Abstract
Cardiac arrest results in significant mortality after initial resuscitation due in most cases to ischemiareperfusion induced brain injury and to a lesser degree myocardial dysfunction. Nitrite has previously been shown to protect against reperfusion injury in animal models of focal cerebral and heart ischemia. Nitrite therapy after murine cardiac arrest improved 22 h survival through improvements in myocardial contractility. These improvements accompanied transient mitochondrial inhibition which reduced oxidative injury to the heart. Based on preliminary evidence that nitrite may also protect against ischemic brain injury, we sought to test this hypothesis in a rat model of asphyxia cardiac arrest with prolonged survival (7 d). Cardiac arrest resulted in hippocampal CA1 delayed neuronal death well characterized in this and other cardiac arrest models. Nitrite therapy did not alter post-arrest hemodynamics but did result in significant (75%) increases in CA1 neuron survival. This was associated with increases in hippo-campal nitrite and S-nitrosothiol levels but not cGMP shortly after therapy. Mitochondrial function 1 h after resuscitation trended towards improvement with nitrite therapy. Based on promising preclinical data, the first ever phase I trial of nitrite infusions in human cardiac arrest survivors has been undertaken. We present preliminary data showing low dose nitrite infusion did not result in hypotension or cause methemoglobinemia. Nitrite thus appears safe and effective for clinical translation as a promising therapy against cardiac arrest mediated heart and brain injury.
Keywords: Nitrite, Cardiac arrest, Ischemia, Reperfusion injury, Brain ischemia, Nitric oxide
Introduction
Cardiac arrest (CA) occurs nearly half a million times annually in the US with approximately 290,000 cases out-of-hospital and 210,000 in-hospital [1,2]. Out-of-hospital cardiac arrest is associated with approximately 90% mortality [1] with survivors also suffering significant neurological disability [3]. Recent changes in guidelines have increased resuscitation rates but failed to improve survival to hospital discharge [4]. Prognosis after cardiac arrest is driven primarily by the degree of reperfusion injury sustained by the brain and heart. In-hospital mortality after successful resuscitation from out-of-hospital cardiac arrest can be attributed to neurological injury in 68% of cases and cardiovascular injury in 23% [5]. Thus there is a significant and enduring need for effective post-resuscitation therapies to mitigate neurological and cardiovascular injury.
It has been noted by multiple investigators that exogenous inhaled nitric oxide has endocrine effects on distant organs which exceed its brief half-life [6,7]. The nitrite anion is present in stable nM levels within the plasma of multiple mammalian species [8] and was first documented by Zweier to be a tissue source of enzyme-independent NO under conditions of ischemia [9]. Gladwin first demonstrated in humans physiologic nitrite consumption accentuated by exercise induced drops in pH and oxygen with associated NO-mediated vasodilator activity [10]. Subsequent work demonstrated the ability of nitrite to act as a NO synthase-independent means of NO delivery targeted specifically to ischemic tissues with vasodilator and cytoprotective effects [11–13]. In less than a decade nitrite therapy has been demonstrated to mitigate reperfusion injury in multiple animal models of ischemiareperfusion in a variety of organs [14,15]. Most relevant to cardiac arrest is evidence that nitrite can protect against reperfusion injury in both brain [16–18] and heart [12,13,19,20].
We previously demonstrated that nitrite therapy after CA resulted in improved 22 h survival [21]. This study utilized a 12 min murine model of asystolic CA resulting in significant myocardial dysfunction simulating the substantial loss of cardiac contractility seen after human CA [22,23]. Nitrite significantly improved cardiac contractility and thereby improved survival in concordance with human clinical data that myocardial dysfunction is a determinant of early mortality [24]. Preliminary evidence of reduced brain injury was noted in this study but due to high mortality, only three pairs of animals were successfully survived to 72 h for histology. Unlike myocardial dysfunction which presents early (4–7 h) after CA and is nearly completely reversed by 24 h [25], global ischemia-reperfusion injury in brain presents as delayed neuronal death, rarely evident by light microscopy before 48–72 h after injury [26] and quite prominent after 7 d [27]. Furthermore in our prior model of cardiac arrest, animals clearly demonstrated post-arrest cardiogenic shock with hypoperfusion based on decreasing blood pressure despite increasing pulse and substantial lactic acidosis [21]. In this setting, it is difficult to ascertain whether nitrite truly provided neuroprotection independent of hypoperfusion from myocardial dysfunction and whether this benefit remained at the point when the majority of delayed neuronal death would be expected to manifest.
Since human cardiac critical care is capable of preventing many post-arrest cardiac deaths, testing the hypothesis that nitrite therapy could provide neuroprotection 7 d after CA in a model marked by less cardiovascular mortality was needed. In this report we present our data on the effects of nitrite therapy in a rat model of cardiac arrest induced brain injury as well as initial studies aimed at characterizing potential mechanisms involved in neuro-protection. We also present preliminary phase I clinical data of nitrite infusions in human CA survivors in preparation for translation of nitrite therapy from bench to bedside in cardiac arrest survivors.
