Abstract
Background
HIV testing male partners of pregnant women may decrease HIV transmission to women and promote uptake of prevention of mother-to-child HIV transmission (PMTCT) interventions. However, it has been difficult to access male partners in antenatal care (ANC) clinics. We hypothesized that home visits to offer HIV testing to partners of women attending ANC would increase partner HIV testing.
Methods
Women attending their first ANC were enrolled, interviewed using smartphone audio-computer assisted self-interviews and randomized to home visits or written invitations for male partners to come to clinic, if they were married or cohabiting, unaccompanied by partners and had no prior couple HIV counseling and testing (CHCT). Enrolled men were offered CHCT (HIV testing and mutual disclosure). Prevalence of CHCT, male HIV seropositivity, couple serodiscordance, and intimate partner violence, reported as physical threat from partner, were compared at 6 weeks.
Results
Among 495 women screened, 312 were eligible, and 300 randomized to clinic-based or home-based CHCT. Median age was 22 years (interquartile range 20, 26), and 87% were monogamous. CHCT was significantly higher in home-visit than clinic-invitation arm (n=128, 85% vs n=54, 36%; p<0.001). Home-arm identified more HIV-seropositive men (12.0 % vs 8.0 %; p= 0.248) and more HIV-discordant couples (14.7% vs 4.7%; p=0.003). There was no difference in intimate partner violence.
Conclusion
Home visits of pregnant women were safe and resulted in more male partner testing and mutual disclosure of HIV status. This strategy could facilitate prevention of maternal HIV acquisition, improve PMTCT uptake, and increase male HIV diagnosis.
Keywords: male partner, couple HIV testing, pregnancy
INTRODUCTION
In developing countries, especially in sub-Saharan Africa, the male partner may strongly influence decisions affecting women’s reproductive health and uptake of prevention of mother-to-child HIV transmission (PMTCT) interventions [1–4]. Women who undergo individual HIV counseling and testing (HCT) without male partner support may not disclose their HIV status to their partner due to fear of accusations of infidelity, violence, abandonment, and loss of economic support [5, 6]. Women who do not secure their male partners’ support are less likely to adhere to comprehensive PMTCT interventions [7, 8]. Lack of male partner HIV testing among serodiscordant couples if the man is HIV infected may also result in maternal HIV seroconversion during pregnancy and breastfeeding, and these acutely infected women are at exceptionally high risk of MTCT [9, 10]. Despite the increasing evidence that couple HCT may increase uptake of PMTCT and benefit maternal and child health, male partner HCT remains low [11–13].
Increasing male partner involvement has been stymied by an inability to access male partners of pregnant women in antenatal care (ANC) clinics. Less than 20% of male partners undergo clinic-based HCT in most low–resource settings, even following adaptations to the ANC clinics to encourage male attendance [8, 13, 14]. One study noted increased uptake of male partner testing following community mobilization, sensitization and written invitation but this only modestly increased testing to 32% [15]. Barriers to male testing in ANC include inadequate infrastructure within the ANC clinics and cultural norms that view ANC clinics as limited to women [16, 17].
In regions with high HIV prevalence, community-wide door-to-door home-based HIV counseling and testing (HBCT) has been feasible and leads to increased uptake of HIV testing [18, 19]. However, in at least three studies, less than 30% of testers reached through door-to-door HBCT were men and few (~4%) of the tested men were partners of women who were pregnant at the time [20–22].
Although some studies suggest an association between HIV infection and experiencing intimate partner violence or relationship instability by women [23, 24], this association was not found in a recent review of data from 10 developing countries [25]. On the contrary, couple counseling and testing may improve communication between couples and their coping with HIV infection [26, 27].
It is unknown if provider-initiated home-based couple HCT offered to women attending ANC clinics as male partner testing and mutual disclosure, would be acceptable and effective in increasing male partner HCT. To evaluate whether home-visits would increase male partner HIV testing among pregnant women attending ANC, we conducted a randomized clinical trial to compare male partner access, uptake and safety of couple HCT between pregnant women visited at home and those whose partners were invited to the clinic.
