Abstract
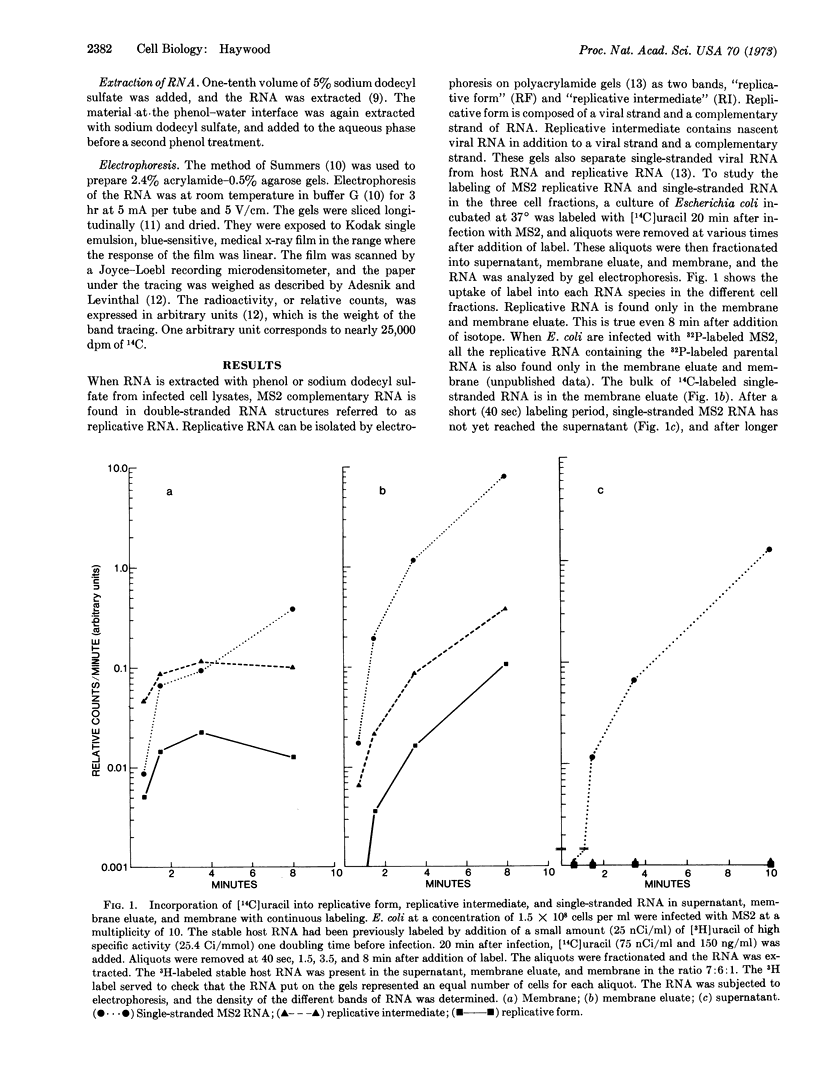
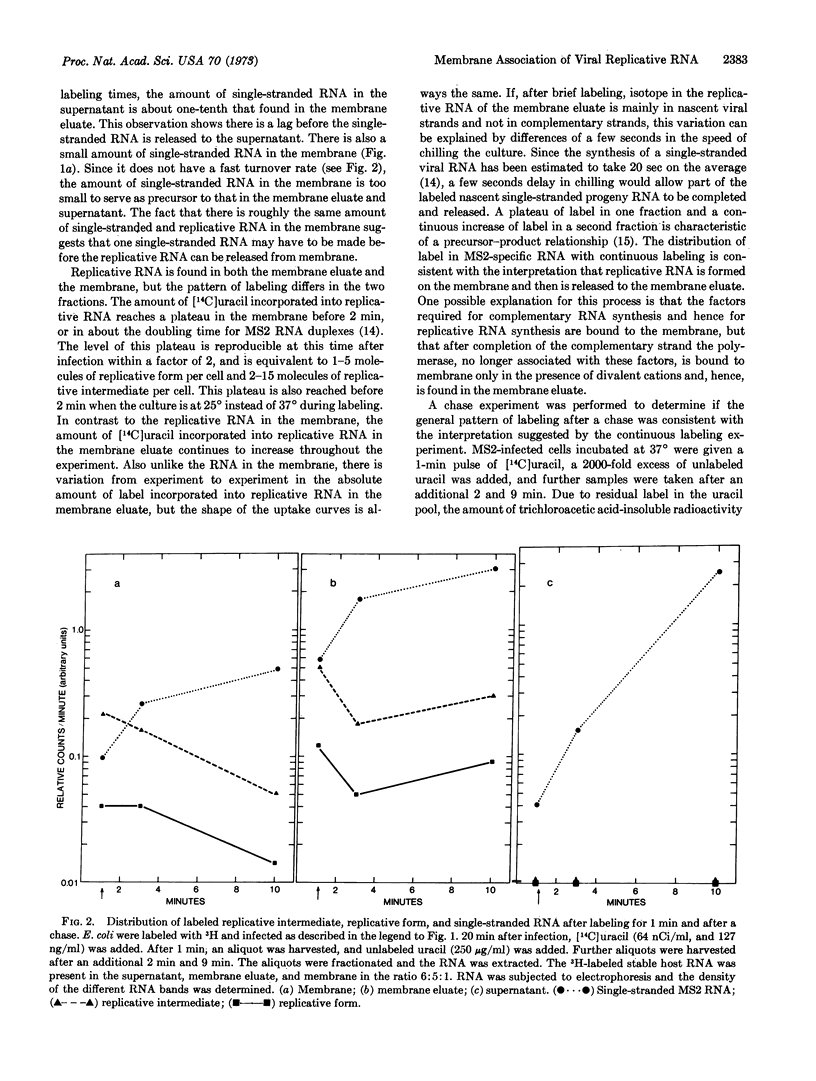
Escherichia coli membranes were isolated in the presence of 6 mM Mg++. They were washed with buffer containing no Mg++ to yield a fraction containing material bound only in the presence of divalent cations, “membrane eluate,” and that bound in the absence of divalent cations, “membrane.” When E. coli infected with bacteriophage MS2 are labeled with [14C]uracil, all MS2 replicative RNA, i.e., the RNA species containing MS2 complementary RNA, is in the membrane eluate and membrane. The amount of [14C]uracil in replicative RNA found in the membrane eluate increases with time of labeling, whereas that in the replicative RNA in the membrane reaches a plateau in 1-2 min. This finding is consistent with a precursor-product relationship. Most of the label entering single-stranded viral RNA comes from the replicative RNA in the membrane eluate. This result suggests that polymerase components or factors required for complementary-strand synthesis are bound to membrane even in the absence of divalent cations and that the polymerase is no longer bound to these factors when it is making the bulk of the progeny single-stranded RNA.
Keywords: complementary-strand synthesis of RNA
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Baron C. Reversible attachment of adenosine triphosphatase to streptococcal membranes and the effect of magnesium ions. Biochemistry. 1968 Feb;7(2):501–507. doi: 10.1021/bi00842a003. [DOI] [PubMed] [Google Scholar]
- Adesnik M., Levinthal C. Synthesis and maturation of ribosomal RNA in Escherichia coli. J Mol Biol. 1969 Dec 14;46(2):281–303. doi: 10.1016/0022-2836(69)90422-7. [DOI] [PubMed] [Google Scholar]
- August J. T., Banerjee A. K., Eoyang L., Franze de Fernandez M. T., Hori K., Kuo C. H., Rensing U., Shapiro L. Synthesis of bacteriophage Q-beta RNA. Cold Spring Harb Symp Quant Biol. 1968;33:73–81. doi: 10.1101/sqb.1968.033.01.013. [DOI] [PubMed] [Google Scholar]