Methods
Rat asphyxial cardiac arrest model
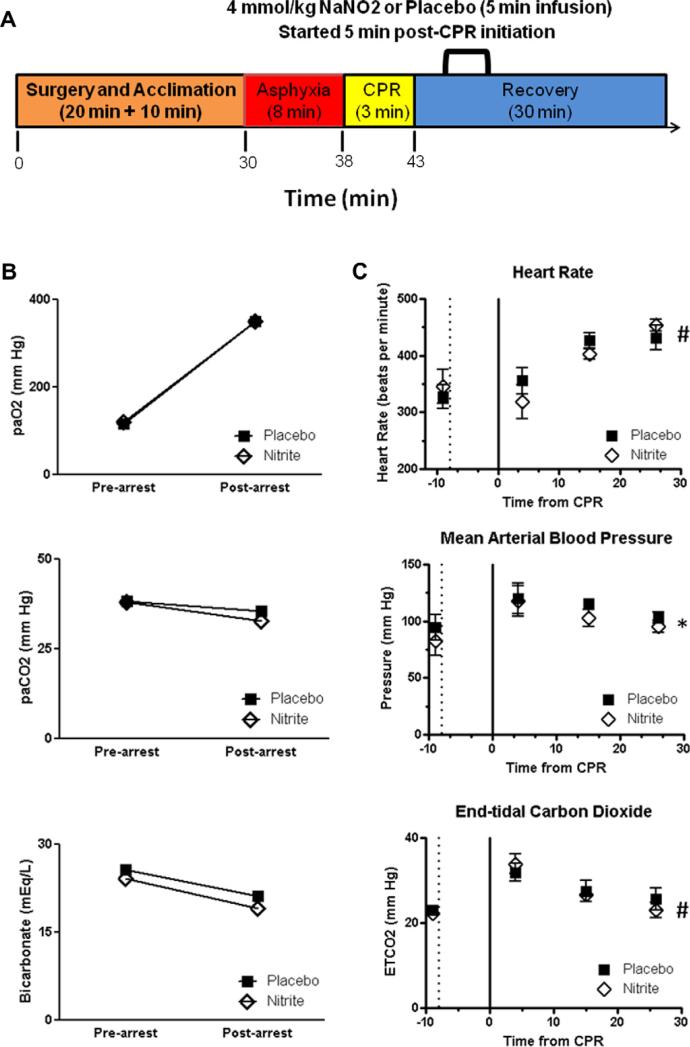
We utilized a rat 8 min asphyxial CA model well characterized by our group [28] and the experiment flow is summarized in Fig. 1A. All procedures were approved by the Institutional Animal Care and Use Committee requirements. Adult male Sprague-Dawley rats (250–350 g) were anesthetized with 3% isoflurane and a 30%:70% mixture of oxygen and N2O followed by endotracheal intubation. Isoflurane was lowered to 1.5% and the femoral vein and artery were cannulated for drug infusion and continuous blood pressure monitoring. Continuous EKG was recorded and rectal and temporalis (head) temperature were monitored and maintained at 36.5–37.0 °C using independent heating lamps. Rats equilibrated at least 10 min from experiment start receiving mechanical ventilation (rate 60 bpm, tidal volume 10 ml/kg) before assessing a blood gas and adjusting ventilation/oxygenation to achieve normal values (PaCO2, 35–45; PaO2 100–150). A continuous recording was made of pulse, mean arterial blood pressure, and exhaled and end tidal CO2 using Powerlab (AD Instruments, Boulder, CO). After equilibration animals received a single dose of vecuronium (1 mg/ kg IV), isoflurane was discontinued and 2 min later asphyxial CA induced by disconnecting the ventilator and occluding the endotracheal tube. Eight minutes after asphyxia initiation, resuscitation was initiated by administering a bolus injection of epinephrine (0.05 mg/kg, i.v.) and sodium bicarbonate (1 meq/kg, i.v.), resuming augmented mechanical ventilation (100% oxygen, rate 80 bpm) and mechanical chest compressions begun at a rate of approximately 200/min until the mean arterial blood pressure reached 60 mmHg. Compressions were delivered for up to 3 min at which point any animal not resuscitated was excluded from the study. Sham animals received anesthesia and surgery as well as similar time acclimation on the ventilator but did not undergo CA.
Fig. 1.
Arterial blood gas and physiological data before and after asphyxia cardiac arrest. (A) Summary of the experimental flow depiting the initial surgery, asphyxia, CPR and recovery periods (times of surgery and recovery are approximate, CPR never exceeded 3 min). Nitrite or saline placebo were infused where indicated. Bottom scale depicts approximate real time from the start of the experiment. Arterial blood gas (B) and physiological (C) data are shown for six pairs of animals randomized to either nitrite or saline placebo. In (C) the dotted line represents the initiation of asphyxia and the solid line at time 0 represents the initiation of cardiopulmonary resuscitation (CPR). There were no statistically significant differences between treatment groups in any of the variables measured. At the end of monitoring, there was a significant increase in heart rate accompanied by increases in mean arterial blood pressure and end-tidal carbon dioxide (ETCO2) thus not indicative of hypoperfusion. * denotes p = 0.027 and # denotes p < 0.0001 on the basis of the time variable by repeated measures ANOVA.
