Abstract
In efforts to assess baroreflex and cardiovascular responses in rats in which substance P (SP) or catecholamine transmission had been eliminated we studied animals after bilateral injections into the nucleus tractus solitarii (NTS) of SP or stabilized SP (SSP) conjugated to saporin (SP-SAP or SSP-SAP respectively) or SAP conjugated to an antibody to dopamine-β-hydroxylase (anti-DBH-SAP). We found that SP- and SSP-SAP eliminated NTS neurons that expressed the SP neurokinin-1 receptor (NK1R) while anti-DBH-SAP eliminated NTS neurons expressing tyrosine hydroxylase (TH) and DBH. The toxins were selective. Thus SP-or SSP-SAP did not eliminate TH/DBH neurons and anti-DBH-SAP did not eliminate NK1R neurons in the NTS. Each toxin, however, led to chronic lability of arterial blood pressure, diminished baroreflex function, cardiac ventricular irritability, coagulation necrosis of cardiac myocytes and, in some animals, sudden death associated with asystole. However, when TH/DBH neurons were targeted and eliminated by injection of 6-hydroxydopamine (6-OHDA), none of the cardiovascular or cardiac changes occurred. The studies reviewed here reveal that selective lesions of the NTS lead to altered baroreflex control and to cardiac changes that may lead to sudden death. Though the findings could support a role for SP or catecholamines in baroreflex transmission neither is proven in that NK1R colocalizes with glutamate receptors. Thus neurons with both are lost when treated with SP- or SSP-SAP. In addition, loss of catecholamine neurons after treatment with 6-OHDA does not affect cardiovascular control. Thus, the effect of the toxins may depend on an action of SAP independent of the effects of the SAP conjugates on targeted neuronal types.
Keywords: Arrhythmia, baroreflex, catecholamine, substance P, sudden death
Introduction
With recognition that the nucleus tractus solitarii (NTS) is the primary site of termination of cardiovascular reflex afferent nerves (Calaresu & Ciriello, 1980;Ciriello, 1983;Dean & Seagard, 1995;Kalia & Mesulam, 1980a;Kalia & Mesulam, 1980b;Mifflin, 1996;Torrealba & Claps, 1988), electrophysiological and anatomical studies contributed to an understanding of reflex transmission from those afferents to NTS neurons and beyond. Amongst the sites to which the NTS projects and from which it receives afferent projections is the cerebral cortex (Owens & Verberne, 1996;Ricardo & Koh, 1978;Ruggiero et al., 1987;Terreberry & Neafsey, 1983;Van der Kooy et al., 1984). Because lesions affecting cortical areas are known to produce autonomic dysfunction and, at times, sudden cardiac death (Samuels, 2007;Talman, 1985), the reciprocal connections could be critically important in clinical medicine.
In studying involvement of the NTS in cardiovascular regulation many early studies utilized electrolytic lesions to produce indiscriminant damage to the NTS to assess the impact of disturbed NTS function on basal cardiovascular control. Such lesions when placed bilaterally in the NTS led to death associated with heart failure and pulmonary edema (Doba & Reis, 1973). Rats in which electrolytic lesions had been placed in the NTS typically died within 6-8 hours of the lesions (Doba & Reis, 1973) though larger animals could survive the lesion with chronic lability of arterial pressure (Nathan & Reis, 1977) as seen also in rats with less extensive (Talman et al., 1980c;Talman et al., 1980a) or more selective (Snyder et al., 1978) lesions. With the advent of interest in putative transmitters involved in baroreflex transmission in the NTS, substances that either activated or inhibited local receptors led to an understanding that chemical rather than structural disturbances in the NTS could lead to fulminating pulmonary edema and death often within minutes of the injection of some agents (Talman et al., 1981). However, these studies and introduction of such agents were not conducive to long-term study though it was clear that chronic interruption of baroreflex function in experimental animals and in man led to lability of arterial pressure regardless of the site at which there had been interruption of the baroreflex (Aksamit et al., 1987;Cowley et al., 1973;Robertson et al., 1993;Talman et al., 1980c;Junqueira & Krieger, 1976). Lability occurred whether the lesion was central or peripheral. It became apparent that use of agents that acted in the NTS at receptors for specific transmitters could provide evidence for the possible role of certain chemicals as putative transmitters in baroreceptor or non-baroreceptor cardiovascular reflex function (Lawrence & Jarrott, 1996;Talman et al., 1980b;Talman, 1994).
We had participated in a number of such studies including ones in which we had sought to identify cardiovascular responses to the microinjection of substance P into the NTS (Talman & Reis, 1981). However, our own studies failed to identify cardiovascular responses to SP in the NTS; and, as a result, we had dismissed a transmitter role for that agent in the NTS. Nonetheless other elegant studies later showed that SP could be involved in modulating, if not overtly transmitting, the baroreflex (Potts et al., 1999;Potts & Fuchs, 2001;Potts et al., 2007;Seagard et al., 2000); and when a toxin that could selectively kill neurons on which SP acted became available we elected to use that toxin (a conjugate of substance P and the ribosome inactivating protein saporin or stabilized substance P conjugated to saporin; SP-SAP or SSP-SAP respectively) to determine if, in the absence of neurons on which SP might act, baroreflexes were compromised and blood pressure regulation was altered. Similarly we sought to kill selectively catecholamine neurons in the NTS with a SAP conjugate directed at neurons expressing dopamine β-hydroxylase (anti-DBH-SAP) in hopes of resolving controversy (Itoh et al., 1992;Snyder et al., 1978) about any role played by those neurons in baroreflex transmission at the level of the NTS and in hopes of comparing and contrasting effects of lesions in one neuronal type with effects from lesions in another.
