Abstract
Echocardiography is a robust tool for assessing cardiac function in both humans and laboratory animals. Conventional echocardiographic measurements, including chamber dimensions, wall thickness, and ejection fraction are routinely obtained to assess cardiac function in mice. Recently, myocardial strain and strain rate measurements have been added to functional assessments to provide additional details on regional abnormalities that are not evident using conventional measurements. To date, all studies of strain and strain rate in mice or rats have involved adult animals. This study serves to outline methods for acquiring echocardiographic images in pediatric mice and to provide myocardial strain and strain rate values for healthy C57BL/6J mice between 3 and 11 weeks old. Between weeks 3 and 11, left ventricular radial strain ranged from 32 to 43% and longitudinal strain ranged from −15 to −19%, with analysis over time showing no significant changes with aging (radial strain, P = 0.192 and longitudinal strain, P = 0.264; n = 4 for each time point evaluated). In conclusion, myocardial strain analysis in pediatric mice is technically feasible and has potential application in studying the pathophysiology of pediatric cardiovascular disease.
Keywords: Pediatric, myocardial strain, myocardial strain rate, cardiac, echocardiography, left ventricle
Echocardiography is a robust tool for assessing heart function in humans and laboratory animals.1,2 Typical measurements provided by echocardiography include chamber dimensions, wall thickness, and ejection fraction (EF). These measurements can be used to determine changes in heart function caused by myocardial injury, disease, genetic modifications, drug treatments, and aging.3 Recently, myocardial strain and strain rate measurements have been used to evaluate regional left ventricular abnormalities that are not visualized with conventional echocardiographic measurements.4–7 Studies of myocardial strain and strain rate to date have been performed on adult mice or rats. This study serves to outline methods for acquiring echocardiographic images of pediatric mice and to provide myocardial strain and strain rate values for healthy C57BL/6J mice between 3 and 11 weeks old, which equate to a human age of 8–10 years.
Strain (ε) is defined as the percentage of deformation an object experiences in relation to its original form. In a simple one-dimensional object, strain is defined as ε = (L − LO)/LO, where LO is the original length and L is the length of the object after deformation. Thus, lengthening of the object is reported as a positive strain value, whereas shortening is reported as a negative value. In three-dimensional objects, a combination of Langrangian and natural strain equations is used to describe cardiac strain in terms of longitudinal, radial, and circumferential strain.8,9
Strain and strain rate10—the change in deformation over the course of time—measurements can detect regional cardiac abnormalities occurring in the absence of changes in chamber dimension, wall thickness, and EF.6,10–12 Migrino and colleagues showed that radial strain measurements identified doxorubicin-induced cardiac dysfunction 4 weeks earlier than fractional shortening measurements.13 Although most research utilizing strain and strain rate has been directed toward observations in adult animals, the ability to detect subtle changes in cardiac function may advance knowledge of postnatal cardiac development and animal models that address the pathophysiology of various human cardiovascular diseases. Accordingly, the objective of this study was to develop a standardized operating procedure for the assessment of strain and strain rate in mice <3 months of age.
Materials and Methods
Animals
All animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, Washington, DC, USA, 2010) and were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio. Two-week-old C57BL/6J mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Mice were maintained in a temperature-controlled environment with a 12-hour light–dark cycle, with water and standard chow ad libitum. Mice were weaned at 3 weeks of age and separated according to gender.
Experimental Design
Left ventricle (LV) function was assessed by echocardiography in mice at 4 ages: 3, 5, 7, and 11 weeks. For each time point, 4 mice were studied (2 male and 2 female). Conventional echocardiographic measurements and strain analyses were obtained for each mouse. After echocardiography, each mouse was sacrificed to collect the heart, lungs, and tibia.
Anesthesia and Animal Preparation
Prior to echocardiography, mice were anesthetized with isoflurane. Unconscious mice were weighed and secured in a supine position on a temperature-controlled restraining board. Anesthesia was maintained with 1–3% isoflurane in oxygen delivered through a nose cone. Body temperature was monitored with a rectal thermometer and was maintained at 33–36°C. Electrocardiogram (ECG) gel was applied to copper electrodes attached to the restraining board, and all 4 mouse paws were gently taped to the stage. All hair in the thoracic region was removed using a depilatory agent, and the area was cleaned with water. Ultrasound gel was applied to the thoracic region to improve sound wave transmission. All mice were maintained at heart rates >400 bpm while images were recorded.
Echocardiography
Echocardiographic images were obtained with a Vevo 2100 system (Visualsonics, Inc., Toronto, Canada) equipped with a MS400 linear array transducer (30 MHz). The transducer was positioned in a stationary stand perpendicular to the mouse. Settings for the instrument are listed in Table I. In brief, a frame rate of >200 frames per minute was maintained for all B-mode and M-mode images. B-mode long-axis parasternal images were recorded when optimal views of the aorta, papillary muscle, and endocardium were visible. M-mode short-axis images were recorded at the level of the papillary muscles and the LV was bisected to obtain the optimal M-mode selection. At least 3 B-mode and M-mode images were captured for each mouse. All images were saved to a local computer and analyzed off line by a technician who was blinded to the animal's age and group. Conventional echocardiographic measurements of the LV included EF, FS, end-diastolic dimension (EDD), end-systolic dimension (ESD), anterior and posterior wall thickness, and mass. For long-axis B-mode measurements, the endocardium was traced semiautomatically beginning from the mitral valve and excluding the papillary muscle. EF and FS were calculated by software using standard computational methods.