Drug therapy and post-resuscitation care
Rats were blindly randomized to sodium nitrite (8 mM in normal saline) or normal saline placebo infused over 5 min in a volume of 500 μl beginning 5 min after resuscitation. By 25 min after resuscitation the catheters were removed and wounds infiltrated with lidocaine and by 60 min animals were taken off mechanical ventilation and placed in a pre-warmed oxygenated recovery chamber. Each animal received 1 ml normal saline i.p. upon placement in the recovery area. Rats in 7 d survival experiments received an additional 3 ml 5% dextrose ½ normal saline s.q. the night of the experiment and every 8 h thereafter until they were clearly ambulating and able to hydrate themselves (generally the next day). Rats were evaluated 24 h post-CA for neurological function using a rat neurological scale described elsewhere [29].
Perfusion and histology
Rats were euthanized 7 d after CA by perfusion under deep isoflurane anesthesia and perfusion and histology performed as previously described [30,31]. Briefly, the chest was opened via median sternotomy and a blunt 16 gauge needle was placed through the left ventricular apex into the aorta and secured with a clamp. The right atrial appendage was excised and the animal was perfused with normal saline followed by FAM (40% formaldehyde, glacial acetic acid, and methanol; 1:1:8 by volume) to initiate fixation. Decapitated heads were fixed overnight in FAM and brains removed from the skull the next day and embedded in paraffin. Coronal sections of 10-mm thicknesses were stained with hematoxylin and eosin and visualized by an investigator blinded to treatment assignment at 40× magnification. Neuronal death was diagnosed based on visual evidence of nuclear pyknosis, karyorrhexis and karyolysis. Neurons with intact nuclei and nucleolar structures were judged to be living. Live and dead neurons were quantified along 18 fields spanning the medial to lateral extent of hippocampus CA1.
Hippocampal nitrite, S-nitrosothiol and cGMP measurements
Rats were subjected to 8 min CA as described above except that 100 μl arterial blood was removed 1 min before CA, 1 min before study drug infusion (4 min after CPR) or 5 min after the end of drug infusion. Blood was immediately mixed in a 4:1 ratio of blood with nitrite preservation solution [32] and samples prepared by methanol extraction. After obtaining the last whole blood measurement, animals were rapidly decapitated and both hippocampi rapidly excised on ice and rinsed in nitrite-free saline to remove any blood coating the surface (none was visible). These lobes were placed into a tissue nitrite preservation solution [21] and homogenized. All samples were stored at −80 °C until subsequent analysis for total nitrite and S-nitrosothiol levels using a tri-iodide based reductive ozone chemiluminescence method with or without the addition of acidified sulfanilamide [33]. The area under the curve was qualified and sulfanilamide treated samples (S-nitrosothiols) were subtracted from untreated samples to quantify nitrite content. In separate experiments, hippocampus was isolated at the same time point (5 min after the end of drug infusion) and placed in 0.1 N HCl. Tissue was homogenized and sonicated prior to storage at −80 °C. Hippocampal cGMP levels were measured after acetylation by colorimetric enzyme immuno-assay (Assay Designs, Ann Arbor, MI). Tissue values were normalized to total protein content (Biorad, Hercules, CA).
Mitochondrial respiration assays
Crude mitochondrial extracts were prepared by differential centrifugation [34] of homogenized hippocampus or the remaining whole brain (minus cerebellum) which had been obtained 1 h after CA. Since brain tissue forms synaptosomes, extracts were permeabilized with digitonin (0.007%) prior to oximetry performed using a heated (30 °C) chamber with a stir bar mounted with a Clark type electrode (Hansatech Instruments, Norfolk, UK). An 0.5 mg aliquot of mitochondria were placed into 400 μl respiration buffer and either 10 mM (final concentration) malate + 20 mM pyruvate or 20 mM succinate provided as substrate. Respiration was measured as the rate of oxygen consumption in pseudo-state 4 prior to addition of ADP (2 mM) which was recorded as state 3 respiration. Respiratory control ratios (RCR = state 3 respiration/state 4 respiration) were considered the measure of mitochondrial function.
Phase I study: nitrite infusion in cardiac arrest survivors
This ongoing study (clinicaltrial.gov NCT01178359) was approved by the University of Washington Institutional Review Board and informed consent obtained from all patients prior to inclusion. Adult survivors of CA at Harborview Medical Center were included in this study after obtaining informed consent from the designated surrogate if the patient was successfully resuscitated and survived to ICU admission, had IV access and was within 12 h of CA. Patients were excluded if they suffered traumatic cardiac arrest, had a known history of dialysis dependent kidney disease, had a “do not resuscitate” order in place, required vasopressor or inotropic support for myocardial dysfunction, had PaO2 < 90% FiO2 of 1.0 or had a systolic blood pressure (BP) < 90 mmHg and/or mean arterial BP < 60 mmHg. In the initial phase of this study, four patients were randomized in a double blinded manner, 3:1 ratio to sodium nitrite (1 mg in 100 ml normal saline) or 100 ml saline placebo infused i.v. over 5 min. Since this is an ongoing blinded study, patient assignments are unknown but safety data on all patients in the first phase have been reviewed as required to permit dose escalation to 9.6 mg i.v. nitrite. Should no significant adverse events occur in this phase, a third and final dose escalation to 14.5 mg nitrite is planned.
