Abstract
Neurocognitive disorders such as dementia and cognitive/motor impairments are among the most significant complications associated with human immunodeficiency virus (HIV) infection, especially in aging populations, yet the pathogenesis remains poorly understood. Activated macrophages and microglia in white matter along with the hallmark multinucleated giant cells are prominent features of HIV encephalitis (HIVE) and of several simian immunodeficiency virus (SIV) models. While infected microglia have been demonstrated in HIVE, this feature is not routinely seen in experimental infections in rhesus macaques using SIV or chimeric simian/HIV (SHIV) strains, limiting utility in HIV-1 pathogenesis and treatment studies. Here, 50 rhesus macaques were inoculated with the CCR5 (R5)-tropic SHIVSF162P3N virus by one of three routes: intravenously (n=9), intrarectally (n=17), or intravaginally (n=24). Forty-three monkeys became viremic, 26 developed AIDS, and 7 (7/26, 27 %) developed giant cell SIVencephalitis (SIVE). Rapid progressor phenotype was evident in five of seven (71 %) macaques with SIVE, and expansion to utilize the CXCR4 coreceptor (X4 coreceptor switch) was observed in four out of seven (57 %). SIVE lesions were present in gray and white matter in the cerebrum, cerebellum, thalamus, and brain stem of affected animals. Lesions were composed of virally infected CD68+, CD163+, and HLA-DR+ macrophages accompanied by white matter damage, necrosis, and astroglial and microglial activation. Importantly, microglial infection was observed, which makes R5 SHIVSF162P3N infection of macaques an attractive animal model not only to study transmission and HIVE pathogenesis but also to conduct preclinical evaluation of therapeutic interventions aimed at eradicating HIV-1 from the central nervous system (CNS).
Keywords: HIV, SHIV, Rhesus macaque, Neuropathogenesis, CCR5, Encephalitis
Introduction
Lentiviral infection of the central nervous system (CNS) can lead to a spectrum of neurologic diseases ranging from mild cognitive impairment to severe dementia and fulminant giant cell encephalitis [human immunodeficiency virus encephalitis (HIVE)/simian immunodeficiency virus encephalitis (SIVE)], with the prevalence of HIV-associated dementia (HAD) and minor cognitive/motor disorders (MCMD) reported as high as 37 % in treated patients with advanced disease (McArthur 2004; Sacktor et al. 2002; Schifitto et al. 2001). Despite progress in treatment, HIV-1 inevitably remerges following cessation of highly active antiretroviral therapy (HAART). The ability of HIV-1 to persist in cellular and tissue reservoirs, including macrophages and microglia in the brain, represents a major hurdle to efforts aimed at eliminating the virus (Deeks and Barre-Sinoussi 2012). Accordingly, to achieve the goal of curing the body of HIV-1 infection, it is critical to understand central nervous system (CNS) latency through comprehensive studies in a highly relevant animal model of neuropathogenesis of AIDS and HAART suppression. In addition, such an animal model will be essential for screening innovative therapeutic interventions aimed at eradicating HIV-1 from the brain.
CD4 is the primary receptor for HIV/SIV, and T lymphocytes are the primary cell type infected early in infection. Later stages of disease, including the development of CNS pathology, are associated with the evolution of HIV/SIV to bind cells expressing low levels of CD4, such as macrophages, which in the terminal stages of infection after lymphocyte depletion, are an important source of circulating free systemic virus. In addition to binding CD4, HIV/SIV strains exhibit variable coreceptor usage including CCR5 (R5) and CXCR4 (X4), and a receptor choice can affect transmissibility and disease progression. In particular, R5-tropic viruses are the most frequent viruses to infect a host across mucosal surfaces (Keele et al. 2008; Salazar-Gonzalez et al. 2009) and to display varying degrees of macrophage tropism. In approximately 50 % of HIV-1 clade B-infected patients, viruses undergo a coreceptor switch late in disease where X4 variants emerge concurrently with rapid CD4+ T cell depletion.
While infected T lymphocytes may be a source of virus entry into the CNS, it is largely held that infected monocyte/macrophages are a major route of entry for virus into the brain. Multiple CNS macrophage populations are targets of HIV including perivascular, meningeal, and choroid plexus macrophages as well as resident microglia (Soulas et al. 2009). Histologically, active brain lesions in treatment-naïve humans include perivascular mononuclear cell accumulation with microglial nodules, multinucleate giant cells, and widespread astrocyte activation. HIV-infected microglia are an important component of HIV neuropathogenesis but have not been a consistent feature of commonly used SIV/simian/HIV (SHIV) model systems in rhesus macaques for HIVE (Gray et al. 2000; Yadav and Collman 2009).