Table I. Vevo 2100 Instrument Settings.
| Parameter | Value |
|---|---|
| Transmit | |
| Frequency (MHz) | 30 |
| Power (%) | 100 |
| Acquisition | |
| Gain (dB) | 27 |
| Display | |
| Dynamic range (dB) | 65 |
| Display Map | C5 |
| Brightness | 50 |
| Contrast | 50 |
| B-mode | |
| Frame rate (fps) | 235 |
| Depth (mm) | 13.00 |
| Width (mm) | 10.36 |
| M-mode | |
| Sweep speed (Hz) | 1200 |
Strain Analysis
Cardiac strain measurements were obtained from long-axis B-mode images using VevoStrain software (Visualsonics). Images selected for strain analysis had well-defined endocardium and epicardium borders and no substantial image artifacts. Image analysis was performed according to manufacturer's instructions.14 Briefly, 3–4 consecutive cardiac cycles at end-expiration were selected. The endocardium and epicardium were traced semiautomatically using VevoStrain software from the anterior wall at the greatest diameter of the ventricle to the papillary muscle. The traces were manually adjusted to ensure adequate tracking of endocardium and epicardium borders during all 3 cardiac cycles. Velocity, displacement, strain, and strain rate were calculated for radial and longitudinal planes (Fig. 1). We calculated values from 6 assigned regional locations: posterior base, mid posterior, posterior apex, anterior base, mid anterior, and anterior apex (Fig. 2). Global average peak values for radial and longitudinal strain and strain rate are reported. Because the apical region of the LV has been shown to exhibit less strain than the mid and base regions of the apex region—a finding substantiated in our studies—we excluded this region from the global strain averages.15,16 The results in Table II indicate that the posterior apex and anterior apex regions exhibit values distinct from other regions and additionally have higher variability as noted by much higher SD values. Global strain averages calculated with and without the apex were similar. However, variability in longitudinal strain averages increased by 25– 38% when the apical region was included (comparison of SD measurements between the 2 groups).
Figure 1.
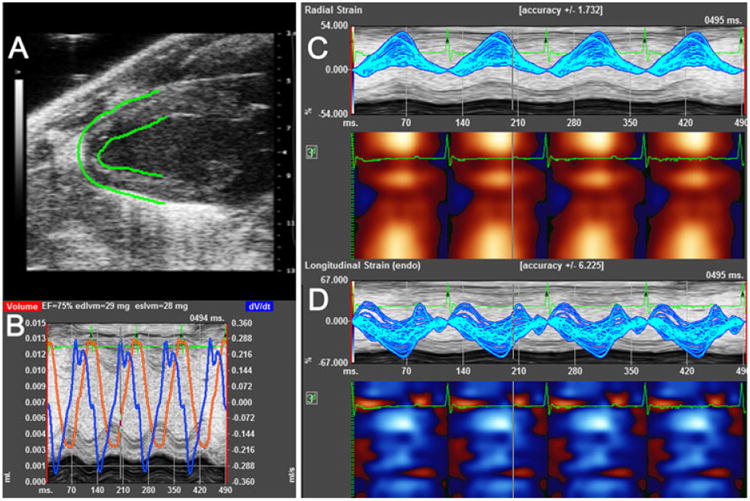
Typical strain measurements. A–D. Images acquired from a healthy 5 week old C57BL/6J mouse. Images were acquired over 4 cardiac cycles. A. B-mode image of left ventricle in long-axis orientation. B. M-mode image with volume (red) and dvol/dtime (blue) values. C. Regional radial strain values represented in linear (upper) and heat map (lower) graphs. D. Regional longitudinal strain values represented in linear (upper) and heat map (lower) graphs.
Figure 2.

Typical regional strain values of a 5-week-old mouse. Software analysis automatically combines strain values into 6 regions – posterior base, mid posterior, posterior apex, anterior base, mid anterior, and anterior apex. Values for radial and longitudinal strain values (left) represent a single contraction as selected in the red boxes (right). Included are time-to-peak values which can be used to detect regional dyssynchrony.