Whole blood mixed in nitrite preservation solution [32] and serum were obtained at baseline (within 5 min of drug infusion start) and 15, 30, 60 and 120 min after infusion start for measurement of nitrite levels as described above. Methemoglobin levels were obtained at baseline and after 60 and 90 min from infusion. Blood pressure measured by automated cuff and heart rate were recorded before infusion, every minute during infusion, and every 5 min for an additional 25 min followed by every 15 min until 2 h. Planned stopping criteria were the occurrence of systolic BP <90 mmHg on two consecutive measurements separated by 1 min or a decrease in systolic BP >15 mmHg on two consecutive measurements or increase in heart rate >10 beats/min for more than 1 min. In the higher dose groups, additional planned stopping criteria are systolic BP <90 mmHg or a decrement of >15 mmHg in more than 3/4 of the patients.
Statistical analysis
Quantitative data was summarized and presented in the text as means ± standard deviation and in graphical form as symbols and error bars denoting means ± standard error. Analysis of variables over time was performed by repeated measures ANOVA. Comparisons of three groups were performed by one-way ANOVA with post-hoc Bonferonni analysis. Comparisons of two groups were performed using Student's unpaired t-test. Statistical significant was considered a p-value <0.05. Data analysis and graph creation was accomplished using Prism v.5.04 (Graphpad Software, La Jolla, CA).
Results
Post-arrest physiology and nitrite effects
An initial cohort of 14 animals were studied for physiology, blood gases and histology after 8 min asphyxial CA (n = 7 each, randomized to placebo or nitrite). In this cohort, 13/14 (93%) animals survived to 7 d. In the course of numerous subsequent experiments conducted for this study and others, mortality occurred only in the initial 15 min due to failure to resuscitate animals with an incidence <10%. In these uncommon incidences animals were excluded from subsequent analysis. Animals were indistinguishable on the basis of their peri-CA characterization. The time from the initiation of asphyxia to loss of pulse was 3.88 ± 0.37 min in placebo treated animals compared to 3.78 ± 0.12 min with nitrite treatment (p > 0.2). Time from CPR initiation to return of spontaneous circulation was 36.17 ± 5.85 s in placebo treated animals compared to 37.33 ± 6.56 s in nitrite treatment (p > 0.2). Thus animals received similar doses of asphyxia and ischemia.
Blood gas (Fig. 1B) and physiological (Fig. 1C) data are shown for six pairs of animals performed on the same days. After CA, both groups of animals had similar increases in oxygenation and slight decreases in carbon dioxide consistent with the hyperventilation and 100% oxygen resuscitation employed (Fig. 1B). Bicarbonate levels were similarly lower in both groups as expected given the metabolic acidosis resultant from global ischemia (Fig. 1B). Both groups had similar increases in heart rate after CA and initial increases in blood pressure and exhaled carbon dioxide consistent with post-resuscitation hyperemia, which approached baseline levels after 25 min of observation (Fig. 1C). This model differs significantly from our previously reported mouse cardiac arrest model, where post-CA myocardial dysfunction was prominent, [21] in that these animals did not have significant uncorrected metabolic acidosis or decrease in cardiac output based on blood pressures or end-tidal carbon dioxide. Animals were therefore able to be survived to 7 d for histology.
Nitrite protects against delayed neuronal death
Analysis of 7 d histology revealed a significant loss (48.0%) of hippocampal CA1 neurons after CA treated with placebo compared to sham (Fig. 2A). Treatment with nitrite nearly completely reversed (75% relative reduction; p < 0.001) neuronal death (Fig. 2B). We found 24 h neurological scores to be minimally reduced and these failed to distinguish groups (data not shown). Very preliminary data (n = 2) using more sophisticated fear conditioning neurobehavioral assessment shows large differences in hippocampal mediated learning between nitrite and placebo treated animals but these studies still await further validation.
Fig. 2.
Seven day hippocampal CA1 histology. (A) Representative sections of approximately the same portion of CA1 are shown for the sham (no CA), placebo and nitrite treated groups. Cardiac arrest resulted in significant cell death compared to sham which was to a large extent reversed by nitrite therapy. (B) Data is summarized for four animals. * denotes p < 0.001 vs. sham and † denotes p < 0.001 vs. placebo by ANOVA with post-hoc Bonferroni comparison.
Nitrite therapy after cardiac arrest is associated with S-nitrosylation
We wished to investigate nitrite signaling along the canonical NO-soluble guanylate cyclase pathway compared to the more recently described S-nitrosylation pathway [35] and to investigate blood and brain distribution of nitrite after therapy. The dose of nitrite infused was 13.3 μmol/kg (based on a median estimated weight of 300 g). This was a significantly higher dose of nitrite than the 1.85 μmol/kg we had previously given mice [21]. This dose was selected since the lower dose in mice had not increased blood nitrite levels significantly over pre-arrest baseline due to nitrite consumption with CA. Our goal whole blood nitrite level after therapy was 15 μM based on dose titration studies performed by others in the past [12,16].
Pre-CA and post-CA whole blood levels of nitrite were nearly identical (ie no nitrite consumption as previously noted). Compared to sham (0.447 ± 0.199 μM) and placebo treatment (0.434 ± 0.218 μM), animals treated with nitrite had higher whole blood nitrite levels (18.97 ± 3.6 μM; p < 0.001, n = 8). Hippocampal tissue nitrite levels (Fig. 3A and B) doubled with nitrite therapy demonstrating effective delivery to brain. Hippocampal S-nitrosothiol levels (Fig. 3C and D) however increased over three-fold with nitrite therapy and could not be detected in the sham (no ischemia) animals. Surprisingly, cGMP levels were not noted to increase in hippocampus after nitrite therapy (Fig. 3E), albeit levels did increase after CA.