In this study, we report giant cell SIV-associated encephalitis in Indian-origin rhesus macaques (RMs) infected with high-dose pathogenic CCR5-tropic SHIVSF162P3N. This isolate was derived through successive and rapid passaging in RMs of SHIVSF162, a CCR5-tropic chimeric molecular clone expressing the envelope of HIV-1SF162 that was recovered from the cerebrospinal fluid (CSF) of a HIV-infected individual with neurologic symptoms and disease (Cheng-Mayer et al. 1989; Ho et al. 2007; Luciw et al. 1995). SHIVSF162P3N infection of RMs exhibited important similarities to HIV-1 infection in humans. These features include the use of CD4 receptor and CCR5 coreceptor for entry, robust transmission via mucosal routes, acute depletion of memory CD4+ T cells in the gut, establishment of persistent infection with a progressive gradual loss of peripheral CD4+ T cells, the potential for CXCR4 coreceptor switch, and AIDS disease development. Importantly, CNS pathology was identified in approximately 30 % of R5 SHIVSF162P3N-infected macaques that developed AIDS, with brain lesions of similar quality and severity to that seen in HIV-1-associated encephalitis in the pre-HAART era. Notably, CD68+, CD163+, and HLA-DR+ macrophages, consistent with an activated phenotype, were frequently infected within the perivascular brain lesions. Moreover, in our model of SIV-associated encephalitis, infected parenchymal microglia were frequently detected, recapitulating a key feature of HIV infection that is absent in most SIV/SHIV macaque models. The highly similar neurological lesions seen in R5 SHIV-infected monkeys and in treatment-naïve HIV-infected people render infection with R5 SHIVSF162P3N, an important animal model not only for studying neurological disease but also for advancing our knowledge of CNS HIV-1 persistence and latency as well
Materials and methods
Animal inoculation and clinical assessments
All inoculations were carried out in adult RMs (Macaca mulatta) of Indian origin individually housed at the Tulane National Primate Research Center (TNRPC) in compliance with its Guide for the care and use of laboratory animals. Animals were confirmed to be serologically negative for simian type D retrovirus, SIV, and simian T cell-lymphotropic virus prior to infection and were screened for the presence of the Mamu-A*01, Mamu-B*17, and Mamu-B*08 class I alleles previously shown to be associated with control of pathogenic SIVmac239 replication using standard PCR with allele-specific primers for cohort characterization (Goulder and Watkins 2008).
Fifty RMs were inoculated with R5 SHIVSF162P3N by either the intravenous (n=9), intrarectal (n=17), or intravaginal route (n=24) (Ho et al. 2007; Ren et al. 2010; Shakirzyanova et al. 2012). The virus stock for intravenous challenge was prepared using human PBMC (HuPBMC) and titered in rhesus PBMC (rhPBMC), while the intrarectal and intravaginal inoculations were performed using two different virus stocks that were propagated and titered in rhPBMC. Whole blood from the inoculated animals was collected weekly for the first 8 weeks, biweekly for another 16 weeks, and monthly thereafter. Plasma viremia was quantified by branched DNA analysis (Siemens Medical Solutions Diagnostic Clinical Lab, Emeryville, CA) and absolute CD4+ and CD8+ cell counts were monitored by Trucount (BD Biosciences, Palo Alto, CA). Animals were euthanized at the end of the study period by intramuscular administration of Telazol and buprenorphine followed by an overdose of sodium pentobarbital, and tissues from multiple sites were collected. Euthanasia was considered to be AIDS related if the animal exhibited peripheral blood CD4+ T cell depletion (<200/mm3), greater than 25% loss of body weight, and combinations of the following conditions: diarrhea unresponsive to treatment, opportunistic infections, peripheral lymph node atrophy, and abnormal hematology (e.g., anemia, thrombocytopenia, or leukopenia).
Neuropathology and immunophenotyping of SHIV-infected cells
Formalin-fixed paraffin-embedded brain sections were processed and stained routinely for hematoxylin and eosin. Sections were also stained for myelin with Luxol fast blue (LFB) with cresyl violet counterstain. Identification of SHIV-infected cells in the CNS was accomplished with double-label in situ hybridization (ISH) for SIVmac and immunohistochemistry for macrophage marker ionized calcium-binding adaptor molecule 1 (Iba-1) (Wako Chemicals, Richmond, VA; rabbit polyclonal, 019-19741, 1:1,000). Additional immunohistochemistry assays were conducted on brain tissues for the following antibodies: glial fibrillary acidic protein (GFAP) (polyclonal; Dako, Carpinteria, CA), CD68 (clone KP-1; Dako), MRP14 (clone Mac387, IgG1; Thermo Scientific, Waltham, MA), CD163 (clone 10D6, IgG1; Thermo Scientific), and HLA-DR (clone LN-3, IgG2b; Leica, Buffalo Grove, IL).
Briefly, tissue sections were deparaffinized in xylene and rehydrated through graded ethanol to Tris-buffered saline (TBS) plus Tween 20. Endogenous peroxidase activity was blocked by incubation in 3 % H2O2 in PBS. Tissue sections were pretreated with reagents from the RiboMap kit (Ventana Medical Systems) and digested with protease 3 (Ventana Medical Systems) for 4 min at 37 °C. Sections were then prehybridized with RiboHyb (Ventana Medical Systems) and hybridized with dig-labeled probe for 6 h at 55 °C. Stringency washes were done using 1.0× sodium chloride-sodium citrate (SSC) (Ventana Medical Systems) at 60 °C. The bound probe was detected with rabbit anti-dig (Sigma-Aldrich, St. Louis, MO; 1:20,000), UMap anti-rabbit alkaline phosphatase conjugate (Ventana Medical Systems), and the chromogen, NBT/BCIP (Ventana Medical Systems). Sections were then incubated with Iba-1 antibody (Wako Chemicals, Richmond, VA; 1:1,000) for 30 min at room temperature followed by biotinylated secondary antibody (GAR-b, Dako; 1:200) for 30 min. Sections were detected using standard avidin-biotin peroxidase complex technique (VECTASTAIN Elite ABC; Vector Laboratories, Burlingame, CA) and counterstained with Nuclear Fast Red (Vector Laboratories). Isotype-matched irrelevant controls were included for all runs.