Table II. Segmental Analyses.
| Age (weeks) | |||||
|---|---|---|---|---|---|
|
|
|||||
| 3 | 5 | 7 | 11 | P-value | |
| Regional radial strain (%) | |||||
| Posterior base | 51.8 ± 7.3 | 36.1 ± 6.4 | 39.6 ± 1.9 | 36.5 ± 2.8 | 0.16 |
| Posterior mid | 37.5 ± 8.7 | 33.9 ± 7.7 | 35.8 ± 3.0 | 28.9 ± 1.8 | 0.09 |
| Posterior apex | 43.3 ± 11.4 | 36.6 ± 9.7 | 27.7 ± 2.8 | 19.0 ± 3.5 | 0.19 |
| Anterior base | 43.0 ± 4.4 | 46.5 ± 5.6 | 37.7 ± 5.6 | 33.4 ± 3.3 | 0.28 |
| Anterior mid | 43.4 ± 2.5 | 44.8 ± 11.0 | 28.3 ± 4.5 | 30.8 ± 4.2 | 0.21 |
| Anterior apex | 34.0 ± 10.6 | 39.4 ± 10.9 | 21.6 ± 1.8 | 18.6 ± 3.4 | 0.24 |
| Regional longitudinal strain (%) | |||||
| Posterior base | −20.2 ± 1.8 | −17.5 ± 4.6 | −25.1 ± 4.7 | −16.2 ± 2.0 | 0.35 |
| Posterior mid | −20.4 ± 2.5 | −20.9 ± 3.6 | −17.0 ± 1.0 | −15.6 ± 5.0 | 0.64 |
| Posterior apex | −25.8 ± 7.3 | −28.0 ± 3.8 | −21.0 ± 2.7 | −18.4 ± 2.2 | 0.45 |
| Anterior base | −21.2 ± 5.3 | −13.6 ± 1.9 | −13.2 ± 4.5 | −12.7 ± 5.3 | 0.50 |
| Anterior mid | −17.0 ± 3.4 | −24.5 ± 3.2 | −14.8 ± 1.7 | −15.7 ± 5.0 | 0.25 |
| Anterior apex | −27.8 ± 8.5 | −30.8 ± 2.9 | −22.9 ± 4.2 | −22.8 ± 4.0 | 0.67 |
| Regional radial strain rate (1/s) | |||||
| Posterior base | 11.3 ± 1.5 | 6.8 ± 0.9 | 7.0 ± 0.9 | 7.9 ± 0.5 | 0.03 |
| Posterior mid | 7.6 ± 1.4 | 7.4 ± 1.2 | 7.6 ± 0.5 | 7.1 ± 0.9 | 0.98 |
| Posterior apex | 10.9 ± 2.4 | 8.2 ± 1.1 | 6.3 ± 0.9 | 5.6 ± 1.0 | 0.11 |
| Anterior base | 8.6 ± 0.9 | 8.7 ± 0.7 | 8.1 ± 1.1 | 6.8 ± 0.5 | 0.42 |
| Anterior mid | 9.1 ± 0.8 | 9.4 ± 2.4 | 6.5 ± 1.1 | 5.9 ± 0.5 | 0.24 |
| Anterior apex | 8.9 ± 2.3 | 9.4 ± 2.8 | 4.4 ± 0.6 | 4.4 ± 0.6 | 0.15 |
| Regional longitudinal strain rate (1/s) | |||||
| Posterior base | 10.6 ± 2.0 | 9.3 ± 2.6 | 10.8 ± 2.2 | 9.7 ± 1.4 | 0.94 |
| Posterior mid | 9.5 ± 3.0 | 10.9 ± 2.1 | 7.6 ± 1.6 | 7.5 ± 2.6 | 0.71 |
| Posterior apex | 8.5 ± 1.2 | 11.1 ± 1.4 | 8.5 ± 1.2 | 5.7 ± 1.0 | 0.052 |
| Anterior base | 9.1 ± 2.0 | 7.0 ± 1.6 | 14.3 ± 3.1 | 7.8 ± 1.1 | 0.10 |
| Anterior mid | 7.0 ± 1.0 | 7.6 ± 2.0 | 6.4 ± 1.3 | 6.4 ± 1.6 | 0.93 |
| Anterior apex | 10.4 ± 2.5 | 11.9 ± 2.6 | 7.2 ± 1.7 | 8.0 ± 1.3 | 0.39 |
Tissue Collection
Each mouse was sedated with 2% isoflurane and injected with 100 μl of heparin (1000 USP U/mL; intraperitoneal). After 5 min, blood was collected from a carotid artery, following which the heart and lungs were removed and flushed in cardioplegia solution (69 mM NaCl, 12 mM NaHCO3, 11 mM glucose, 30 mM 2,3-butanedione monoxime, 10 mM EGTA, 0.001 mM nifedipine, 50 mM KCl, and 100 units heparin were dissolved in 0.9% saline adjusting pH to 7.4 and volume to 1 L) to remove the blood and arrest the heart in diastole.17 The atria and right ventricle were dissected from the LV. The LV was sectioned into base, mid, and apical regions in cross-sectional slices. The lungs, right ventricle, and LV were weighed. The left tibia was removed and soaked overnight in 2M KOH, and tibia length was measured using a digital caliper.
Statistical Analysis
All data are represented as mean ± standard error of the mean (SEM). One-way ANOVA with Student Newman Keuls posttest was used to determine statistical differences among the different time points. A P value <0.05 was considered statistically significant. For all plots, R2 values and correlation curves were calculated by performing a linear regression on the 2 variables denoted.