METHODS
Study design and setting
This was a randomized single blind clinical trial in which pregnant women seeking antenatal care underwent block randomization to either a homevisit or an invitation to bring their male partner to the clinic for couple HCT. The study was conducted in a rural resource-constrained high HIV-prevalence setting in Nyanza province, Kenya at the Ahero Sub-district Hospital. The hospital provides PMTCT as well as comprehensive HIV care services, including antiretroviral therapy.
Study procedures
All pregnant women presenting to the clinic for their first ANC visit were screened for study eligibility. Women were eligible if they were ≥18 years old, in stable relationships, and unaccompanied by their male partners during their first ANC visit. Eligible women could not have received couple HCT in the current pregnancy, prior to their first ANC visit. HIV positive women were ineligible if they had previously disclosed their HIV status to their male partners. Women were enrolled if they understood and consented for randomization to a home-visit or clinic-invitation for proposed couples HCT, and planned to stay with their male partners in the study area for ≥ 6 weeks after enrollment.
Eligible women were interviewed through Audio Computer Assisted Self Interview (ACASI), randomized to either home-visit or to clinic-invitation to bring their male partner to clinic for couples HCT and subsequently received routine HCT and antenatal care. A trained community health worker, who was also an experienced HIV counsellor, immediately accompanied women who were randomized to the home HCT arm to their homes to access the male partner and invite his participation in the study. If the male partner was not available an appointment was scheduled. The counsellors obtained a physical address and global positioning system (GPS) coordinates for return visits. Pregnant women allocated to the clinic-based arm were given a written note inviting their male partners for reproductive health education and couple HCT at the ANC clinic and they also provided details of their physical home address. Women in the clinic HCT arm were encouraged to schedule appointments by calling the study counsellor or walking in on any clinic day for couple HCT. In both arms, if the man had not been reached two weeks after the enrollment of the woman, a phone call reminder was made and/or text message reminder sent to the woman to reschedule appointments. In both trial arms, male partners were enrolled after providing written informed consent, after which they were individually interviewed and then offered couple HCT. Male partners were considered not reachable if they had not been traced by the time the pregnant women were due for 6-week follow-up.
Prenatal and couple HCT were conducted according to Kenya National HIV AIDS and STIs Control Programme guidelines. Each woman’s HIV status was known to the woman and provider following the enrollment visit but was only disclosed to their male partner after both had undergone couple HCT.
A 6-week follow-up visit either at home or at the clinic, as preferred by individual participant, was conducted to assess relationship status. Participants were reminded of their follow-up dates two weeks prior to the appointment through a phone call and short text message. Subjects were traced through cell phone contacts or home-visits using the GPS coordinates and/or physical address. Loss to follow-up occurred if participants were not reached two weeks after their scheduled follow-up.
Audio computer assisted interviews (ACASI)
Study counsellors demonstrated to each study participant the correct use of a smartphone (Google Nexus S) for self-interview. Pregnant women underwent individual ACASI in a private room at the clinic. Invited male partners underwent ACASI at the clinic while those enrolled at home were interviewed at a private location within their homes. The interviews were conducted in the preferred language, either English or Dholuo (the local dialect), and obtained information on sociodemographics, reproductive health decisions, HIV risk factors, and uptake of HIV prevention interventions. At follow-up, participants were interviewed either at home or in the clinic, on stability of their relationships and intimate partner violence
Randomization and blinding
An independent statistician used a computer-generated list of random numbers using Stata 11.0 (StataCorp, College Station, TX) statistical software in random blocks of 20 and a ratio of 1:1 to allocate study arms. The randomly generated numbers were contained in opaque sealed envelopes sequentially numbered with participant study identification numbers. The independent statistician was then called to issue the study arm corresponding to the random numbers.