Full-length SIVmac ISH probe was used for maximum sensitivity in detecting viral RNA and DNA and was performed on the Discovery Ultra platform (Ventana Medical Systems, Tucson, AZ) as previously described (Annamalai et al. 2010).
Results
Outcomes of SHIVSF162P3N infection in rhesus macaques including development of SIVE
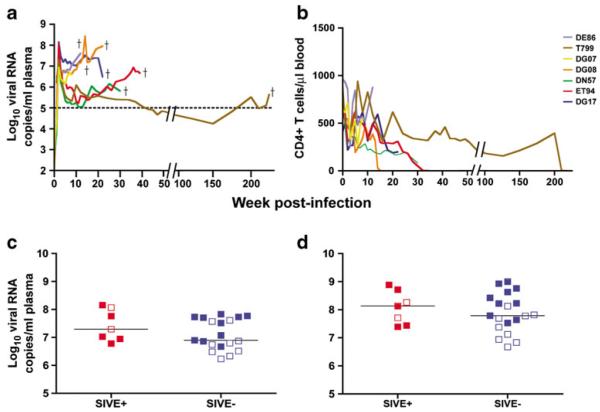
Of the 43 Indian-origin RMs that became viremic after inoculation with R5 SHIVSF162P3N via the intravenous (IV), intrarectal (IR), or intravaginal (IVag) route, 26 RMs (60 %) progressed to develop AIDS, including 8 from the intravenous group, 13 from the IR group, and 5 from the IVag group (Suppl Table 1). Out of 26, seven (27 %) developed SIVE (Table 1), including two from the IV group, four from the IR group, and one from the IVag group. High and sustained levels of virus replication were seen in the seven SIVE animals (Fig. 1a), with the development of clinical signs that met the criteria for study endpoint including the development of opportunistic infections, low CD4+ T cell counts, and/or marked weight loss (Fig. 1b, other data are not shown). A rapid progressor (RP) phenotype, characterized by high levels of virus replication and weak or undetectable antiviral antibody response, was evident in five of seven (57 %) macaques with SIVE (71.4 %), and coreceptor switch was documented in four of the seven (57 %) SIVE monkeys (Table 1). Consistent with findings in HIV-1-infected humans, precipitous peripheral CD4 loss was seen towards end-stage disease in three of the four SIVE macaques with coreceptor switch (Fig. 1b).
Table 1.
Properties of subtype B R5 SHIVSF162P3N-infected macaques with SIVE
| Animal # | Age (years) | Weight (kg) | Route | Dose (TCID50) | Time to necropsy (weeks) | CoR switch | CSF viral load (RNA copies/ml) |
|---|---|---|---|---|---|---|---|
| DE86 | 5.9 | 9.8 | IV | 3,000 | 12a | Yes | 33,214 |
| T799 | 14.9 | 7.1 | IV | 3,000 | 224 | Yes | NA |
| DG07 | 6.9 | 8.5 | IR | 10,000 | 7a | 10,586 | |
| DG08 | 7.4 | 8.6 | IR | 10,000 | 20a | Yes | 40,386 |
| DN57 | 6.4 | 9.7 | IR | 10,000 | 30a | 120,000 | |
| ET94 | 7.4 | 8.5 | IR | 10,000 | 39 | Yes | 114,429 |
| DG17 | 8.9 | 5.8 | IVag | 1,000 | 22a | NA |
Age in years and weight in kilograms indicated are at the time of necropsy (indicated in weeks postinoculation). Routes of inoculation are IV, IR, and IVag. Animals with CoR switch from CCR5 to CXCR4 are indicated
IV intravenous, IR intrarectal, IVag intravaginal, CoR coreceptor, NA serum not available for testing
Macaques meeting criteria for a rapid progressor status
Fig. 1.
Virologic and immunologic parameters of SHIVSF162P3N infection. Temporal analysis of viral load (a) and peripheral CD4+ T cell counts (b) in the plasma of macaques with SIVE. Dagger indicates time of euthanasia. Peak plasma (c) and end-stage CSF (d) viral load in infected rhesus macaques that progressed to AIDS, divided as those with SIVE (plus sign) and those without SIVE (minus sign). Solid symbols in c and d designate rapid progressors
There was no significant difference between the levels of peak or cumulative virus in the plasma between animals with and without SIVE when all animals were pooled regardless of progressor phenotype (Fig. 1c, other data are not shown). Age of the macaques at the time of virus inoculation, cumulative viral load up to the time of euthanasia, CD4 nadir, and switch or expansion from CCR5 to CXCR4 usage also did not differ between the SHIVSF162P3N-infected AIDS macaques with and without a RP phenotype or SIVE (Suppl Table 1). RT-PCR for SIV gag sequences in the CSF collected at the time of necropsy for five of the seven AIDS animals with SIVE showed the presence of SHIV RNA, including DG07, which was euthanized at only 7 weeks postinfection (Fig. 1d, Table 2). Although CSF viral loads trended higher in the SIVE cases compared to those without, the difference was not statistically significant (p=0.10) (Fig. 1d).
Table 2.