Results
C57BL/6J mice from 3 to 11 weeks of age were assessed for cardiac function. Body weight ranged from 7.1 to 28.6 g, consistent with the rapid postnatal growth phase. The heated restraining board with built-in ECG electrodes was designed primarily for adult mice; however, the electrodes were spaced adequately to make contact with all 4 paws, even for the smallest of the 3-week-old mouse imaged (Fig. 3).
Figure 3.
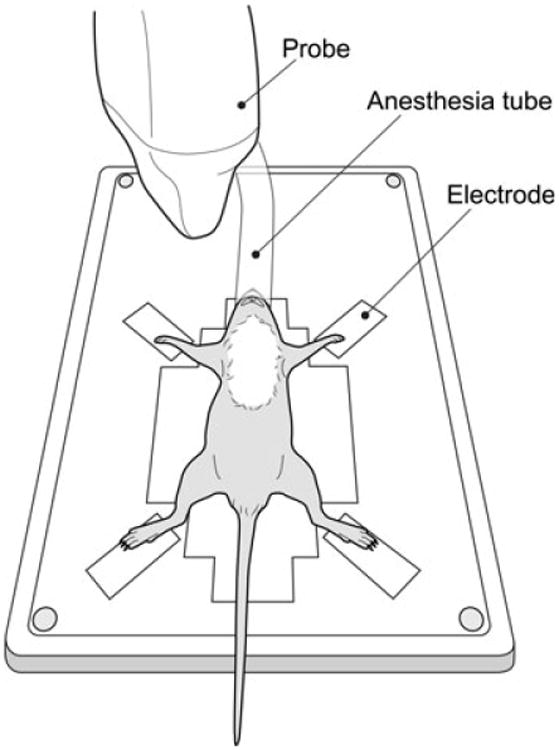
Three-week-old mouse positioned on a heated restraining board. The mouse is large enough such that all 4 paws can make contact with built-in copper electrodes used for electrocardiogram acquisition. This mouse is receiving anesthesia via a nose cone. All hair in the thoracic region has been removed using a depilatory agent.
Echocardiography
Quality echocardiographic images were acquired for all mice and importantly in mice as young as 3 weeks old and weighing as little as 7.1 g. We successfully obtained images on 16/16 mice evaluated. As many of the conventional echocardiographic measurements and all strain analyses used long-axis images, it was imperative to acquire images with minimal artifacts (i.e. ribs or fat). While having minimal impact on conventional measurements, artifacts can substantially affect computer-assisted identification of endocardium and epicardium borders, which in turn introduces error into strain and strain rate measurements. During image acquisition, artifacts could normally be avoided by angling the transducer such that it was directed more laterally to avoid the sternum (Fig. 4). It is important to note that changing the angle of the transducer to improve long-axis images sometimes resulted in lower quality short-axis images in younger (i.e. 3–5 weeks of age) mice.
Figure 4.
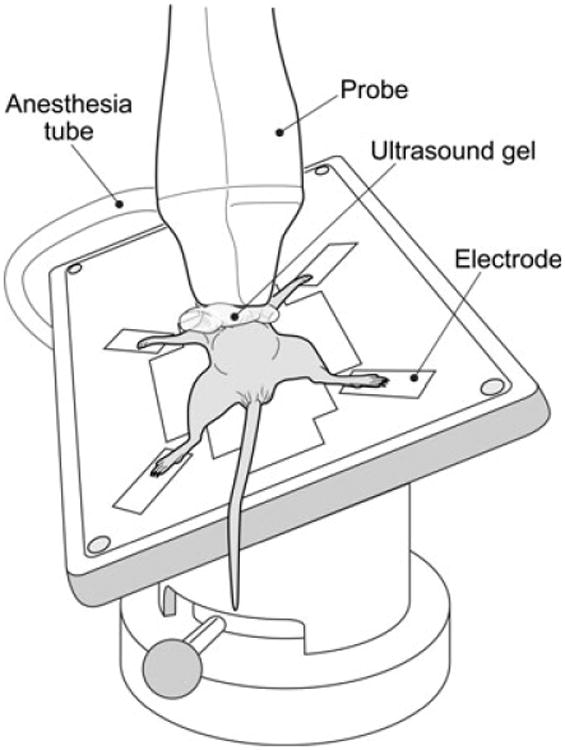
The angles of the solid-state transducer and heated stage have been adjusted such that artifacts caused by the sternum can be avoided. A foam block is used to help keep the mouse in position. Ultrasound gel has been applied between the transducer and mouse to improve sound wave transmission.
Conventional Measurements
All of the following conventional echocardiographic measurements were evaluated in our study: left ventricular EF, FS, EDD, ESD, posterior wall thickness in diastole (LVPWd), and posterior wall thickness in systole (LVPWs). Results for mice ages 3–11 weeks are listed in Table III. All images were acquired and analyzed by the same trained technician.