Fig. 3.
Hippocampal nitrite, S-nitrosothiol and cGMP levels. Hippocampi were rapidly excised from sham or post-arrest animals randomized to nitrite or placebo upon infusion completion. (A) A representative tracing of data output from the nitric oxide analyzer demonstrates the increased area under the curve for similar volumes of hippocampal homogenate from nitrite treated animals compared to placebo. The summary data for eight animals is shown graphically below (B). Representative curves from samples pre-treated with acidified sulfanilamide (C) demonstrate the increase in S-nitrosothiols after nitrite therapy and are likewise summarized below (D). Significant increases in nitrite (B) and S-nitrosothiol (D) content were noted in the brains of nitrite-treated compared to placebo and sham animals. (E) Similarly timed hippocampal homogenates analyzed for cGMP content did not reveal significant differences. All results normalized to protein content. * Denotes p < 0.05 and # denotes p < 0.01 by ANOVA.
Effects of nitrite therapy on mitochondrial function after cardiac arrest
Since mitochondrial dysfunction with associated energetic failure, free radical production and release of pro-apoptotic factors, is a frequently invoked mechanism for delayed neuronal death after global ischemia [36], we investigated mitochondrial function 1 h after arrest and therapy with either nitrite or placebo. The RCR incorporates both the state 4 rate of deleterious non-specific oxygen consumption (uncoupling or free radical production) with the effective ATP generating state 3 respiration rate. The RCR of mitochondria isolated from post-arrest animals treated with nitrite was in most cases better with nitrite therapy though this did not rise to the level of statistical significance (Fig. 4). Hippocampal mitochondria demonstrated the strongest trends towards improved function (Fig. 4A and B) whereas the remainder of the brain had a trend towards improved complex I mediated function (malate/pyruvate substrate; Fig. 4C) but not complex II (succinate; Fig. 4D) mediated function. These results strongly suggest improved mitochondrial function, particularly in the hippocampus where selective vulnerability to ischemic injury is greatest and histological neuroprotection by nitrite therapy is evident.
Fig. 4.
Nitrite therapy improves post-CA mitochondrial function. Respiratory control ratios (RCR) were calculated using the complex I substrates malate and pyruvate (A and C) or the complex II substrate succinate (B and D). Mitochondria were isolated from hippocampus (A and B) or the remaining whole brain (C and D) of non-arrested shams or 1 h post-arrest animals treated with nitrite or placebo (n = 4 each group). Nitrite therapy showed trends (p = 0.05–0.15) towards improved function in nearly all groups assessed except complex II dependent function in whole brain (D) where all results were similar. These effects appeared more prominent in hippocampus (A and B) than the remainder of brain (C and D).
Phase I nitrite infusion in cardiac arrest survivors
Cardiac arrest randomized controlled trials, like many trials of emergency research, often require waiver, delay or use of surrogate informed consent to enroll patients within the therapeutic window [37]. To justify such a stance ethically requires safety data on the drug being tested. In an effort to generate such data, we have begun a pilot phase I trial of nitrite infusion in cardiac arrest survivors to look for adverse effects. The most anticipated such effects would be hypotension due to excessive vasodilation and methemoglobinemia. The design of this trial is a stepwise increase in the dose of nitrite infused after assessment of adverse effects at each dose level. We recently completed the initial dose phase, 1 mg nitrite which represents 0.2 μg/kg (based on 70 kg body weight).
Nitrite infusion at this dose did not appear to cause significant hypotension or tachycardia (Fig. 5A). Mean methemoglobin levels were 0.74 ± 0.14% over time and the highest recorded methemoglobin level was 1.1%. Patients had normal hemoglobin levels (13.9 ± 1.4 g/dL). Nitrite infusion increased plasma and blood levels in three of four patients (one received placebo; Fig. 5B) but the degree of increase was in all cases less than fivefold from the patient's baseline. Even this modest increase though may be sufficient to provide cardioprotection based on our cardiac arrest data in mice [21].
Fig. 5.
Effects of nitrite infusion into cardiac arrest patients. (A) Hemodynamic effects (mean arterial blood pressure, blue, on top and heart rate, red, below) and (B) plasma or whole blood nitrite levels are shown over a 120 min period in cardiac arrest patients who were randomized in a 3:1 ratio to receive nitrite or placebo in a double-blind fashion. Each row represents one patient. The infusion consisted of 1 mg nitrite in 100 ml saline (n = 3) or 100 ml saline placebo (n = 1) and was infused over the initial 5 min represented by the bar on the top of each graph. No evidence of significant hypotension or tachycardia was noted at this dose. Nitrite levels increased at most three fold during the monitoring period in 3/4 patients (likely those dosed with active drug) but the magnitude of the increase did not exceed five times the baseline.
Discussion
We demonstrate that nitrite therapy given after CA enters the brain and results in neuroprotection in the absence of physiological adverse effects. This neuroprotection appears to be associated more so with S-nitrosation of proteins rather than the activation of the canonical soluble guanylate cyclase signaling pathways. Finally, we provide the first ever data on nitrite infusion into human cardiac arrest survivors early in resuscitation and demonstrate safety at our initial infusion dose. This work supplements our earlier findings of cardioprotection and improved myocardial contractility and survival with nitrite therapy after CA and lays the early groundwork for bench to bedside translation of nitrite therapy as a post-resuscitation modality.