Summary of IHC results and severity scoring
| Animal # | Route | RP | Tissue | SIV (ISH) | CD68 | GFAP | CD163 | Iba-1 |
|---|---|---|---|---|---|---|---|---|
| ET94 | IR | N | 1d FC | ++ | +++ | + | ++ | ++ |
| 1e TH | ++++ | ++++ | +++ | ++++ | ++++ | |||
| 1f OC | ++++ | ++++ | ++ | ++++ | +++ | |||
| DG17 | IVag | Y | 1d FC | ++ | +++ | ++ | +++ | +++ |
| 1e TH | +++ | +++ | ++ | +++ | ++++ | |||
| 1f OC | +++ | +++ | ++ | +++ | +++ | |||
| DN57 | IR | N | 1d PFC | 0 | 0 | + | 0 | 0 |
| 1e TH/PUT | + | ++ | 0 | ++ | ++ | |||
| 1f OC | +++ | +++ | + | +++ | NP | |||
| 1g CB | ++++ | ++++ | + | ++++ | ++++ | |||
| 1h BS | ++++ | ++++ | + | NP | ++++ | |||
| DG08 | IR | Y | 1d PFC | 0 | 0 | 0 | +/− | 0 |
| 1e TH/PUT | 0 | 0 | + | 0 | + | |||
| 1f OC | 0 | 0 | + | +/− | + | |||
| 1g CB | +++ | +++ | ++ | ++++ | +++ | |||
| 1h BS/CB | +++ | +++ | + | ++++ | +++ | |||
| DE86 | IV | Y | 1c FC | ++ | ++ | NP | NP | NP |
| 1d TH | +/− | +(focal) | + | + | + | |||
| 1e OC | +++ | +++ | + | ++ | +++ | |||
| 1f CB | +/− | +(focal) | 0 | ++ | + | |||
| 1g BS | + | ++ | ++ | ++ | ++ | |||
| T799 | IV | N | 1d FC | 0 | + | + | + | ++ |
| 1e BG | +++ | ++ | ++ | ++ | ++ | |||
| 1h BS | 0 | ++++ | ++ | +++ | +++ | |||
| DG07 | IR | N | 1c FC | 0 | NP | NP | 0 | NP |
| 1d MFB | 0 | NP | NP | 0 | NP | |||
| 1f MHB | +/− | NP | NP | +/− | NP | |||
| 1g OC | 0 | NP | NP | 0 | NP |
Subjective semiquantitative severity scoring for the presence of SIVantigen by in situ hybridization and macrophage markers (CD68, CD163, Iba-1) or the astrocyte marker glial fibrillary acidic protein by immunohistochemistry. Lesions in examined brain sections range from 0=no change over control brain sections to ++++=most severe SIV-associated giant cell lesions observed. Animals are listed in order of overall lesion severity
GFAPglial fibrillary acidic protein; PFCprefrontal cortex; FCfrontal cortex; MFBmid-forebrain; THthalamus with temporal cortex and, in some cases, PUT putamen, as noted; OC occipital cortex; MB midbrain; MHB mid-hindbrain; CB cerebellum; BS brain stem; NP not performed
Virus detection in the CNS and three distinct CNS lesion patterns
Histologic examination of brain tissues revealed that SIV-associated giant cell neuropathology in the R5 SHIVSF162P3N SIVE cohort was found throughout all brain sections (Table 2) and was often severe with marked tissue destruction. The lesions observed in the infected macaques mirrored that previously reported for pre-HAART HIVE in infected patients and were characterized by perivascular mononuclear cell accumulation with microglial nodules, multinucleated giant cells, and widespread astrocyte activation around lesions.
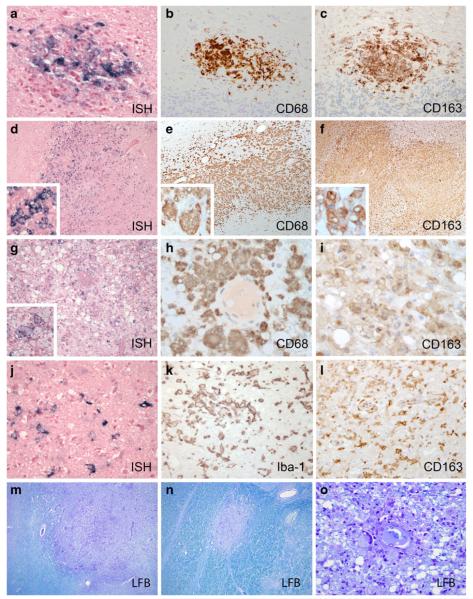
Lesions in infected rhesus with SIVE were divided into three main histopathologic patterns, here designated perivascular histiocytic with MNGCs, severe parenchymal with white matter damage, and chronic burnt-out (Fig. 2). The perivascular type was composed of perivascular lesions similar to those seen in other macaque models of SIV, which were predominantly located within cerebral white matter and were comprised of activated CD68+/HLA-DR+ SHIV-infected macrophages and multinucleated giant cells (Fig. 2a, b). CD163, the cellular scavenger receptor for hemoglobin-haptoglobin complex and a marker of activated monocyte/macrophage lineage cells, was abundantly expressed by infiltrated monocyte/macrophages and activated microglia (Fig. 2c). The second type was characterized by very large, expansive lesions that extended far beyond perivascular spaces into deep gray and white matter parenchyma and were observed in areas including the thalamus, basal ganglia, brain stem, and cerebellum (Fig. 2d–f). These lesions were unlike the classical SIVmac239/251 lesions based on their size, the marked activation of surrounding microglia and astroglia, the widespread expansion of SHIV-infected cells into the parenchyma, and extensive white matter damage (Fig. 2m, n). The third type of lesion was comprised of chronic “burnt-out” lesions centered on vessels surrounded by large numbers of vacuolated macrophage/microglia that contained scant amounts of virus based on ISH (Fig. 2g). These activated cells were morphologically consistent with phagocytic macrophages (Fig. 2h, i) and contained phagocytosed cellular and myelin debris in their cytoplasm (Fig. 2o). In addition, R5 SHIV brain sections contained marked white matter damage (decreased Luxol fast blue myelin staining, Fig. 2m–o), axonal damage (spheroid formation), and neuronal dropout. All three types of lesions were detected in ET94 and DG17, with varying degrees of perivascular and parenchymal lesions seen in DN57, DG08, DE86, and T799, and only mild perivascular lesions were observed in DG07.