Table III. Conventional Echocardiographic Measurements.
| Age (weeks) | |||||
|---|---|---|---|---|---|
|
|
|||||
| 3 | 5 | 7 | 11 | P-value | |
| Morphometric parameters | |||||
| Body weight (g) | 7.8 ± 0.3 | 18.3 ± 1.2* | 20.7 ± 1.0* | 24.5 ± 2.0* | <0.01 |
| Tibia length (mm) | 11.3 ± 0.1 | 14.8 ± 0.2* | 16.1 ± 0.1* | 16.1 ± 0.9* | <0.01 |
| LV mass (mg) (necropsy) | 27 ± 1 | 61 ± 4* | 72 ± 6* | 84 ± 7* | <0.01 |
| LV mass (mg) (ECHO†) | 28.4 ± 5.9 | 41.9 ± 6.8 | 73.7 ± 12.7 | 79.8 ± 18.2 | <0.01 |
| Conventional echocardiographic measurements | |||||
| Ejection fraction (%) | 75 ± 1 | 70 ± 3 | 60 ± 1* | 61 ± 2* | <0.01 |
| Fractional shortening (%) | 44 ± 1 | 39 ± 2 | 33 ± 2* | 34 ± 2* | <0.01 |
| LVEDD (mm) | 2.7 ± 0.1 | 3.5 ± 0.1* | 4.3 ± 0.1* | 4.0 ± 0.2* | <0.01 |
| LVESD (mm) | 1.5 ± 0.04 | 2.1 ± 0.10* | 2.9 ± 0.1* | 2.7 ± 0.2* | <0.01 |
| LVPWd (mm) | 0.4 ± 0.04 | 0.5 ± 0.04 | 0.6 ± 0.03* | 0.6 ± 0.02* | <0.01 |
LV = left ventricle; LVEDD = left ventricle end-diastolic dimension; LVESD = left ventricular end-systolic dimension; LVPWd = left ventricular posterior wall thickness.
P < 0.01 as compared to 3 weeks.
LV mass calculated from echocardiographic measurements.
Cardiac Strain, Strain Rate, and Segmental Analyses
We successfully obtained values for radial and longitudinal strain for all mice evaluated (n = 4 for time points at 3, 5, 7, and 11 weeks). Similar to studies in adult mice,15,16 the apical region of the LV exhibited different strain and strain rate values compared to the mid and basal regions (Fig. 5 and Table II); we therefore excluded the apical region for measurements of global LV strain and strain rate. Our data are therefore in agreement with previous publications that have also argued for the exclusion of apical data when reporting global strain and strain rate values.15,16 From 3 to 11 weeks of age, no statistically significant differences in radial or longitudinal strain rate were detected for the ages studied. In addition, there were no statistically significant changes in these measurements when the data are analyzed segmentally, with the exception of radial strain rate in the posterior base. This outlier is likely the result of small sample size and may lose significance with analysis of larger numbers of mice. Results for radial and longitudinal strain and strain rate are represented in Fig. 6, including a regression analysis of radial strain versus age, with an R2 of 0.29 in 6E. Although, there appears to be a downward trend in radial strain that is dependent on age, the linear regression of radial strain as a variable dependent on age suggests otherwise. As with other analyses, the observed trend may disappear with the analysis of greater numbers of animals.
Figure 5.
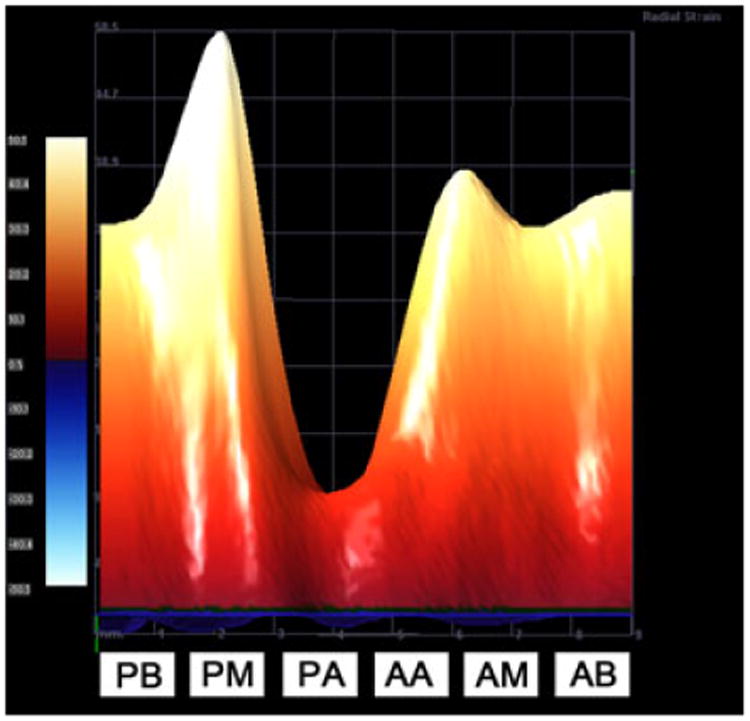
3D representation of radial strain. VevoStrain software can provide a 3D heat map representation of strain values. This image was acquired from a healthy 11 week old mouse and represents the maximum radial strain values of the left ventricle during a single contraction. It is important to note that radial strain values in the apical regions are much lower than basal or mid regions. PB = posterior base, PM = posterior mid, PA = posterior apex, AA = anterior apex, AM = anterior mid, AB = anterior base).