Materials and Methods
Prior to performance of any studies protocols were approved by the institutional animal care and use committees of the University of Iowa and of the Iowa City Veterans Affairs Healthcare System. Both committees found the work in compliance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). In all studies reviewed here we utilized adult male Sprague Dawley rats, instrumented either for recording arterial pressure (AP), mean arterial pressure (MAP), and heart rate (HR) in the anesthetized animal or by radiotelemetry monitoring (DSI, St. Paul, MN) in the awake, freely moving animal (Nayate et al., 2008;Riley et al., 2002;Talman & Nitschke Dragon, 2004). All efforts were made to eliminate any potential pain or distress that might have been experienced by the animal subjects of our studies.
As previously described, microinjections were made into the NTS through a dorsal cervical incision and partial occipital craniotomy with direct exposure of the vagal complex that lies near the dorsal surface of the medulla oblongata (Nayate et al., 2008). Injections were made through glass micropipettes placed at predetermined (Nayate et al., 2008) coordinates (0.4 mm rostral to the calamus scriptorius, 0.5 mm lateral to the midline, and 0.5 mm below the dorsal surface of the brain stem at that site) into the NTS with each agent being delivered in solutions with phosphate buffered saline (PBS, pH 7.4) as the solvent. Injections included SSP-SAP (3ng in 100 nl), anti-DBH-SAP (42 ng in 200 nl), 6-hydroxydopamine (6-OHDA; 1μg in 400 nl), or the vehicle PBS (100 nl) alone. Baroreflex function was compared between study groups 7 days after injections had been made into the NTS in animals anesthetized with chloralose by determining reflex bradycardic responses to graded increases in AP following IV injection of phenylephrine (.0625 – 1 μg) and reflex tachycardic responses to graded decreases in AP following IV injection of sodium nitroprusside (0.25 - 4μg). In some animals we utilized sequence analysis of baroreflex activity (Stauss et al., 2006) as previously reported (Talman et al., 2012).
Toxic effects of injectates were assessed 7 days after the injection by analysis of their effects on immunoreactivity (IR) at the sites of injection (Lin et al., 2012;Lin et al., 2008;Nayate et al., 2008;Talman et al., 2012). In such cases animals only received unilateral injections of agents so that qualitative comparisons could be made between the injected and non-injected side of the NTS. Immunofluorescent confocal microscopy was used to analyze changes in IR within neurons expressing the neurokinin 1 receptor (NK1R), dopamine β-hydroxylase (DBH), tyrosine hydroxylase (TH), neuronal nitric oxide synthase (nNOS), N-methyl-D-aspartate 1 receptors (NMDAR1), and vesicular glutamate transporter 2 (VGLUT2). Intensity of immunoreactivity (IR) on the injected side was compared to that of the control side in the same section. Intensities of IR were graded as increased (↑), decreased (↓), or no obvious changes (-).
Results
We found that microinjection of SSP-SAP selectively reduced NK1R-IR in the NTS. (Fig. 1) While this toxic conjugate did not lead to loss of TH, DBH or nNOS-IR, it did appreciably reduce IR for NMDAR1 (Fig. 2) and increased nNOS and VGLUT2 in fibers in the NTS (Lin et al., 2012). In contrast, microinjection of anti-DBH-SAP into the NTS appreciably reduced DBH- and TH-IR (Fig. 3) while leaving unaffected, NMDAR1- , and NK1R-IR (See Table I). Microinjection of 6-OHDA into the NTS, like anti-DBH-SAP, led to a loss of DBH and TH-IR at the site of injections (Fig. 4) with the decline in TH/DBH being comparable for the two toxins (Table I).
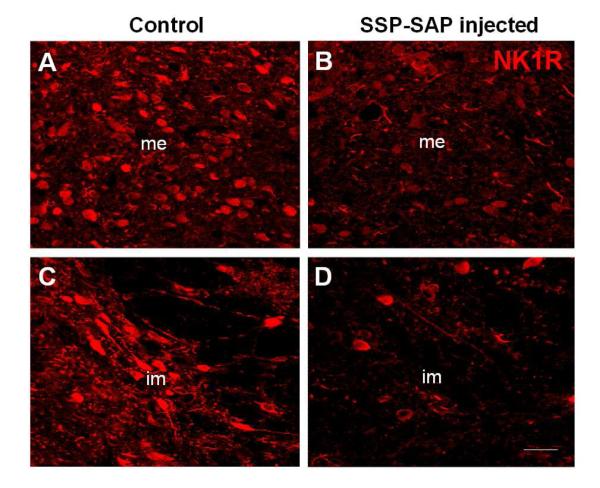
Fig. 1.