The principal investigator and data analyst were blinded to the study arm. The study counsellors and women were unaware of the randomization assignment during enrollment, interviews and HCT. Post randomization, it was not practical to blind the counsellors and study participants due to the nature of the study. Participating men adhered to the study arm randomly assigned to their female partners.
Sample size
To detect a 50% increase in male partner tracing and uptake of couple HCT between the invited men and those visited at home, we determined that 268 pregnant women (134 per study arm) would provide at least 80% power assuming a two-sided test and type I error rate of 5%. We increased our sample size to 300 to account for ~10% loss to follow-up.
Study Outcome
The primary study outcomes were number of male partners reached, interviewed and couple tested for HIV at 6 weeks. Secondary outcomes were male HIV status, couple serodiscordance, post couple HCT relationship stability and intimate partner violence at follow-up.
Statistical analysis
Smartphone ACASI data was saved on an Open Data Kit (ODK) collect, then downloaded and submitted to an ODK aggregate database. Stored data were imported into Stata 12, which was used for analyses. The primary analysis was conducted on an intent-to-treat basis and included all randomized pregnant women. Chi-square tests were used to compare between trial arms: proportions of men who were interviewed and underwent couple HCT; baseline characteristics, including HIV prevalence, and intimate partner violence; and at follow-up the prevalence of relationship stability and intimate partner violence. Couple and male partner HIV status among those tested were compared using modified intention-to-treat analyses.
Ethical Statement
Institutional Review Boards at University of Washington, University of Nairobi and Kenyatta National Hospital approved the study protocol. All study participants provided written informed consent in either the local language (Dholuo) or English. The community advisory board provided oversight and advice. The study protocol was registered at ClinicalTrials.gov, registration number, NCT01620073.
RESULTS
Population characteristics
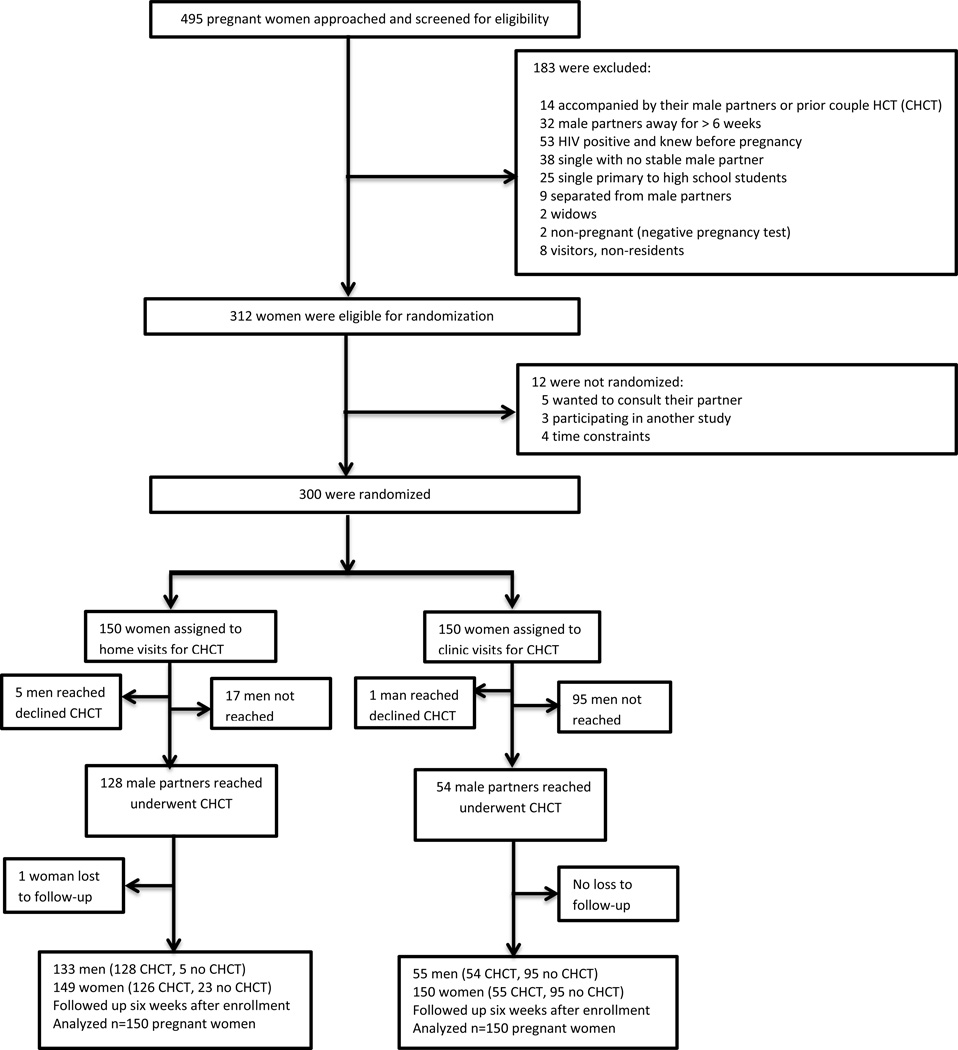