Figure 6.
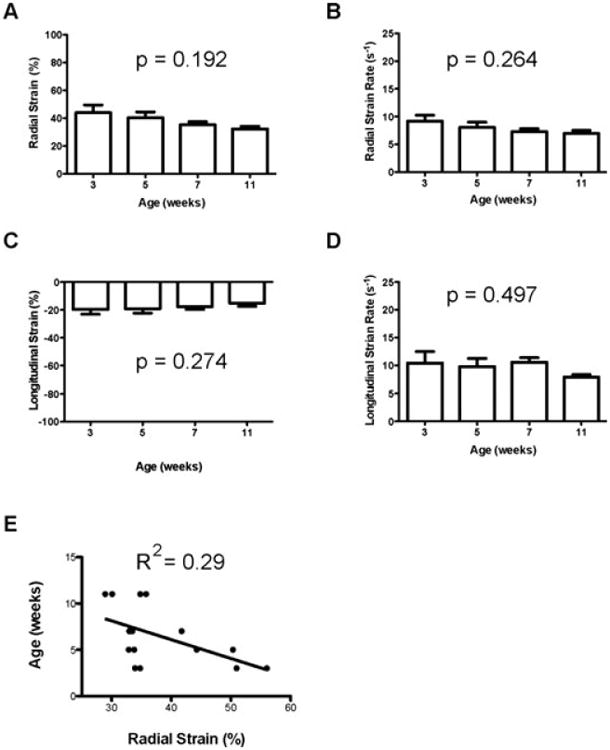
Strain and strain rate measurements. A–E. Measurements of healthy C57BL/6J mice (n = 4 per age group). A. Radial strain versus age. B. Radial strain rate versus age C. longitudinal strain versus age. D. Longitudinal strain rate versus age. E. Linear regression of age versus radial strain. A–D. Values are represented as mean ± SEM. All values were analyzed using one-way ANOVA over all ages.
Discussion
Echocardiography is an effective tool for noninvasive imaging of hearts in mice, from embryonic through pediatric development.18–20 However, conventional echocardiographic measurements, such as chamber dimensions, wall thickness, and EF cannot reproducibly assess regional wallmotion abnormalities.13 Strain and strain rate are considered superior alternatives to avoid these shortcomings,11 but to date most studies have concentrated on obtaining these measurements in adult mice or at single time point during their early development. This study demonstrates that strain and strain rate remain consistent from 3 to 11 weeks of age in C57BL/6J mice, and can serve as reliable noninvasive measurements for assessing cardiac function in pediatric mice.
As the majority of existing literature details echocardiographic analysis in adult mice, it was imperative to determine if conditions for echocardiography on adult mice were applicable to mice as young as 3 weeks old. Using 2% isoflurane for anesthesia appeared to have no adverse effects on cardiac function on all of the age groups tested, as exhibited by normal heart rates (400–550 bpm). It was important to maintain body temperatures between 33–36°C during echocardiography so as not to affect baseline heart rate. No deaths occurred as a result of the study protocol, indicating that conditions typically recommended for assessment of adults are applicable to pediatric mice.14
An evaluation of conventional echocardiographic measurements on all images used for strain and strain rate analysis was performed to ensure that images corresponded well to normal values for C57BL/6J mice of similar ages. For a healthy adult mouse, an EF of 60–80%14,20–22 and FS of 30–40% has been reported.21,22 As shown in Table III, young mice in this study exhibited similar values. It is interesting to note that there was a significant development-related decline in both EF and FS, which has been reported previously.23,24
Using an add-on software package, longitudinal, radial, and circumferential strain and strain rate can be measured from B-mode images. Long-axis images can be assessed for longitudinal and radial parameters, and short-axis images can be assessed for radial and circumferential parameters. Previous studies have indicated that circumferential values are substantially affected by the angle of the transducer and obtaining reproducible results is more difficult than with radial or longitudinal measurements.10 Consequentially, our study concentrates on longitudinal and radial values measured in long-axis images. Throughout the investigation, radial strain measured in the apical region of the LV was more variable and typically lower than similar measurements obtained from the basal or mid regions. Studies in humans demonstrate a gradient of higher to lower strain from the basal to the apical regions of the LV, and data from our study corresponds to these observations.15,16 Thus, values obtained from the apical region were excluded when calculating global strain and strain rate to obtain a more precise assessment of cardiac function.
During postnatal development of mice, significant changes in body and heart mass occur. A goal of this study was to determine if similar significant changes occur in strain and strain rate in mice as they reach adulthood. Based on data represented in Fig. 6, it appears that for both longitudinal and radial orientations, strain and strain rate remain constant from 3 to 11 weeks of age. No statistically significant changes were detected in either strain or strain rate for the 4 age groups tested in this study. Although there appears to be an age-dependent decline in radial strain rate (Fig. 6A), a linear regression indicates there is no statistically significant correlation between age and radial strain (Fig. 6E). These results should be taken with some caution due to the small sample size and the possibility of resulting type I error.