Representative confocal images of immunofluorescent labeling of the medial (me) and intermediate (im) subnuclei of the NTS on the control side where no injection was given (left) and on the injected side where SSP-SAP had been injected (right). The un-injected side served as an intra-animal intra-section control. Note the marked reduction in immunostaining for NK1R in the NTS on the treated side. The scale bar = 20 μm. From Nayate et al as permitted (Nayate et al., 2008)
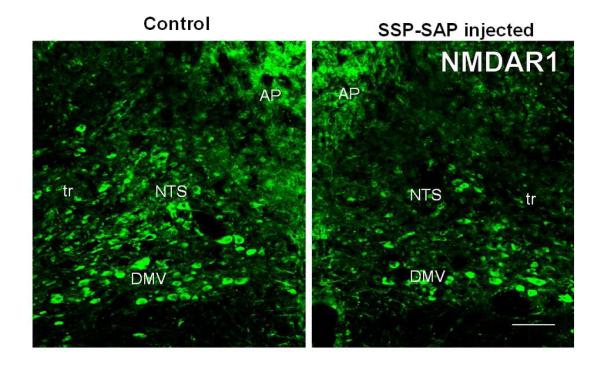
Fig. 2.
Confocal images of immunofluorescent staining showing decreases in NMDAR1-IR in the NTS on the SSP-SAP injected side (right) when compared to that of the control (un-injected; left) side. Abbreviations: AP, area postrema; DMV, dorsal motor nucleus of vagus; NTS, nucleus tractus solitarii; tr, tractus solitarius. The scale bar = 100 μm. From Lin et al as permitted (Lin et al., 2012)
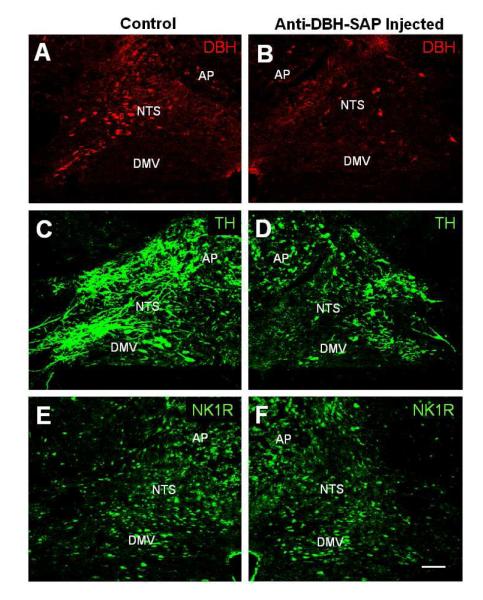
Fig. 3.
Confocal images showing immunofluorescent staining of DBH (A and B), TH (C and D), and NK1R (E and F) in the rat NTS after anti-DBH-SAP was injected unilaterally into the NTS. Compared to the un-injected side (A and C), the injected NTS (B and D) shows a decrease in DBH- and TH-IR. No obvious change in NK1R-IR was seen in the injected side (F) when compared to that of the control side (E). Abbreviations: as in Fig. 2. Scale bar = 100 μm. From Talman et al as permitted (Talman et al., 2012)
Table I.
Relative Changes in Immunoreactivity for Neuronal Markers in the NTS
| SSP-SAP | anti-DBH-SAp | 6-OHDA | PBS | |
|---|---|---|---|---|
| NK1R | ↓ | - | - | - |
| NMDAR1 | ↓ | - | - | - |
| VGLUT2 | ↑ ↑ | - | - | - |
| nNOS | ↑ ↑ | ↑ ↑ | ↑ | - |
| DBH | - | ↓ | ↓ | - |
| TH | ↑ ↑ | ↓ | ↓ | - |
Overall immunoreactivity (IR), which included IR observed in all cells and fibers, of the injected side was visually compared to that of the control side in the same section and assigned levels of increase (↑ or ↑↑), decrease (↓) or no obvious changes (-) .
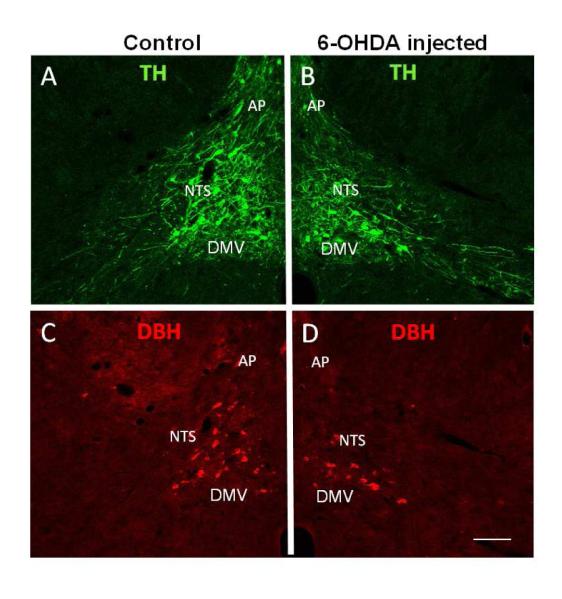
Fig. 4.