- BRITTEN R. J., McCARTHY B. J. The synthesis of ribosomes in E. coli. II. Analysis of the kinetics of tracer incorporation in growing cells. Biophys J. 1962 Jan;2:49–55. doi: 10.1016/s0006-3495(62)86840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Abrams A. Isolation of a bacterial membrane protein, nectin, essential for the attachment of adenosine triphosphatase. J Biol Chem. 1971 Mar 10;246(5):1542–1544. [PubMed] [Google Scholar]
- Billeter M. A., Libonati M., Viñuela E., Weissmann C. Replication of viral ribonucleic acid. X. Turnover of virus-specific double-stranded ribonucleic acid during replication of phage MS2 in Escherichia coli. J Biol Chem. 1966 Oct 25;241(20):4750–4757. [PubMed] [Google Scholar]
- Bishop D. H., Claybrook J. R., Pace N. R., Spiegelman S. An analysis by gel electrophoresis of Q-beta-RNA complexes formed during the latent period of an in vitro synthesis. Proc Natl Acad Sci U S A. 1967 May;57(5):1474–1481. doi: 10.1073/pnas.57.5.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Haywood A. M. Cellular site of Escherichia coli ribosomal RNA synthesis. Proc Natl Acad Sci U S A. 1971 Feb;68(2):435–439. doi: 10.1073/pnas.68.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M., Cramer J. H., Shoemaker N. L. Host-virus interaction in ribonucleic acid bacteriophage-infected Escherichia coli. I. Location of "late" MS2-specific ribonucleic acid synthesis. J Virol. 1969 Oct;4(4):364–371. doi: 10.1128/jvi.4.4.364-371.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M., Sinsheimer R. L. The replication of bacteriophage MS2. V. Proteins specifically associated with infection. J Mol Biol. 1965 Dec;14(2):305–326. doi: 10.1016/s0022-2836(65)80184-x. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B., Sinsheimer R. L. The replication of bacteriophage MS2. VII. Non-conservative replication of double-stranded RNA. J Mol Biol. 1967 Jun 14;26(2):169–179. doi: 10.1016/0022-2836(67)90289-6. [DOI] [PubMed] [Google Scholar]
