of allantoin, allantoate, ureidoglycolate, or urea to the medium, (ii) no increase in activity was observed unless the added compound could be metabolized to allophanate, (iii) strains lacking allophanate hydrolase contained large amounts of allantoinase even in the absence of added urea, and (iv) the urea analogue, formamide, was capable of inducing allantoinase synthesis in wild-type strains but would not serve this function in a strain lacking urea carboxylase.
Keywords: allophanic acid, urea carboxylase, allantoin degradation defective mutants
Full text
PDF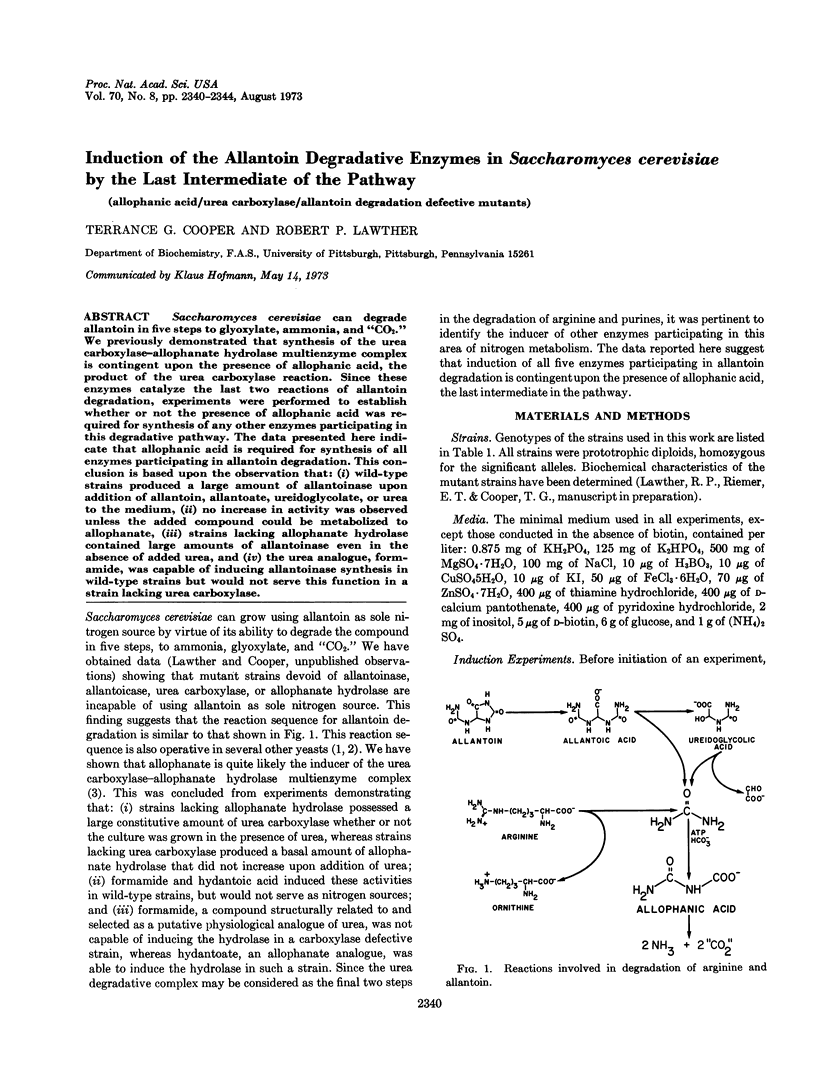



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choi K. S., Lee K. W., Roush A. H. The assay of yeast ureidoglycolatase. Anal Biochem. 1966 Dec;17(3):413–422. doi: 10.1016/0003-2697(66)90177-1. [DOI] [PubMed] [Google Scholar]
- DI CARLO F. J., SCHULTZ A. S., KENT A. M. The mechanism of allantoin catabolism by yeast. Arch Biochem Biophys. 1953 Jun;44(2):468–474. doi: 10.1016/0003-9861(53)90064-2. [DOI] [PubMed] [Google Scholar]
- DOMNAS A. Amide metabolism in yeasts. II. The uptake of amide and amide like compounds by yeast. J Biochem. 1962 Sep;52:149–154. [PubMed] [Google Scholar]
- LEE K. W., ROUSH A. H. ALLANTOINASE ASSAYS AND THEIR APPLICATION TO YEAST AND SOYBEAN ALLANTOINASES. Arch Biochem Biophys. 1964 Dec;108:460–467. doi: 10.1016/0003-9861(64)90427-8. [DOI] [PubMed] [Google Scholar]
- PALLERONI N. J., STANIER R. Y. REGULATORY MECHANISMS GOVERNING SYNTHESIS OF THE ENZYMES FOR TRYPTOPHAN OXIDATION BY PSEUDOMONAS FLUORESCENS. J Gen Microbiol. 1964 May;35:319–334. doi: 10.1099/00221287-35-2-319. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Scotto P., Magasanik B. Exogenous and endogenous induction of the histidine-degrading enzymes in Aerobacter aerogenes. J Biol Chem. 1965 Nov;240(11):4331–4337. [PubMed] [Google Scholar]
- Sumper M., Riepertinger C. Structural relationship of biotin-containing enzymes. Acetyl-CoA carboxylase and pyruvate carboxylase from yeast. Eur J Biochem. 1972 Sep 18;29(2):237–248. doi: 10.1111/j.1432-1033.1972.tb01980.x. [DOI] [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Degradation of allantoin by Pseudomonas acidovorans. Biochim Biophys Acta. 1966 Feb 14;113(2):292–301. doi: 10.1016/s0926-6593(66)80068-1. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Urea carboxylase and allophanate hydrolase. Two components of adenosine triphosphate:urea amido-lyase in Saccharomyces cerevisiae. J Biol Chem. 1972 Mar 10;247(5):1349–1353. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. Urea carboxylase from Saccharomyces cerevisiae. Evidence for a minimal two-step reaction sequence. J Biol Chem. 1973 Jan 10;248(1):325–330. [PubMed] [Google Scholar]