We employed a rat model of CA with significant neuronal loss in the well characterized selectively vulnerable hippocampal CA1 region. This model and its injury pattern reflect pathological findings in human cardiac arrest survivors [27] and the higher survival better approximates the clinical situation where most deaths after successful resuscitation from CA are due to neurological injury [5]. Thus the findings of robust neuroprotection at a time when pathological neuronal loss would be expected to be fully evident are highly significant and act as important supplement to our earlier results [21]. Nitrite therapy within this study was delivered approximately 4 min after animals had been resuscitated. This is a more realistic time window for pre-hospital drug delivery than upon initiation of resuscitation as we previously reported. The dose of nitrite used in this model (13.3 μmol/kg) was significantly higher than previously (1.8 μmol/kg) and the blood levels produced more closely approximated the “optimal” dose based on prior observations in focal heart, liver and brain ischemia-reperfusion [12]. Formal dose-effect and pharmacodynamic investigations are presently underway but it is clear that higher doses (53 μmol/kg) lose protection and trend towards harm (data not shown) underscoring the importance of establishing the correct optimal dose.
From a patient safety perspective, our animal and human data are extremely important. The absence of hemodynamic effects attributable to this higher dose nitrite infusion in our rat model is reassuring. The dose delivered in this rat study was 13.3 μmol/kg over 5 min or 2.7 μmol/kg/min. This dose is significantly less than what has already been given to human volunteers in a number of studies both in terms of total nitrite delivery and infusion rate [10,38,39]. This dose has failed to produce hemodynamic embarrassment or significant methemoglobinemia. Significant hypotension has been noted in short infusions such as ours when blood nitrite levels exceeded 10 μM [38] or in longer term infusions when blood levels were as low as 1 μM [39]. Of note, in the latter example over 90 mg of nitrite had been delivered to the subject before the onset of hypotension.
Since cardiac arrest survivors have a much higher potential for hemodynamic instability, we designed our phase 1 trial to be extremely conservative and our initial dose represents an infusion of 0.04 μmol/kg/min (2.86 μg/kg/min) of nitrite over 5 min. It is reassuring though to see that this dose did not cause adverse effects while there were small increases in blood nitrite which are proportionally in the range of what we know to be protective in our preclinical data. The final planned dose in this phase I study will deliver 0.6 μmol/kg/min (41.4 μg/kg/min) of nitrite over 5 min is predicted to yield a blood nitrite level of 5-10 μM based on Dejam's report that a 28 μg/kg/min infusion resulted in a 5 min whole blood level of 4.6 μM [38]. This infusion is expected to be just under the level at which hypotension will be problematic yet still near the level where protection was observed in animal studies [12,16].
The increase in hippocampal nitrite levels after therapy is important in providing evidence of effective drug delivery. This confirms data from a primate model of subarachnoid hemorrhage where intravenous nitrite infusion produced increases in CSF nitrite and S-nitrosothiols [40]. It is therefore clear that nitrite crosses the blood brain barrier in this model of brain injury and the significantly increased production of S-nitrosothiols confirms ischemic nitrite reduction [10,41–44] with consequent NO formation. This is confirmed by the absence of measurable S-nitrosothiols in the non-ischemic sham group and the substantial decrease in S-nitrosothiols in the ischemic placebo group where lower levels of endogenous nitrite produced lower levels of S-nitrosothiols. Although it's unclear from our work which enzyme catalyzed nitrite reduction, an intriguing possibility in the brain is neuroglobin [45] which is present in high levels. Formation of S-nitrosothiols has important signaling implications [46] both in regulating mitochondrial function [44,47,48] and apoptosis [49,50] and suggests potential mechanisms for nitrite mediated neuroprotection.
The lack of increases in cGMP levels in the brain with nitrite therapy is surprising and differs from the findings of Jung and colleagues [16] who reported significant increases utilizing a very similar immunoassay technique. These investigators noted the increases 1 d after simulated stroke and reperfusion with nitrite therapy. The persistent delayed response is interesting and likely reflects mechanisms beyond acute nitrite conversion to NO (eg NO synthase stimulation) since nitrite reduction would be largely complete within the first few hours after therapy. Given the difficulty of measuring cGMP and potential problems with degradation even under acidified conditions due to the assays long completion time, this result will await further validation over a time course. Nonetheless, the implication of our finding is that early nitrite-mediated neuroprotective signaling is mediated more through S-nitrosylation than the canonical NO–cGMP pathway. Again, this preliminary result will require further investigation to identify specific targets of S-nitrosylation which may explain the observed protection.
On a mechanistic basis, nitrite therapy has been associated with antioxidant effects when given upon reperfusion. Reductions in markers of oxidative and nitrosative stress have been noted in brain [16,17]. In other tissue types, including heart after cardiac arrest, nitrite therapy has been associated with a transient mitochondrial complex I inhibition with reduced free radical production at reperfusion and improved long term mitochondrial function and oxidant injury [21,51]. Free radical injury burst occurs primarily within the first 30 min of reperfusion [52] which we confirmed in our prior cardiac arrest model [21], yet protection against brain injury has been noted with application of therapeutic hypothermia hours after resuscitation from CA [53,54]. Clearly this implies other important mechanisms of brain injury and a substantial body of work implicates programmed cell death (perhaps due to energetic failure) and excitotoxicity as principle culprits [36].