Fig. 2.
Neuropathologic features of SIVE in brains of R5 SHIVSF162P3N-infected macaques. a–c Perivascular histiocytic lesions (case ET94, frontal cortex) are positive for SIV by ISH (a), and CD68 (b) and CD163 (c) by immunohistochemistry with frequent multinucleated giant cells surrounded by activated microglia observed. d–f Severe parenchymal lesions with white matter damage (case ET94, thalamus) also contain SIV+ (d), CD68+ (e), and CD163+ (f) cells with gemistocytic astrocytes and occasional neutrophils. g–i In chronic burnt-out lesions (case ET94, occipital cortex), infected cells contain a scant amount of virus by SIV ISH (g), but abundant CD68 (h) and CD163+ (i) cells invade deeply into the surrounding parenchyma. j Microglia with rod-shaped nuclei are infected in all three types of lesions (SIV ISH+). k, l Activated microglia. k Iba-1-positive and l CD163-positive activated microglia are located distant from the center of a perivascular lesion. Luxol fast blue (LFB) staining of myelin is decreased in severe parenchymal lesion (m), lesion in the white matter tract (n), and in burnt-out lesion with phagocytosed myelin within macrophages (o)
In summary, the observed types of histologic lesions induced by R5 SHIVSF162P3N are consistent with descriptions of moderate to severe pre-HAART HIVE (Budka et al. 1987; Michaels et al. 1988; Sharer 1992; Wiley et al. 1986). In addition, unique features were seen in these infected macaques that have occasionally been noted in untreated human cases, including the presence of vacuolated macrophages, necrosis with a neutrophil component to the encephalitis, and extensive white matter damage (Budka et al. 1987). Some lesions also featured neuronal apoptosis and vascular proliferation. Severity of CNS pathology among the SIVE animals is summarized in the following rank order: ET94, DG17 > DN57 > DG08 > DE86, and T799 > DG07 (Table 2).
Microglial and astroglial activation
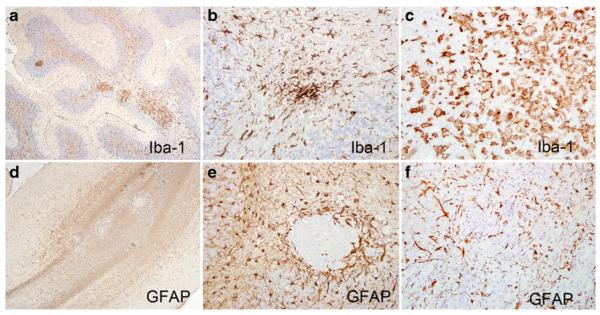
SIVE lesions in SHIVSF162P3N-infected macaques were associated with widespread glial activation. Iba-1, a marker of microglial activation (Ohsawa et al. 2004), was robustly expressed by a spectrum of early activated microglia from ramified to those with retracted branches (Fig. 3a, b) and those fully activated with amoeboid morphology and phagocytic vacuoles. GFAP, an intermediate filament in astrocytes, was markedly upregulated (Fig. 3d–f) compared to uninfected rhesus brain (data not shown) in astrocytes around perivascular lesions (Fig. 3d, e) and throughout large parenchymal lesions (Fig. 3f). Astrocytic processes were frequently thickened consistent with marked activation.
Fig. 3.