Confocal images of double immunofluorescent staining showing decreases in TH-IR (B) and DBH-IR (D) in the NTS after injection of 6-OHDA into this area when compared with the control side (A and C, respectively) in the same section. Abbreviations: as in Fig. 2. Scale bar = 100 μm.
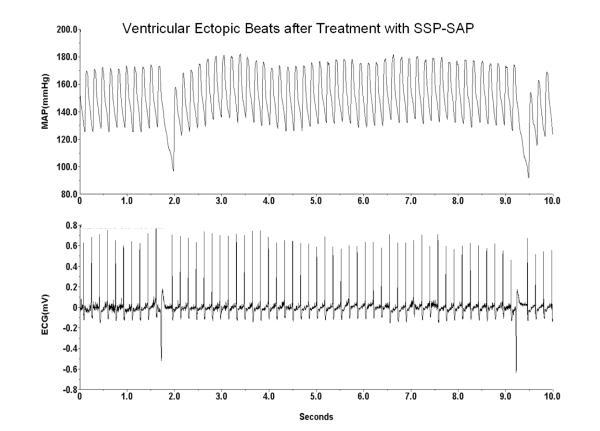
In our initial study in which we injected an SP-saporin (SP-SAP) conjugate we found that the baroreflex was significantly attenuated over the course of the 1-2 weeks that we followed the animals after the injection (Riley et al., 2002) (Fig. 5). In those studies baroreflexes were analyzed in animals anesthetized with chloralose (40 mg/kg IV for induction and 20 mg/kg per hour for maintenance). Not knowing how the toxin would affect treated animals and seeking to assure that the animals did not suffer pain or distress, we regularly assessed each animal every day from the time of injection till the end of the study. After recovery from surgery the animals had an uneventful course and could not be differentiated outwardly from controls in which placebo had been injected into the NTS. However, to our surprise, approximately 30% of treated animals died suddenly and without warning. We conjectured that the apparent sudden death might mimic that seen in humans with central nervous system dysfunction and sought to determine if treated rats instrumented with radiotelemetry monitors would manifest chronic changes in control of blood pressure, heart rate, and ECG. Treated animals developed labile arterial pressure and some, again without warning, suddenly died with cardiac asystole (Nayate et al., 2008) (Fig. 6). We have subsequently found that treated animals also manifest an increased frequency of ventricular ectopic beats (Fig. 7), but no animal died due to a ventricular tachyarrhythmia (Nayate et al., 2008). All that died did so in asystole. Furthermore, we found that animals with the greatest degree of lability of arterial pressure would manifest microscopic necrotic sites in the heart whilst those with less lability did not (Fig. 8). After the initial study we had conjectured that the sudden death was indeed a cardiac death, which was not related specifically to SP transmitter pathways in the NTS but more due to interruption of central baroreflex function instead. Thus, we sought to determine if another toxic conjugate, here saporin conjugated to an antibody to the norepinephrine synthesizing enzyme dopamine β hydroxylase (anti-DBH-SAP), would have similar effects on vascular and cardiovascular control (Talman et al., 2012). While SSP-SAP had reduced the number of NTS neurons expressing the NK1R with no reduction in those expressing DBH or TH, anti-DBH-SAP significantly reduced the number of NTS neurons expressing DBH or TH while not affecting those with the NK1R (See Fig. 3). However, cardiovascular effects produced by bilateral injection of anti-DBH-SAP were identical to those produced by SSP-SAP. Treated animals again displayed lability of arterial pressure (Fig. 9) and diminished baroreflex responses assessed either by changes in arterial pressure or through sequence analysis and again some animals died suddenly in asystole (Talman et al., 2012) though with an increased incidence of ectopic ventricular contractions as well. No animals died with a ventricular tachyarrhythmia and none demonstrated ventricular arrhythmias during air jet stress. Labile treated animals demonstrated multifocal sites of cardiac myocytolysis like that also seen in animals treated with SSP-SAP. Given that each saporin toxin produced similar cardiovascular changes we sought to compare and contrast effects when catecholamine neurons were affected by anti-DBH-SAP with those when the same neuronal types, those expressing TH and DBH, were affected by 6-OHDA, a toxin that does not contain saporin. We found that 6-OHDA, while affecting TH- and DBH-IR in the NTS as had anti-DBH-SAP had no effect on lability of arterial pressure or baroreflex activity and animals did not manifest ventricular ectopy, asystole or sudden death.
Fig. 5.