As echocardiographic analysis in pediatric mice evaluates functional and anatomic parameters in the context of a rapidly growing animal, it is important to account for these changes when assessing more complex measurements such as ventricular strain and strain rate. Pediatric mice exhibit age-dependent changes in parameters such as LV mass, contractility, and blood pressure.25,26 Our data indicate that echocardiography can be used to detect statistically significant changes in anatomic measurements such as LV mass. For example, LV mass from necropsy and calculated from echocardiographic measurements is shown in Table III. The data obtained are equivalent and a linear regression of the two methods for assessment of LV mass indicates they correlate well with an R2 value of 0.82. Although our study, conducted in a small number of pediatric mice, indicates there is no statistically significant change in strain or strain rate as the animals age, it is important to correlate these values with parameters related to animal growth. Furthermore, LV mass and tibia length exhibit no statistically significant correlation with either strain or strain rate (Fig. 7). Thus, in contrast to other echocardiographic parameters that change with age (i.e. EF, SF, and LV mass), strain and strain rate remain relatively stable in the growing and developing mouse and represent attractive output measurements in disease models. Finally, the analytical tools available allow the detection and graphical depiction of strain and strain rate differences (Fig. 5) in the context of pediatric pathophysiology.
Figure 7.
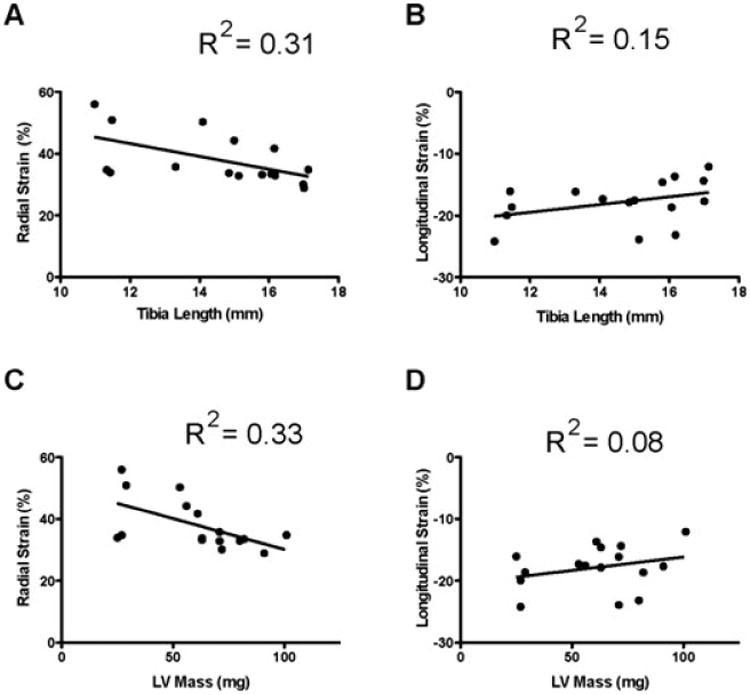
Strain and strain rate measurements and their correlation to LV mass and tibia length. A. and B. Tibia length. C. and D. LV mass.
Conclusions
While strain and strain rate have been shown to provide more detail of cardiac function than conventional echocardiographic measurements in mice, few studies have included pediatric animals. In this study, we determined that protocols and equipment used for acquiring and analyzing images in adult mice were feasible for pediatric mice. Data indicate that apical regions of the LV should be excluded from global measurements due to increased variability in measurements in the region. Values for strain and strain rate, both radial and longitudinal, do not significantly change in mice from 3 to 11 weeks of age. Stability in cardiac function as assessed by strain and strain rate in healthy pediatric mice will be a powerful resource for detecting subtle phenotypic changes and response to injury in murine pediatric models.