Microglial and astroglial activation in R5 SHIVSF162P3N-infected macaques. Brain sections were analyzed by immunohistochemistry for activated microglia (Iba-1, a–c) and astrocytes [glial fibrillary acidic protein (GFAP), d–f]. Low magnification images demonstrate an extent of microglial (a) and astroglial (d) activation (original magnification, ×4). Extensive microglial activation exhibited by upregulated Iba-1 and microglial nodule (b) and amoeboid morphology (c) (original magnification, ×20). Higher magnification of GFAP+-activated astrocytes around a discrete lesion (e, original magnification, ×20) and throughout a large expansive region (f, original magnification, ×10)
Multiple macrophage subtypes and microglial infection within lesions
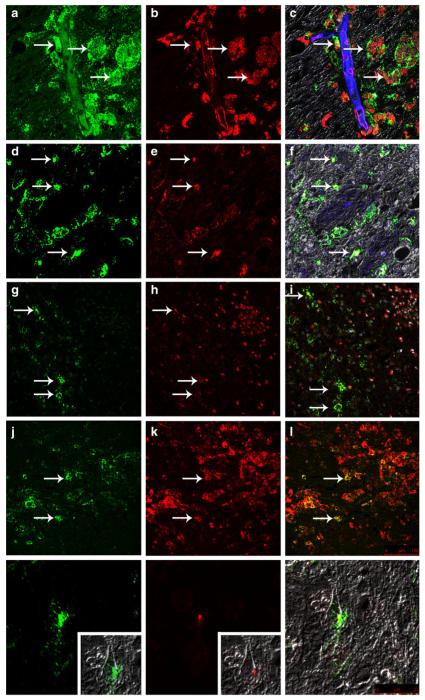
Consistent with reports that macrophage activation plays a central role in HIV/SIV CNS disease (Zink et al. 1997), macrophages within lesions were CD68+, CD163+, and HLA-DR+ and were robustly infected (Fig. 4). All three types of histologic lesions contained macrophages positive for all three markers. In addition to infiltrating macrophages within perivascular lesions, SHIVSF162P3N infected resident microglia, identified based on their in situ-positive rod-shaped nuclei (Fig. 2j), cellular morphology, upregulated Iba-1 and CD163 expression (Fig. 2k, l), and distance from the central perivascular lesion (Fig. 4m–o). A subset of activated macrophage/microglia that were Mac387/MRP14 immunopositive were present in most lesions but were particularly prominent at the periphery or leading edge of the large expansive lesions (Fig. 4h). Based on their location in the parenchyma distant from blood vessels and their morphology, some of these cells are likely recently activated resident microglia. Based also on double-label confocal microscopy, occasional Mac387+/MRP14+ macrophage/microglia were infected with virus (Fig. 4g–i).
Fig. 4.
Characterization of R5 SHIVSF162P3N-infected macrophage subpopulations and microglia. Immunofluorescence and confocal laser microscopy of CD68+ (a–c), CD163+ (d–f), Mac387+ (g–i), and HLA-DR+ (j–l) macrophage/microglia in the brain. An infected CD68+ microglia in a perineuronal location in the gray matter (m–o). a, d, g, j, m SIV p27 protein (green). b, n CD68 (red). e CD163 (red). h Mac387 (red). k HLA-DR (red). c, f, i, l, o Overlay with glut1 endothelial marker (blue) and colocalization of infected macrophages (yellow, c, f, i, l) or microglia (o). a–f, m–o Original magnification, ×80. g–l Original magnification, ×40
Discussion
Despite major advances in treatment regimens for humans infected with HIV-1, disease recurrence is a major complication in patients following cessation of medication due to tissue and cellular reservoirs of HIV. One such proposed reservoir is the CNS. Macrophages and microglia in the brain represent the major sites of productive virus infection by HIV, and there are some reports that astrocytes and endothelia may undergo restricted or latent infection (Churchill et al. 2009; Trillo-Pazos et al. 2003). Compartmentalization of virus in the CNS and emergence of CD4-independent viral strains contributes to the likelihood of infection of resident microglia. In addition, macrophage/microglial activation plays a central role in the mechanisms of HIV/SIV CNS disease by persistent secretion of damaging proinflammatory mediators in the CNS such as TNF-α and IFN-γ that occurs despite antiretroviral therapy (Annamalai et al. 2010; Eden et al. 2007; Gonzalez-Scarano and Martin-Garcia 2005; Yilmaz et al. 2008; Zink et al. 1997, 2010). These cytokines may provide a microenvironment conducive to viral replication and/or ongoing inflammatory cell turnover in the CNS, thereby contributing to virus maintenance and egress from the brain.
We have characterized CNS infection of a SHIV isolate, SHIVSF162P3N, which encodes highly relevant HIV-1 envelope glycoprotein determinants of neuroinvasion and neuropathogenesis, notably the ability to bind and infect CD4low monocyte/macrophage lineage. CNS pathology was identified in ~30 % of R5 SHIVSF162P3N-infected AIDS macaques, resulting in three different histologic patterns of brain lesions with varying degrees of accompanying white matter damage. Activated CD68+, CD163+, and HLA-DR+ macrophages were frequently infected within the perivascular and parenchymal brain lesions. Moreover, infected parenchymal microglia were readily detectable, recapitulating a key feature of HIV infection that is absent in most SIV/macaque models. In several of the cases examined in this study (e.g., DG08, DN57, ET94), the most severely affected sections were in present in deep gray matter structures of the brain (e.g., the basal ganglia, thalamus, brain stem, cerebellar peduncle). This virus distribution pattern is reminiscent of early observations in untreated HIV patients (Masliah et al. 1996; Wiley et al. 1998).
SHIVSF162P3N replicates robustly in primary macrophages, which represent the major viral target in the brain and a potential cellular reservoir in this distinct compartment. The virus induced clinical signs of encephalitis in a short time frame, in one case as little as 7 weeks postinoculation (DG07), consistent with the notion that HIV/SIV enters the CNS rapidly and efficiently after intravenous or mucosal transmission. One animal, T799, despite having large lesions consistent with SIVE, had only scant amounts of viral nucleic acid by ISH in brain tissues. This animal was euthanized at 224 weeks postinfection, much later than any other animal in the cohort. It is possible that these brain lesions represent a residual damage at previous sites of inflammation following depletion of the productively infected cells.