Between July 2012 and February 2013, 495 women attending their first ANC visit were screened. Of the eligible 312 (63%) pregnant women, 300 (96.2%) were randomly assigned to home-based (n=150) and clinic-based (n=150) groups (Figure 1). Among 183 ineligible women, reasons for ineligibility included: known HIV positive prior to current pregnancy (n=53), not planning to be in the area for ≥ 6 weeks (n=32), single or separated women with no stable partner (n=72), widows (n=2), visitors or non-residents (n=8), already presented with their male partners or had previously undergone couple HCT in current pregnancy (n=14) and negative urine pregnancy test (n=2). Twelve (3.8%) of the eligible women were not enrolled because they wanted to consult their male partners (n=5), were in a hurry (n=4) or were participating in another study (n=3).
Figure 1.
Enrollment and follow-up of pregnant women seeking prenatal care
The two groups had similar baseline characteristics (Table 1). Median age of women was 22 years (interquartile range 20, 26) and 68.3% were aged ≤25 years. Overall, 48 (16%) of the 300 randomized women were HIV positive, 21 (14%) in the home-based arm and 27 (18%) in the clinic-based arm. About two-thirds (67.3%) had primary education or lower, and only 6% reported post-secondary level of education, while 95.7% were married of whom 91.3% were in monogamous partnerships. Most (74.7%) women reported a daily household income <$2, and the majority (74.3%) used cell phones daily. Whereas a substantial number of women (28.7%) perceived that their male partners had concurrent sexual relationships only 5 (1.7%) women reported self-concurrent sexual partnerships. At enrollment, 23.0% of women reported being physically threatened by their male partner in the preceding six months and 2.7% experienced forced sex from their male partner in that same time period. Although interest in voluntary medical circumcision of male partner was high among women, (>70%), only one-in-three women reported that their male partners were circumcised. Seventeen percent of women were primigravida, 28.7% primiparous and 54.3% multiparous. One woman in the home-based arm separated from her male partner for social reasons not related to couple HCT and was lost to follow-up.
Table 1.