- Mueller K., Bremer H. Rate of synthesis of messenger ribonucleic acid in Escherichia coli. J Mol Biol. 1968 Dec;38(3):329–353. doi: 10.1016/0022-2836(68)90390-2. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Peterson R. F., Kievitt K. D., Ennis H. L. Membrane protein synthesis after infection of Escherichia coli B with phage T4: the rIIB protein. Virology. 1972 Nov;50(2):520–527. doi: 10.1016/0042-6822(72)90403-5. [DOI] [PubMed] [Google Scholar]
- Racker E. Resolution and reconstitution of the inner mitochondrial membrane. Fed Proc. 1967 Sep;26(5):1335–1340. [PubMed] [Google Scholar]
- Razin S. Reconstruction of biological membranes. Biochim Biophys Acta. 1972 Apr 18;265(2):241–296. [PubMed] [Google Scholar]
- Spiegelman S., Haruna I., Holland I. B., Beaudreau G., Mills D. The synthesis of a self-propagating and infectious nucleic acid with a purified enzyme. Proc Natl Acad Sci U S A. 1965 Sep;54(3):919–927. doi: 10.1073/pnas.54.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Pace N. R., Mills D. R., Levisohn R., Eikhom T. S., Taylor M. M., Peterson R. L., Bishop D. H. The mechanism of RNA replication. Cold Spring Harb Symp Quant Biol. 1968;33:101–124. doi: 10.1101/sqb.1968.033.01.015. [DOI] [PubMed] [Google Scholar]
- Stavis R. L., August J. T. The biochemistry of RNA bacteriophage replication. Annu Rev Biochem. 1970;39:527–560. doi: 10.1146/annurev.bi.39.070170.002523. [DOI] [PubMed] [Google Scholar]
- Summers W. C. The process of infection with coliphage T7. I. Characterization of T7 RNA by polyacrylamide gel electrophoretic analysis. Virology. 1969 Oct;39(2):175–181. doi: 10.1016/0042-6822(69)90037-3. [DOI] [PubMed] [Google Scholar]
- Weintraub S. B., Frankel F. R. Identification of the T4rIIB gene product as a membrane protein. J Mol Biol. 1972 Oct 14;70(3):589–615. doi: 10.1016/0022-2836(72)90561-x. [DOI] [PubMed] [Google Scholar]