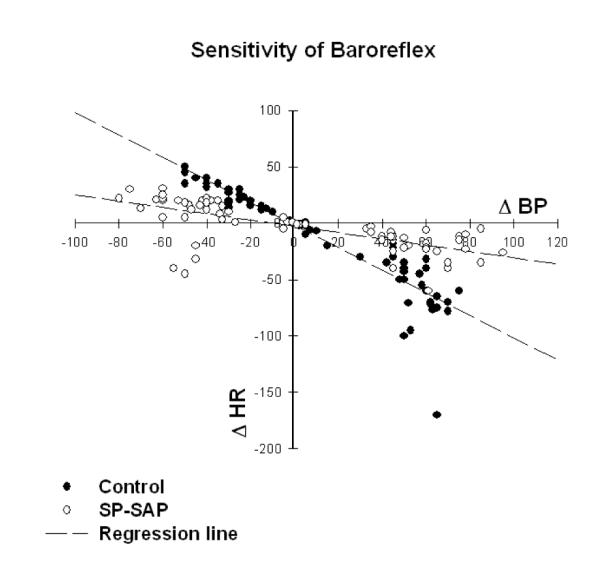
Baroreflex sensitivity was significantly (p < 0.0001) reduced after bilateral injection of SP-SAP into the NTS. The baroreflex was tested in 8 rats anesthetized with chloralose. Reflex bradycardia in response to pressor effects of varying doses of phenylephrine and tachycardia in response to depressor effects of nitroprusside were assessed and analyzed by regression analysis. Because there was no significant difference in baroreflex responses at 1 week (n=5) and 2 weeks (n=3) after administering SP-SAP, data were combined in this analysis. Baroreflex sensitivity was also decreased in animals treated with anti-DBH-SAP (not shown here) whether measured by assessing changes in HR following pressor or depressor responses to phenylephrine or nitroprusside respectively or by sequence analysis (Talman et al., 2012). From Riley et al as permitted (Riley et al., 2002)
Fig. 6.
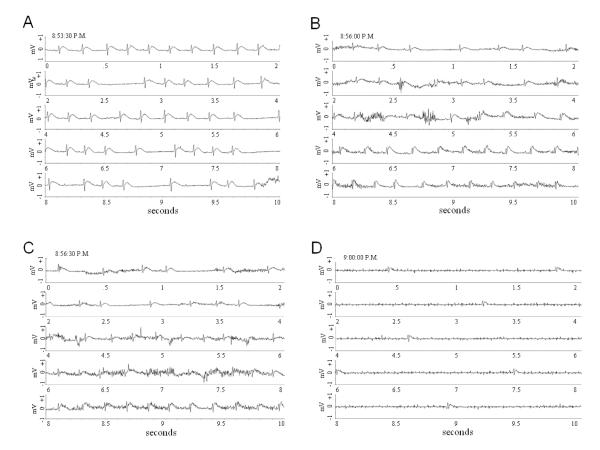
ECG recordings show progression from a normal sinus rhythm at 8:53:30 PM (A) to asystole (D) over the course of 6.5 minutes. Each segment of this figure depicts a continuous 10 second recording with the time at the beginning of the record posted in the top left hand corner of each. From Nayate et al as permitted (Nayate et al., 2008)
Fig. 7.
Animals treated with SSP-SAP (shown here) or anti-DBH-SAP (not shown), but not those treated with 6-OHDA, showed an increased frequency of ventricular ectopic beats on ECG monitoring of the awake freely moving animal.
Fig. 8.
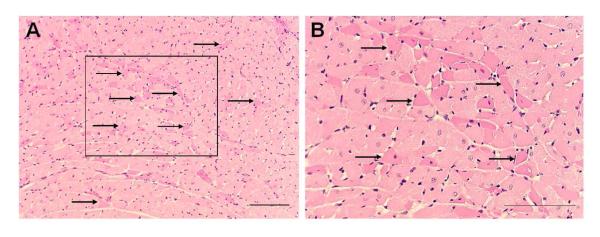
Eosinophilia of discrete cardiac myocytes (indicated by arrows) was seen diffusely at low (A) and high (B) power magnification in sections of left ventricular myocardium stained with hematoxylin and eosin. The box in A is seen at higher magnification in B. The scale bars = 50 μm. From Nayate et al as permitted (Nayate et al., 2008).
Fig. 9.
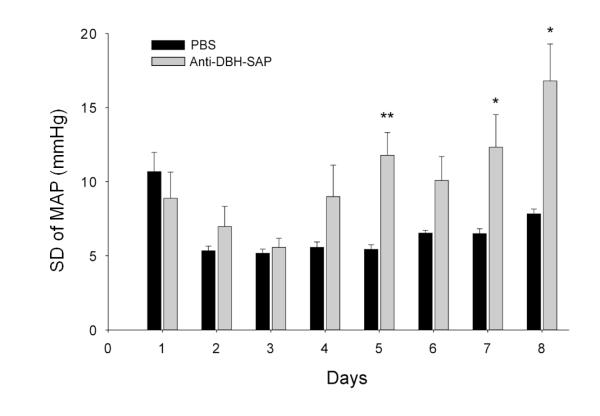
In animals in which anti-DBH-SAP had been injected bilaterally into the NTS lability (SD of MAP) began to rise 4 days after injection and reached significantly (**p < 0.01) increased levels on Day 5 when compared with those of control rats. Comparison of the SD at individual days revealed significant differences between treated and control animals also on Day 7 (*p < 0.05) and Day 8 (*p < 0.05). The SD of MAP for Day 1 is shown but was not included in analysis because it was artificially elevated as a result of data collection as animals were recovering from anesthesia. From Talman et al as permitted (Talman et al., 2012)
Discussion
Our studies showed that use of saporin conjugates to kill neurons with the NK1R or the biosynthetic enzymes TH and DBH in the NTS attenuated baroreflex responses and led to cardiac arrhythmias, sudden death and myocardial lesions. In contrast toxic effects on TH/DBH neurons when produced by another toxin had no such cardiovascular effects. The absence of a cardiovascular effect when TH/DBH neurons are injured by 6-OHDA despite comparable loss of those markers for noradrenergic neurons supports observations from an earlier study (Itoh et al., 1992) that drew the same conclusion and suggested that cardiovascular effects reported earlier (Snyder et al., 1978) when 6-OHDA had been injected into the NTS were due to non-specific effects of the toxic doses used in those earlier studies. Though the current studies showed a correlation between specific neuronal damage and disturbed cardiovascular reflex control it was not, from these studies, possible to define a lesion-response curve based upon the degree of loss of each cellular marker and the degree of attenuation of the baroreflex. To do so would have necessitated use of considerably more animals and fully quantitaive techniques for assessing loss of proteins studied here with immunofluorescent microscopy.