Baseline characteristics of enrolled pregnant women by study arm
| Characteristic | Home-based visits N=150 |
Clinic-based visits N=150 |
P value | |||
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Median | 22 | 22 | 0.309 | |||
| Interquartile range | 20,25 | 20,26 | ||||
| Age (years) - no (%) | ||||||
| ≤25 | 103 | (68.7) | 102 | (68.0) | 0.901 | |
| HIV status | ||||||
| HIV positive | 21 | (14.0) | 27 | (18.0) | 0.345 | |
| Highest education level -no (%) | ||||||
| Primary or lower | 103 | (68.7) | 99 | (66.0) | 0.622 | |
| Secondary (some or complete) | 39 | (26.0) | 41 | (27.3) | 0.794 | |
| Post secondary | 8 | (5.3) | 10 | (6.7) | 0.627 | |
| Marital status | ||||||
| Monogamous | 131 | (87.3) | 131 | (87.3) | 1.000 | |
| Polygamous | 12 | (8.0) | 13 | (8.7) | 0. 862 | |
| Unmarried | 7 | (4.7) | 6 | (4.0) | 0.777 | |
| Economic status | ||||||
| Daily household income ≥$2 | 113 | (75.3) | 111 | (74.1) | 0.791 | |
| Daily cell phone use | 107 | (71.3) | 116 | (77.3) | 0.234 | |
| Sexual partnerships | ||||||
| Perceived partner concurrency | 46 | (30.7) | 40 | (26.7) | 0.444 | |
| Casual sex when partner away | 2 | (1.3) | 3 | (2.0) | 0.652 | |
| Physically threatened past 6 months | ||||||
| None | 101 | (67.3) | 107 | (71.0) | 0.453 | |
| Study partner | 34 | (22.7) | 36 | (24.0) | 0.785 | |
| Experienced forced sex | ||||||
| None | 144 | (96.0) | 146 | (97.3) | 0.520 | |
| Study partner | 5 | (3.3) | 3 | (2.0) | 0.474 | |
| Family member | 1 | (0.7) | 1 | (0.7) | 1.000 | |
| Male circumcision | ||||||
| Supports VMMC | 116 | (77.3) | 120 | (80.0) | 0.785 | |
| Partner undergone circumcision | 47 | (31.3) | 51 | (34.0) | 0.622 | |
| Number of previous pregnancies | ||||||
| 0 | 21 | (14.0) | 30 | (20.0) | 0.367 | |
| 1 | 45 | (30.0) | 41 | (27.3) | 0.610 | |
| ≥2 | 84 | (56.0) | 79 | (52.7) | 0.562 | |
$ United States dollars, VMMC= voluntary male medical circumcision
Male partner access and uptake of couple HIV counseling and testing
A higher proportion of male partners were seen by health care workers in the homevisit arm (n=133, 88.7%) compared to the clinic-invitation arm (n=55, 36.7%), p<0.001) (Table 2). Similarly a substantially higher proportion of women and their male partners underwent couple HCT at home (n=128, 85.3%) compared to those in clinic (n=54,36.0%), p<0.001). Male partners were more than twice as likely to be reached and to undergo couple HCT using the provider-initiated home HCT strategy compared to ANC clinic invitation (Table 2). Because far fewer men got tested in the clinic arm, more HIV-positive and more HIV-negative men were identified in the home-test arm. Eighteen (12%) male partners of 150 women in the home arm were HIV positive compared to 12 (8%) male partners of 150 women randomized to the clinic arm (p=0.2). One hundred and ten (73%) of male partners of women randomized to the home arm were HIV-negative compared to 42 (28%) of women randomized to the clinic arm.
Table 2.