Jung and colleagues [17] have been the only ones to do a formal time course examining the efficacy of nitrite therapy. In this report, nitrite therapy was delivered as late as 6 h after experimental stroke and had benefits 3 h later independently and 4.5 h later in conjunction with memantine. Late benefits such as this are difficult to explain on the basis of reduction in free radical injury. To this extent, our findings of preserved and likely improved mitochondrial function after cardiac arrest with nitrite therapy provide one mechanism whereby nitrite therapy may stave off subsequent delayed neuronal death. In addition S-nitrosylation is known to be able to impact apoptotic signaling [49,50,55] which could produce a pro-survival phenotype resulting in reduced neuronal loss at 7 d. This pro-survival phenotype may also be associated with improved mitochondrial function and reduced cytochrome c release both of which may be mediated through a single effector such as protein kinase C [30,56,57]. Taken as a whole, our preliminary results are suggestive of neuroprotective mechanisms that are not without precedent and require further investigation.
Conclusion
Nitrite therapy offers significant promise as an anti-ischemic therapy in cardiac arrest. We have demonstrated cardioprotection and now neuroprotection after cardiac arrest. Nitrite's ability to deliver NO in ischemic regions and S-nitrosate proteins provides multiple mechanisms for protection including reductions in oxidative injury, improved mitochondrial function, improved blood flow and pro-survival cell signaling. Nitrite's stability in non-ischemic blood also prevent large scale non-specific NO release and likely underlie the lack of hypotension observed in preclinical and now clinical cardiac arrest models. Taken as a whole, nitrite is a safe and potent weapon against a process which causes significant mortality and morbidity and clearly deserves attention for clinical translation.
Acknowledgments
Funding sources
This study and Dr. Dezfulian were supported by the State of Florida Department of Health James and Esther King Biomedical Foundation New Investigator Research Award and NINDS K08NS069817.
Abbreviations
- CA
cardiac arrest
- NO
nitric oxide
- FAM
formaldehyde-glacial acetic acid-methanol
- RCR
[mitochondrial] respiratory control ratio
References
- 1.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–1431. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, Carr BG, Mitra N, Bradley SM, Abella BS, Groeneveld PW, t.A.H.A.G.W.T.G.-R Investigators, incidence of treated cardiac arrest in hospitalized patients in the United States. Critical Care Medicine. 2011;39:2401–2406. doi: 10.1097/CCM.0b013e3182257459. 10.1097/ CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wachelder EM, Moulaert VRMP, van Heugten C, Verbunt JA, Bekkers SCAM, Wade DT. Life after survival: long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation. 2009;80:517–522. doi: 10.1016/j.resuscitation.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 4.Robinson S, Swain AH, Hoyle SR, Larsen PD. Survival from out-of-hospital cardiac arrest in New Zealand following the 2005 resuscitation guideline changes. Resuscitation. 2010;81:1648–1651. doi: 10.1016/j.resuscitation.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 6.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proc. Natl. Acad. Sci. USA. 2008;105:11430–11435. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannon RO, 3rd, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J. Clin. Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic. Biol. Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 9.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 10.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 11.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat. Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 12.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemiareperfusion damage. Proc. Natl. Acad. Sci. USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovas. Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 17.Jung K-H, Chu K, Lee S-T, Park H-K, Kim J-H, Kang K-M, Kim M, Lee SK, Roh J-K. Augmentation of nitrite therapy in cerebral ischemia by NMDA receptor inhibition. Biochemical and Biophysical Research Communications. 2009;378:507–512. doi: 10.1016/j.bbrc.2008.11.081. [DOI] [PubMed] [Google Scholar]
- 18.Jung K-H, Chu K, Lee S-T, Sunwoo J-S, Park D-K, Kim J-H, Kim S, Lee SK, Kim M, Roh J-K. Effects of long term nitrite therapy on functional recovery in experimental ischemia model. Biochem. Biophys. Res. Commun. 2010;403:66–72. doi: 10.1016/j.bbrc.2010.10.116. [DOI] [PubMed] [Google Scholar]
- 19.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, 3rd, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Bailen M, Aguayo de Hoyos E, Ruiz-Navarro S, Diaz-Castellanos MA, Rucabado-Aguilar L, Gomez-Jimenez FJ, Martinez-Escobar S, Moreno RM, Fierro-Roson J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66:175–181. doi: 10.1016/j.resuscitation.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez MM, Berg RA, Nadkarni VM, Vianna CB, Kern KB, Timerman S, Ramires JA. Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation. 2008;117:1864–1872. doi: 10.1161/CIRCULATIONAHA.107.740167. [DOI] [PubMed] [Google Scholar]
- 24.Chang WT, Ma MH, Chien KL, Huang CH, Tsai MS, Shih FY, Yuan A, Tsai KC, Lin FY, Lee YT, Chen WJ. Postresuscitation myocardial dysfunction: correlated factors and prognostic implications. Intensive Care Med. 2007;33:88–95. doi: 10.1007/s00134-006-0442-9. [DOI] [PubMed] [Google Scholar]