Acknowledgments
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000149. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. (G.J.A.–KL2 Scholar)
References
- 1.Rottman JN, Ni G, Brown M. Echocardiographic evaluation of ventricular function in mice. Echocardiography. 2007;24:83–89. doi: 10.1111/j.1540-8175.2006.00356.x. [DOI] [PubMed] [Google Scholar]
- 2.Scherrer-Crosbie M, Thibault HB. Echocardiography in Translational Research: Of Mice and Men. J Am Soc Echocardiogr. 2008;21:1083–1092. doi: 10.1016/j.echo.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ram R, Mickelsen DM, Theodoropoulos C, et al. New approaches in small animal echocardiography: Imaging the sounds of silence. Am J Physiol Heart Circ Physiol. 2011;301:H1765–H1780. doi: 10.1152/ajpheart.00559.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kepez A, Akdogan A, Sade LE, et al. Detection of sub-clinical cardiac involvement in systemic sclerosis by echocardiographic strain imaging. Echocardiography. 2008;25:191–197. doi: 10.1111/j.1540-8175.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 5.Derumeaux G, Ichinose F, Raher MJ, et al. Myocardial alterations in senescent mice and effect of exercise training: A strain rate imaging study. Circ Cardiovasc Imaging. 2008;1:227–234. doi: 10.1161/CIRCIMAGING.107.745919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham TP, Dimaano VL, Liang HY. Role of tissue doppler and strain echocardiography in current clinical practice. Circulation. 2007;116:2597–2609. doi: 10.1161/CIRCULATIONAHA.106.647172. [DOI] [PubMed] [Google Scholar]
- 7.Friedberg MK, Mertens L. Echocardiographic assessment of ventricular synchrony in congenital and acquired heart disease in children. Echocardiography. 2013;30:460–471. doi: 10.1111/echo.12110. [DOI] [PubMed] [Google Scholar]
- 8.D'hooge J, Heimdal A, Jamal F, et al. Regional strain and strain rate measurements by cardiac ultrasound: Principles, implementation and limitations. Eur J Echocardiogr. 2000;1:154–170. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- 9.Han HC, Fung YC. Direct measurement of transverse residual strains in aorta. Am J Physiol. 1996;270:H750–H759. doi: 10.1152/ajpheart.1996.270.2.H750. [DOI] [PubMed] [Google Scholar]
- 10.Bauer M, Cheng S, Jain M, et al. Echocardiographic speckle-tracking based strain imaging for rapid cardiovascular phenotyping in mice. Circ Res. 2011;108:908–916. doi: 10.1161/CIRCRESAHA.110.239574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bijnens BH, Cikes M, Claus P, et al. Velocity and deformation imaging for the assessment of myocardial dysfunction. Eur J Echocardiogr. 2009;10:216–226. doi: 10.1093/ejechocard/jen323. [DOI] [PubMed] [Google Scholar]
- 12.Marwick TH. Measurement of strain and strain rate by echocardiography. J Am Coll Cardiol. 2006;47:1313–1327. doi: 10.1016/j.jacc.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 13.Migrino RQ, Aggarwal D, Konorev E, et al. Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med Biol. 2008;34:208–214. doi: 10.1016/j.ultrasmedbio.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VevoStrain (TM) Analysis. Manufacturer manual. Available at www.visualsonics.com.
- 15.Knutsen AK, Ma N, Taggar AK, et al. Heterogeneous distribution of left ventricular contractile injury in chronic aortic insufficiency. Ann Thorac Surg. 2012;93:1121–1127. doi: 10.1016/j.athoracsur.2011.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellerin D, Sharma R, Elliott P, et al. Tissue Doppler, strain, and strain rate echocardiography for the assessment of left and right systolic ventricular function. Heart. 2003;89(Suppl III):9–17. doi: 10.1136/heart.89.suppl_3.iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michael LH, Ballantyne CM, Zachariah JP, et al. Myocardial infarction and remodeling in mice: Effect of reperfusion. Am J Physiol. 1999;277:H660–H668. doi: 10.1152/ajpheart.1999.277.2.H660. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan S, Baldwin HS, Aristizabal O, et al. Noninvasive, in utero imaging of mouse embryonic heart development with 40-MHz echocardiography. Circulation. 1998;98:912–918. doi: 10.1161/01.cir.98.9.912. [DOI] [PubMed] [Google Scholar]
- 19.Bose AK, Mathewson JW, Anderson BE, et al. Initial Experience with High Frequency Ultrasound for the Newborn C57BL Mouse. Echocardiography. 2007;24:412–419. doi: 10.1111/j.1540-8175.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- 20.Spurney CF, Lo CW, Leatherbury L. Fetal mouse imaging using echocardiography: A review of current technology. Echocardiography. 2006;23:891–899. doi: 10.1111/j.1540-8175.2006.00335.x. [DOI] [PubMed] [Google Scholar]
- 21.Stypmann J, Engelen MA, Troatz C, et al. Echocardiographic assessment of global left ventricular function in mice. Lab Anim. 2009;43:127–137. doi: 10.1258/la.2007.06001e. [DOI] [PubMed] [Google Scholar]
- 22.Stypmann J. Doppler ultrasound in mice. Echocardiography. 2007;24:97–112. doi: 10.1111/j.1540-8175.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 23.Corrigan N, Brazil DP, McAuliffe FM. High-frequency ultrasound assessment of the murine heart from embryo through to juvenile. Reprod Sci. 2009;17:147–157. doi: 10.1177/1933719109348923. [DOI] [PubMed] [Google Scholar]
- 24.Wiesmann Frank, JR, Hiller Karl-Heinz, Rommel Eberhard, et al. Development changes of cardiac function and mass assessed with MRI in neonatal, juvenile, and adult mice. Am J Physiol Heart Circ Physiol. 2000;278:H652–H657. doi: 10.1152/ajpheart.2000.278.2.H652. [DOI] [PubMed] [Google Scholar]
- 25.Ghanem A, Roll W, Hashemi T, et al. Echocardiographic assessment of left ventricular mass in neonatal and adult mice: Accuracy of different echocardiographic methods. Echocardiography. 2006;23:900–907. doi: 10.1111/j.1540-8175.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- 26.Tiemann K, Weyer D, Djoufack PC, et al. Increasing myocardial contraction and blood pressure in C57BL/6 mice during early postnatal development. Am J Physiol Heart Circ Physiol. 2003;284:H464–H474. doi: 10.1152/ajpheart.00540.2002. [DOI] [PubMed] [Google Scholar]