Male partner access, HIV counseling and testing and relationship status
| Variable | Home visits | Clinic visits | RR | 95%CI | P value | ||
|---|---|---|---|---|---|---|---|
| Overall male partner tracing and couple HIV testing | |||||||
| Male partners reached | 133/150 | (88.7) | 55/150 | (36.7) | 2.42 | 1.94–3.01 | <0.001 |
| Male partners tested | 128/150 | (85.3) | 54/150 | (36.0) | 2.37 | 1.90–2.96 | <0.001 |
| Male partner HIV status | |||||||
| Overall male partner HIV status including those not reached or tested | |||||||
| Male partner HIV positive | 18/150 | (12.0) | 12/150 | (8.0) | 1.5 | 0.74–3.00 | 0.248 |
| Male partner HIV negative | 110/150 | (73.3) | 42/150 | (28.0) | 2.62 | 1.99–3.45 | <0.001 |
| Overall male partner HIV status among the subset who had couple HIV counseling and testing | |||||||
| Male partner HIV positive | 18/128 | (14.1) | 12/54 | (22.2) | 0.63 | 0.33–1.22 | 0.175 |
| Female HIV positive | 21/150 | (14.0) | 27/150 | (18.0) | 0.67 | 0.34–1.34 | 0.345 |
| Couple HIV status | |||||||
| Overall couple HIV status among all couples including those not reached or tested | |||||||
| HIV seroconcordant negative | 99/150 | (66.0) | 39/150 | (26.0) | 2.53 | 1.89–3.40 | <0.001 |
| HIV seroconcordant positive | 7/150 | (4.7) | 8/150 | (5.3) | 0.88 | 0.33–2.35 | 0.791 |
| HIV serodiscordant | 22/150 | (14.7) | 7/150 | (4.7) | 3.14 | 1.38–7.13 | 0.003 |
| Couple HIV status unknown | 22/150 | (14.7) | 96/150 | (64.0) | 0.23 | 0.15–0.34 | <0.001 |
| Couple HIV status among the subset who had couple HIV counseling and testing | |||||||
| HIV seroconcordant negative | 99/128 | (77.3) | 39/54 | (72.2) | 1.07 | 0.89–1.30 | 0.461 |
| HIV seroconcordant positive | 7/128 | (5.5) | 8/54 | (14.8) | 0.37 | 0.14–0.97 | 0.036 |
| HIV serodiscordant | 22/128 | (17.2) | 7/54 | (13.0) | 1.33 | 0.60–2.92 | 0.477 |
| Relationship status at 6 weeks post-randomization | |||||||
| Physically threatened in the past six months | 22/150 | (14.7) | 26/150 | (17.3) | 0.85 | 0.50–1.42 | 0.529 |
| Physically threatened due to couple testing | 3/150 | (2.00) | 3/150 | (2.00) | 1.0 | 0.21–4.88 | 1.000 |
| Worsened relationship since enrollment | 6/150 | (4.00) | 7/150 | (5.00) | 0.86 | 0.29–2.49 | 0.778 |
| Improved relationship since enrollment | 100/141* | (69.9) | 42/143† | (29.8) | 2.35 | 1.78–3.09 | <0.001 |
| Same relationship status since enrollment | 43/150 | (28.9) | 99/150 | (66.9) | 0.43 | 0.33–0.57 | <0.001 |
| Forced sex since enrollment | 2/150 | (1.33) | 0/150 | (0.00) | ․ | ․ | 0.156 |
RR=Relative risk, CI=confidence interval,
9 missing values,
7 missing values
At the couple level, significantly more couples were likely to find out that they were HIV concordant negative if tested at home (66.0%) compared to clinic (26.0%). There was no difference in the proportions of couples who found out they were concordant positive (4.7% at home versus 5.3% at the clinic) (Table 2). However, couples were three times as likely to learn that they were HIV serodiscordant if they were in the home-based (14.7%) compared to the clinic arm (4.7%). Home visit arm participants were also significantly less likely to have undetermined couple HIV status (14.7%) compared to the clinic arm (64.0%).
Restricting analyses to the subset in each arm who underwent testing, the male partner HIV prevalence among couples tested at home was 14.1% (18 of 128) compared to 22.0 % (12 of 54) of those tested at the clinic (p=0.2) (Table 2). HIV serodiscordance was identified in 15.9% (29 of 182) of couples and was not statistically significantly different between the two arms: 17.2% in the home-based arm and 13.0% in the clinic-based arm (p=0.477). Among the discordant couples, 15 HIV negative women had HIV positive partners while 14 HIV positive women had HIV negative partners. Couples tested at the clinic were more likely to be HIV concordant positive (14.8%) than those tested at home (5.5%). Similar proportions of couples who underwent testing at home (77.3%) and at the clinic (72.2%) were concordant HIV negative.