In general, brain lesions were comprised CD68+, CD163+, and HLA-DR+ macrophages, consistent with an activated phenotype, and were frequently infected. Microglial infection and expansive gray matter lesions were also features of this cohort that have not been generally reported in SIVmac-infected rhesus. A subset of activated Mac387+/MRP14+ macrophages was also present within the lesions, notably more concentrated at the outer or leading edges of the larger lesions. Although infrequent, R5 SHIVSF162P3N was detected in rare Mac387/MRP14+ macrophages, a subset not usually reported to be infected with SIV (Soulas et al. 2011).
Since the vast majority of HIV transmission occurs with R5 viruses via mucosal surfaces, mucosal transmission of SHIVSF162P3N accurately models sexual transmission in humans and is useful for studying features of viral transfer that cannot be recapitulated in intravenous inoculations, including the founder effect. An interesting feature of the CNS disease manifestation in this cohort of the SHIVSF162P3N-infected RMs is that the most severe brain lesions were seen in four of the animals that were mucosally inoculated. This observation may be the result of interactions with this R5-tropic SHIV swarm and the unique immune microenvironment of the vaginal or rectal mucosa, which may promote selection of viruses with particular traits. Furthermore, while CCR5 to CXCR4 coreceptor switch was documented in the blood and lymph nodes in four of the seven SIVE animals, preliminary analysis of Env variants in the brain and CSF of the macaques with SIVE showed only R5-tropic viruses (these authors, unpublished observations). Studies in larger cohorts of animals are needed to address if neuropathology is indeed more severe with SHIVSF162P3N mucosal inoculations and to understand the mechanism of X4 virus exclusion from the brain.
In summary, we characterize CNS disease in RMs infected with R5-tropic SHIVSF162P3N, which exhibit several features of HIVE rarely seen in SIVmac251- or SIVmac239-infected rhesus, especially the detectable infection of microglia. Because macrophage and microglia are long-lived cells and because antiretroviral drug access to the CNS is limited, viral decay in the brain may be slower than that in the periphery after the commencement of antiretroviral therapy (Schnell et al. 2011; Staprans et al. 1999). Additionally, CNS and brain macrophages and microglia may be less sensitive (Aquaro et al. 2002; Gunthard et al. 2001) and less efficient at uptake of certain antiretroviral drugs (Letendre et al. 2008; Perno et al. 1994; Wynn et al. 2002). Appropriate animal models of CNS infection in AIDS, therefore, are critically needed to evaluate the effectiveness of various treatment regimens in crossing the blood-brain barrier and for testing strategies aimed at eradicating viral sanctuaries in the brain. This study identifies an important new model system for studying HIV neuropathology, drug suppression, and residual virus in the CNS.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health grant R01AI46980 (CCM). Additional support was provided by the Tulane National Primate Research Center Base grant RR00164 (JB), the New England Primate Research Center Base grant P51OD011103-51 (NEPRC), and the National Institutes of Health T32 training grant T32OD011064 (SW).
Footnotes
Conflict of Interest The authors Carole Harbison, Ke Zhuang, Agegnehu Gettie, James Blanchard, Heather Knight, Peter Didier, Cecilia Cheng-Mayer, and Susan Westmoreland declare that they have no conflict of interest.
Contributor Information
Carole Harbison, Division of Comparative Medicine and Pathology, New England Primate Research Center, Harvard Medical School, Southborough, MA 01772, USA.
Ke Zhuang, Aaron Diamond AIDS Research Center, New York, NY, USA.
Agegnehu Gettie, Aaron Diamond AIDS Research Center, New York, NY, USA.
James Blanchard, Tulane National Primate Research Center, Tulane University Medical Center, Covington, LA, USA.
Heather Knight, Division of Comparative Medicine and Pathology, New England Primate Research Center, Harvard Medical School, Southborough, MA 01772, USA.
Peter Didier, Tulane National Primate Research Center, Tulane University Medical Center, Covington, LA, USA.
Cecilia Cheng-Mayer, Aaron Diamond AIDS Research Center, New York, NY, USA.
Susan Westmoreland, Division of Comparative Medicine and Pathology, New England Primate Research Center, Harvard Medical School, Southborough, MA 01772, USA.