Previously published studies utilizing SP antagonists injected into the NTS suggested that inhibition of SP transmission led to loss of baroreflex function (Seagard et al., 2000). That added to the thought that SP may be a transmitter in the baroreflex pathway within the NTS but that conclusion would depend upon the pure selectivity of the SP antagonist used in the study and, in fact, the antagonist had been shown to act at non-SP receptors as well (Jensen et al., 1984). Our studies, while showing attenuation of baroreflexes after damage to NTS NK1R neurons upon which SP might act, still did not prove that SP transmission was integral to baroreflex function in that NK1R-IR colocalized with receptors for other transmitters (Lin et al., 2008), most particularly the transmitter glutamate that is integral to baroreflex transmission (Lawrence & Jarrott, 1996;Talman et al., 1980b), and thus death of NK1R-IR neurons would have simultaneously removed glutamate transmission from the region.
Each of the toxins that produced attenuation of the baroreflex led in some animals to sudden death and diffuse areas of cardiac myocytolysis that resembled damage seen in humans who have sustained sudden death as a result of central lesions such as subarachnoid hemorrhage (Samuels, 2007). However, in the human condition coagulation necrosis, as seen in our study, is not as prominent as is so-called “contraction band necrosis” (Karch & Billingham, 1986). Also in the human condition associated with subarachnoid hemorrhage, sudden death is thought to be more often associated with ventricular tachycardia or ventricular fibrillation (Estanol & Marin, 1975) than with asystole as seen in each of our studies in the NTS though asystole may be seen more often than is generally thought (Noritomi et al., 2006). Sudden death, particularly that resulting from ventricular tachyarrhythmias does not of necessity have an associated central lesion. Instead it may occur under conditions of extreme stress as has been reported during major earthquakes (Muller & Verrier, 1996;Voridis et al., 1983;Watanabe et al., 2005) and by implication during periods of inescapable social stress (Cannon, 1942). Our unpublished studies using air jet stress applied to animals with NTS neuronal lesions from SSP-SAP or anti-DBH-SAP failed to show a predilection to sudden death or enhanced arrhythmias as a result of the stress. However, sudden death that may be more associated with asystole also occurs during seizures, so-called sudden unexplained death in epilepsy (SUDEP). Given that neural connections between the forebrain and NTS have been clearly defined we conjecture that disturbances at the level of NTS may mediate asystolic sudden death and a central site that may participate both in the arrhythmogenic nature of central lesions and in development of overt cardiac damage with such lesions. Our findings raise the possibility that saporin could be having an effect on central cardiovascular control independent of its effect on specific neuronal types. Further study is progressing to define that toxic effect, which, if present, might occur at select doses of saporin given that others did not report cellular or systemic changes following injection of saporin alone into the NTS (Abdala et al., 2006).