- 25.Laurent I, Monchi M, Chiche J-D, Joly L-M, Spaulding C, Bourgeois B.e., Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut J-F. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2002;40:2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 26.Kirino Takaaki. Delayed neuronal death. Neuropathology. 2000;20:95–97. doi: 10.1046/j.1440-1789.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- 27.Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992;85:79–87. doi: 10.1007/BF00304636. [DOI] [PubMed] [Google Scholar]
- 28.Dave KR, Raval AP, Prado R, Katz LM, Sick TJ, Ginsberg MD, Busto R, Perez-Pinzon MA. Mild cardiopulmonary arrest promotes synaptic dysfunction in rat hippocampus. Brain Res. 2004;1024:89–96. doi: 10.1016/j.brainres.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 29.Neumar RW, Bircher NG, Sim KM, Xiao F, Zadach KS, Radovsky A, Katz L, Ebmeyer E, Safar P. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation. 1995;29:249–263. doi: 10.1016/0300-9572(94)00827-3. [DOI] [PubMed] [Google Scholar]
- 30.Raval AP, Dave KR, Prado R, Katz LM, Busto R, Sick TJ, Ginsberg MD, Mochly-Rosen D, Perez-Pinzon MA. Protein kinase C delta cleavage initiates an aberrant signal transduction pathway after cardiac arrest and oxygen glucose deprivation. J. Cereb. Blood Flow Metab. 2005;25:730–741. doi: 10.1038/sj.jcbfm.9600071. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J. Cereb. Blood Flow Metab. 1997;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacArthur PH, Shiva S, Gladwin MT. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J. Chromatogr. B. 2007;851:93–105. doi: 10.1016/j.jchromb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Shiva S, Brookes PS, Patel RP, Anderson PG, Darley-Usmar VM. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc. Natl. Acad. Sci. USA. 2001;98:7212–7217. doi: 10.1073/pnas.131128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 36.Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh M, Dailey MW, Callaway CW. Surrogate consent by family members for out-of-hospital cardiac arrest research. Acad. Emerg. Med. 2001;8:851–853. doi: 10.1111/j.1553-2712.2001.tb00220.x. [DOI] [PubMed] [Google Scholar]
- 38.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, 3rd, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates. endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 39.Pluta RM, Oldfield EH, Bakhtian KD, Fathi AR, Smith RK, DeVroom HL, Nahavandi M, Woo S, Figg WD, Lonser RR. Safety and feasibility of long-term intravenous sodium nitrite infusion in healthy volunteers. PLoS One. 2011;6:e14504. doi: 10.1371/journal.pone.0014504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pluta RM, Dejam A, Grimes G, Gladwin MT, Oldfield EH. Nitrite infusions to prevent delayed cerebral vasospasm in a primate model of subarachnoid hemorrhage. Jama. 2005;293:1477–1484. doi: 10.1001/jama.293.12.1477. [DOI] [PubMed] [Google Scholar]
- 41.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milsom AB, Patel NSA, Mazzon E, Tripatara P, Storey A, Mota-Filipe H, Sepodes B, Webb AJ, Cuzzocrea S, Hobbs AJ, Thiemermann C, Ahluwalia A. Role for endothelial nitric oxide synthase in nitrite-induced protection against renal ischemia-reperfusion injury in mice. Nitric oxide. 2010;22:141–148. doi: 10.1016/j.niox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite Reductase Function of Deoxymyoglobin. Oxygen Sensor and Regulator of Cardiac Energetics and Function. Circ. Res. 2007 doi: 10.1161/CIRCRESAHA.107.152488. CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 44.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 45.Petersen MG, Dewilde S, Fago A. Reactions of ferrous neuroglobin and cytoglobin with nitrite under anaerobic conditions. J. Inorg. Biochem. 2008;102:1777–1782. doi: 10.1016/j.jinorgbio.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 47.Dahm CC, Moore K, Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J. Biol. Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 48.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ. Res. 2007;101:1155–1163. doi: 10.1161/CIRCRESAHA.107.155879. [DOI] [PubMed] [Google Scholar]
- 49.Benhar M, Stamler JS. A central role for S-nitrosylation in apoptosis. Nat. Cell Biol. 2005;7:645–646. doi: 10.1038/ncb0705-645. [DOI] [PubMed] [Google Scholar]
- 50.Melino G, Bernassola F, Knight RA, Corasaniti MT, Nistico G, Finazzi-Agro A. S-nitrosylation regulates apoptosis. Nature. 1997;388:432–433. doi: 10.1038/41237. [DOI] [PubMed] [Google Scholar]
- 51.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J. Exp. Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bolli R, Patel BS, Jeroudi MO, Lai EK, McCay PB. Demonstration of free radical generation in “stunned” myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J. Clin. Invest. 1988;82:476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia. 2002:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 54.The Hypothermia after Cardiac Arrest Study, Group, Mild Therapeutic Hypothermia to Improve the Neurologic Outcome after Cardiac Arrest. New England Journal of Medicine. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 55.Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, Stamler JS. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 56.Basu A, Sivaprasad U. Protein kinase C[epsilon] makes the life and death decision. Cell. Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bright R, Raval AP, Dembner JM, Perez-Pinzon MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J. Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]