References
- Annamalai L, Bhaskar V, Pauley DR, Knight H, Williams K, Lentz M, Ratai E, Westmoreland SV, Gonzalez RG, O’Neil SP. Impact of short-term combined antiretroviral therapy on brain virus burden in simian immunodeficiency virus-infected and CD8+ lymphocyte-depleted rhesus macaques. Am J Pathol. 2010;177:777–791. doi: 10.2353/ajpath.2010.091248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquaro S, Calio R, Balzarini J, Bellocchi MC, Garaci E, Perno CF. Macrophages and HIV infection: therapeutical approaches toward this strategic virus reservoir. Antivir Res. 2002;55:209–225. doi: 10.1016/s0166-3542(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Budka H, Costanzi G, Cristina S, Lechi A, Parravicini C, Trabattoni R, Vago L. Brain pathology induced by infection with the human immunodeficiency virus (HIV). A histological, immunocytochemical, and electron microscopical study of 100 autopsy cases. Acta Neuropathol. 1987;75:185–198. doi: 10.1007/BF00687080. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C, Weiss C, Seto D, Levy JA. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci U S A. 1989;86:8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Barre-Sinoussi F. Public health: towards a cure for HIV. Nature. 2012;487:293–294. doi: 10.1038/487293a. [DOI] [PubMed] [Google Scholar]
- Eden A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray F, Adle-Biassette H, Brion F, Ereau T, le Maner I, Levy V, Corcket G. Neuronal apoptosis in human immunodeficiency virus infection. J Neurovirol. 2000;6(Suppl 1):S38–S43. [PubMed] [Google Scholar]
- Gunthard HF, Havlir DV, Fiscus S, Zhang ZQ, Eron J, Mellors J, Gulick R, Frost SD, Brown AJ, Schleif W, Valentine F, Jonas L, Meibohm A, Ignacio CC, Isaacs R, Gamagami R, Emini E, Haase A, Richman DD, Wong JK. Residual human immunodeficiency virus (HIV) Type 1 RNA and DNA in lymph nodes and HIV RNA in genital secretions and in cerebrospinal fluid after suppression of viremia for 2 years. J Infect Dis. 2001;183:1318–1327. doi: 10.1086/319864. [DOI] [PubMed] [Google Scholar]
- Ho SH, Tasca S, Shek L, Li A, Gettie A, Blanchard J, Boden D, Cheng-Mayer C. Coreceptor switch in R5-tropic simian/human immunodeficiency virus-infected macaques. J Virol. 2007;81:8621–8633. doi: 10.1128/JVI.00759-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw PA, Pratt-Lowe E, Shaw KE, Levy JA, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proc Natl Acad Sci U S A. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Ge N, Mucke L. Pathogenesis of HIV-1 associated neurodegeneration. Crit Rev Neurobiol. 1996;10:57–67. doi: 10.1615/critrevneurobiol.v10.i1.30. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Michaels J, Sharer LR, Epstein LG. Human immunodeficiency virus type 1 (HIV-1) infection of the nervous system: a review. Immunodefic Rev. 1988;1:71–104. [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem. 2004;88:844–856. doi: 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- Perno CF, Aquaro S, Rosenwirth B, Balestra E, Peichl P, Billich A, Villani N, Calio R. In vitro activity of inhibitors of late stages of the replication of HIV in chronically infected macrophages. J Leukocyte Biol. 1994;56:381–386. doi: 10.1002/jlb.56.3.381. [DOI] [PubMed] [Google Scholar]
- Ren W, Tasca S, Zhuang K, Gettie A, Blanchard J, Cheng-Mayer C. Different tempo and anatomic location of dual-tropic and X4 virus emergence in a model of R5 simian-human immunodeficiency virus infection. J Virol. 2010;84:340–351. doi: 10.1128/JVI.01865-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifitto G, Kieburtz K, McDermott MP, McArthur J, Marder K, Sacktor N, Palumbo D, Selnes O, Stern Y, Epstein L, Albert S. Clinical trials in HIV-associated cognitive impairment: cognitive and functional outcomes. Neurology. 2001;56:415–418. doi: 10.1212/wnl.56.3.415. [DOI] [PubMed] [Google Scholar]
- Schnell G, Joseph S, Spudich S, Price RW, Swanstrom R. HIV-1 replication in the central nervous system occurs in two distinct cell types. PLoS Pathog. 2011;7:e1002286. doi: 10.1371/journal.ppat.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakirzyanova M, Tsai L, Ren W, Gettie A, Blanchard J, Cheng-Mayer C. Pathogenic consequences of vaginal infection with CCR5-tropic simian-human immunodeficiency virus SHIVSF162P3N. J Virol. 2012;86:9432–9442. doi: 10.1128/JVI.00852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharer LR. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992;51:3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Soulas C, Conerly C, Kim WK, Burdo TH, Alvarez X, Lackner AA, Williams KC. Recently infiltrating MAC387(+) monocytes/macrophages a third macrophage population involved in SIV and HIV encephalitic lesion formation. Am J Pathol. 2011;178:2121–2135. doi: 10.1016/j.ajpath.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulas C, Donahue RE, Dunbar CE, Persons DA, Alvarez X, Williams KC. Genetically modified CD34+ hematopoietic stem cells contribute to turnover of brain perivascular macrophages in longterm repopulated primates. Am J Pathol. 2009;174:1808–1817. doi: 10.2353/ajpath.2009.081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans S, Marlowe N, Glidden D, Novakovic-Agopian T, Grant RM, Heyes M, Aweeka F, Deeks S, Price RW. Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS. 1999;13:1051–1061. doi: 10.1097/00002030-199906180-00008. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdis-section. Brain Pathol. 2003;13:144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Dailey P, Achim CL. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8:277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn HE, Brundage RC, Fletcher CV. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs. 2002;16:595–609. doi: 10.2165/00023210-200216090-00002. [DOI] [PubMed] [Google Scholar]
- Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. J Neuroimmune Pharmacol. 2009;4:430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz A, Price RW, Spudich S, Fuchs D, Hagberg L, Gisslen M. Persistent intrathecal immune activation in HIV-1-infected individuals on antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:168–173. doi: 10.1097/QAI.0b013e31815ace97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Amedee AM, Mankowski JL, Craig L, Didier P, Carter DL, Munoz A, Murphey-Corb M, Clements JE. Pathogenesis of SIVencephalitis. Selection and replication of neurovirulent SIV. Am J Pathol. 1997;151:793–803. [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Brice AK, Kelly KM, Queen SE, Gama L, Li M, Adams RJ, Bartizal C, Varrone J, Rabi SA, Graham DR, Tarwater PM, Mankowski JL, Clements JE. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis. 2010;202:161–170. doi: 10.1086/653213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.