Acknowledgements
The work presented in this review was funded by NIH R01 HL59593 and HL088090 and in part by VA Merit Review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abdala AP, Schoorlemmer GH, Colombari E. Ablation of NK1 receptor bearing neurons in the nucleus of the solitary tract blunts cardiovascular reflexes in awake rats. Brain Research. 2006;1119:165–173. doi: 10.1016/j.brainres.2006.08.059. [DOI] [PubMed] [Google Scholar]
- Aksamit TR, Floras JS, Victor RG, Aylward PE. Paroxysmal hypertension due to sinoaortic baroreceptor denervation in humans. Hypertension. 1987;9:309–314. doi: 10.1161/01.hyp.9.3.309. [DOI] [PubMed] [Google Scholar]
- Calaresu FR, Ciriello J. Projections to the hypothalamus from buffer nerves and nucleus tractus solitarius in the cat. American Journal of Physiology. 1980;239:R130–R136. doi: 10.1152/ajpregu.1980.239.1.R130. [DOI] [PubMed] [Google Scholar]
- Cannon WB. “Voodoo” death. American Anthropologist. 1942;44:169–181. doi: 10.2105/ajph.92.10.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriello J. Brainstem projections of aortic baroreceptor afferent fibers in the rat. Neuroscience Letters. 1983;36:37–42. doi: 10.1016/0304-3940(83)90482-2. [DOI] [PubMed] [Google Scholar]
- Cowley AW, Liard JF, Guyton AC. Role of the baroreceptor reflex in daily control of arterial blood pressure and other variables in dogs. Circulation Research. 1973;32:564–576. doi: 10.1161/01.res.32.5.564. [DOI] [PubMed] [Google Scholar]
- Dean C, Seagard JL. Expression of c-fos protein in the nucleus tractus solitarius in response to physiological activation of carotid baroreceptors. Neuroscience. 1995;69:249–257. doi: 10.1016/0306-4522(95)00217-7. [DOI] [PubMed] [Google Scholar]
- Doba N, Reis DJ. Acute fulminating neurogenic hypertension produced by brainstem lesions in the rat. Circulation Research. 1973;32:584–593. doi: 10.1161/01.res.32.5.584. [DOI] [PubMed] [Google Scholar]
- Estanol BV, Marin OSM. Cardiac arrhythmias and sudden death in subarachnoid hemorrhage. Stroke. 1975;6:382–386. doi: 10.1161/01.str.6.4.382. [DOI] [PubMed] [Google Scholar]
- Itoh H, Alper RH, Buñag RD. Baroreflex changes produced by serotonergic or catecholaminergic lesions in the rat nucleus tractus solitarius. The Journal of Pharmacology and Experimental Therapeutics. 1992;261:225–233. [PubMed] [Google Scholar]
- Jensen RT, Jones SW, Folkers K, Gardner JD. A synthetic peptide that is a bombesin receptor antagonist. Nature. 1984;309:61–63. doi: 10.1038/309061a0. [DOI] [PubMed] [Google Scholar]
- Junqueira LF, Krieger EM. Blood pressure and sleep in the rat in normotension and in neurogenic hypertension. Journal of Physiology (London) 1976;259:725–735. doi: 10.1113/jphysiol.1976.sp011491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M, Mesulam M-M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. the cervical vagus and nodose ganglion. Journal of Comparative Neurology. 1980a;193:435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M, Mesulam M-M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. Journal of Comparative Neurology. 1980b;193:467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Karch SB, Billingham ME. Myocardial contraction bands revisited. Human Pathology. 1986;17:9–13. doi: 10.1016/s0046-8177(86)80150-2. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Jarrott B. Neurochemical modulation of cardiovascular control in the nucleus tractus solitarius. Progress in Neurobiology. 1996;48:21–53. doi: 10.1016/0301-0082(95)00034-8. [DOI] [PubMed] [Google Scholar]
- Lin LH, Taktakishvili OM, Talman WT. Colocalization of neurokinin-1, N-methyl-D-aspartate, and AMPA receptors on neurons of the rat nucleus tractus solitarii. Neuroscience. 2008;154:690–700. doi: 10.1016/j.neuroscience.2008.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-H, Nitschke Dragon D, Talman WT. Collateral damage and compensatory changes after injection of a toxin targeting neurons with the neurokinin-1 receptor in the nucleus tractus solitarii of rat. Journal of Chemical Neuroanatomy. 2012;43:141–148. doi: 10.1016/j.jchemneu.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 1996;271:R870–R880. doi: 10.1152/ajpregu.1996.271.4.R870. [DOI] [PubMed] [Google Scholar]
- Muller JE, Verrier RL. Triggering of sudden death-lessons from an earthquake. The New England Journal of Medicine. 1996;334:460–461. doi: 10.1056/NEJM199602153340709. [DOI] [PubMed] [Google Scholar]
- Nathan MA, Reis DJ. Chronic labile hypertension produced by lesions of the nucleus tractus solitarii in the cat. Circulation Research. 1977;40:72–81. doi: 10.1161/01.res.40.1.72. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Nayate A, Moore SA, Weiss R, Taktakishvili O, Lin L-H, Talman WT. Cardiac Damage after Lesions of the Nucleus Tractus Solitarii. Am.J.Physiol Regul.Integr.Comp Physiol. 2008;296:R272–R279. doi: 10.1152/ajpregu.00080.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritomi DT, de Cleva R, Beer I, Dalbem AG, Liborio AB, Frota NA, Gama-Rodrigues JJ. Doctors awareness of spontaneous subarachnoid haemorrhage as a cause of cardiopulmonary arrest. Resuscitation. 2006;71:123–124. doi: 10.1016/j.resuscitation.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Owens NC, Verberne AJM. An electrophysiological study of the medial prefrontal cortical projection to the nucleus of the solitary tract in rat. Experimental Brain Research. 1996;110:55–61. doi: 10.1007/BF00241374. [DOI] [PubMed] [Google Scholar]
- Potts JT, Fong AY, Anguelov PI, Lee S, McGovern D, Grias I. Targeted deletion of neurokinin-1 receptor expressing nucleus tractus solitarii neurons precludes somatosensory depression of arterial baroreceptor-heart rate reflex. Neuroscience. 2007;145:1168–1181. doi: 10.1016/j.neuroscience.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JT, Fuchs IE. Naturalistic activation of barosensitive afferents release substance P in the nucleus tractus solitarius of the cat. Brain Research. 2001;893:155–164. doi: 10.1016/s0006-8993(00)03308-4. [DOI] [PubMed] [Google Scholar]
- Potts JT, Fuchs IE, Li JH, Leshnower B, Mitchell JH. Skeletal muscle afferent fibres release substance P in the nucleus tractus solitarii of anaesthetized cats. Journal of Physiology (London) 1999;514:829–841. doi: 10.1111/j.1469-7793.1999.829ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Research. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Riley J, Lin L-H, Chianca DA, Jr., Talman WT. Ablation of NK1 receptors in rat nucleus tractus solitarii blocks baroreflexes. Hypertension. 2002;40:823–826. doi: 10.1161/01.hyp.0000042089.34004.cf. [DOI] [PubMed] [Google Scholar]
- Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda-Garcia R, Robertson RM. The diagnosis and treatment of baroreflex failure. The New England Journal of Medicine. 1993;20:1449–1455. doi: 10.1056/NEJM199311113292003. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Mraovitch S, Granata AR, Anwar M, Reis DJ. A role of insular cortex in cardiovascular function. Journal of Comparative Neurology. 1987;257:189–207. doi: 10.1002/cne.902570206. [DOI] [PubMed] [Google Scholar]
- Samuels MA. The brain-heart connection. Circulation. 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Hopp FA. Modulation of the carotid baroreceptor reflex by substance P in the nucleus tractus solitarius. Journal of the Autonomic Nervous System. 2000;78:77–85. doi: 10.1016/s0165-1838(99)00060-0. [DOI] [PubMed] [Google Scholar]
- Snyder DW, Nathan MA, Reis DJ. Chronic lability of arterial pressure produced by selective destruction of the catecholamine innervation of the nucleus tractus solitarii in the rat. Circulation Research. 1978;43:662–671. doi: 10.1161/01.res.43.4.662. [DOI] [PubMed] [Google Scholar]
- Stauss H, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK. Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. American Journal of Physiology: Heart and Circulatory Physiology. 2006 doi: 10.1152/ajpheart.00228.2006. [DOI] [PubMed] [Google Scholar]
- Talman WT. Cardiovascular Regulation and Lesions of the Central Nervous System. Annals of Neurology. 1985;18:1–12. doi: 10.1002/ana.410180102. [DOI] [PubMed] [Google Scholar]
- Talman WT. Neuroactive substances in the control of cardiovascular and visceral responses: an overview. In: Barraco RA, editor. Nucleus of the solitary tract. CRC Press, Inc; Boca Raton, FL: 1994. pp. 233–244. [Google Scholar]
- Talman WT, Alonso DR, Reis DJ. Chronic lability of arterial pressure in the rat does not evolve into hypertension. Clinical Science. 1980a;59:405s–407s. doi: 10.1042/cs059405s. [DOI] [PubMed] [Google Scholar]
- Talman WT, Dragon DN, Jones SY, Moore SA, Lin LH. Sudden death and myocardial lesions after damage to catecholamine neurons of the nucleus tractus solitarii in rat. Cell Mol.Neurobiol. 2012;32:1119–1126. doi: 10.1007/s10571-012-9835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talman WT, Nitschke Dragon D. Transmission of arterial baroreflex signals depends on neuronal nitric oxide synthase. Hypertension. 2004;43:820–824. doi: 10.1161/01.HYP.0000120848.76987.ef. [DOI] [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Evidence for L-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science. 1980b;209:813–815. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Acute hypertension after the local injection of kainic acid into the nucleus tractus solitarii of rats. Circulation Research. 1981;48:292–298. doi: 10.1161/01.res.48.2.292. [DOI] [PubMed] [Google Scholar]
- Talman WT, Reis DJ. Baroreflex actions of substance P microinjected into the nucleus tractus solitarii in rat: a consequence of local distortion. Brain Research. 1981;220:402–407. doi: 10.1016/0006-8993(81)91233-6. [DOI] [PubMed] [Google Scholar]
- Talman WT, Snyder DW, Reis DJ. Chronic lability of arterial pressure produced by destruction of A2 catecholaminergic neurons in rat brainstem. Circulation Research. 1980c;46:842–853. doi: 10.1161/01.res.46.6.842. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. Rat medial frontal cortex: a visceral motor region with a direct projection to the solitary nucleus. Brain Research. 1983;278:245–249. doi: 10.1016/0006-8993(83)90246-9. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Claps A. The carotid sinus connections: A WGA-HRP study in the cat. Brain Research. 1988;455:134–143. doi: 10.1016/0006-8993(88)90122-9. [DOI] [PubMed] [Google Scholar]
- Van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. Journal of Comparative Neurology. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Voridis EM, Mallios KD, Papantonis TM. Holter monitoring during 1981 Athens earthquakes. Lancet. 1983;1:1281–1282. doi: 10.1016/s0140-6736(83)92737-x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Kodama M, Okura Y, Aizawa Y, Tanabe N, Chinushi M, Nakamura Y, Nagai T, Sato M, Okabe M. Impact of earthquakes on Takotsubo cardiomyopathy. Journal of the American Medical Association. 2005;294:305–307. doi: 10.1001/jama.294.3.305. [DOI] [PubMed] [Google Scholar]