Intimate partner violence and relationship stability
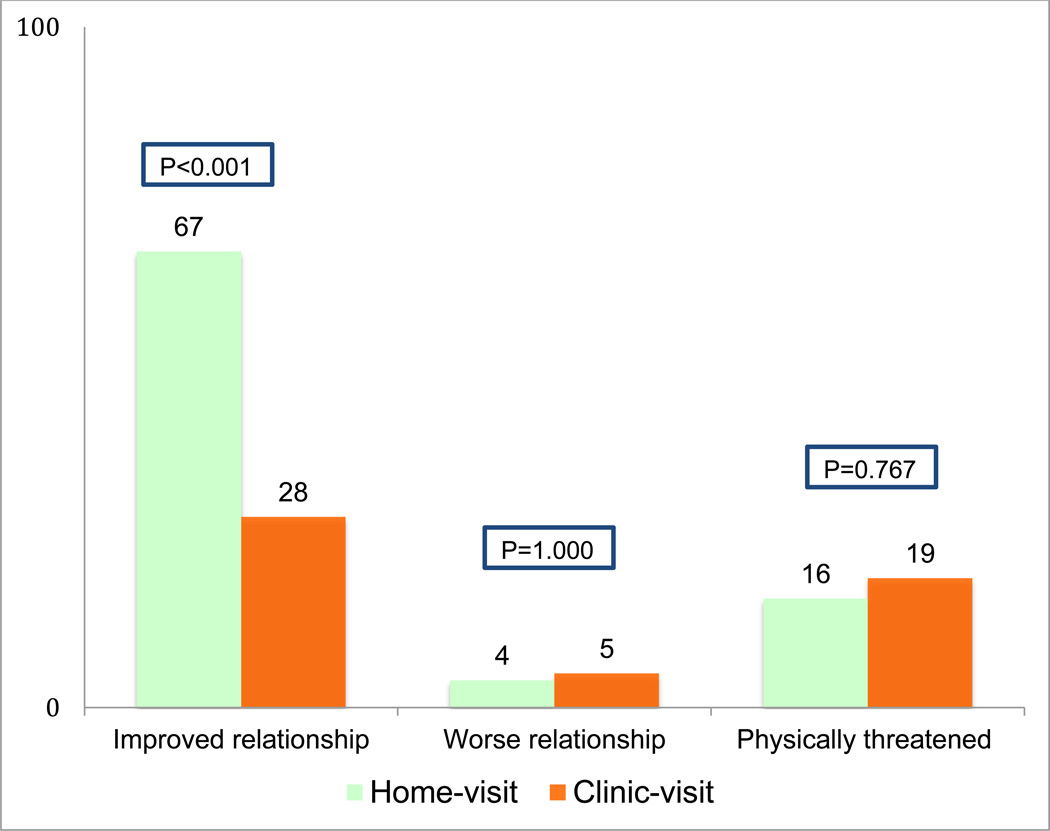
Intimate partner violence, reported as physical threats from male partners during the last 6 months, did not vary markedly at follow up from those reported at enrollment, (14.7% versus 22.7% at enrollment in home arm compared to 17.3% versus 24.0% at enrollment at the clinic) (Table 1 and Table 2). In each arm, 2% of women associated the physical threat they had experienced to couple HCT. Improved quality of the relationship was more than twice as likely to be reported by women whose partners were tested at home (67.1%), than those whose partners were tested at the clinic (28.4%) (Table 2, Figure 2). However when restricted to women whose partners were tested, this difference was not statistically significant (RR 1.10 [95%CI 0.90–1.34], p=0.329). Worsening quality of relationships was not statistically significantly different between the study arms (4.0% at home vs 5.0% at the clinic) (Figure 2).
Figure 2.
Couple relationship status reported by participating women at follow-up
DISCUSSION
In this randomized clinical trial among pregnant women seeking antenatal care in a high HIV prevalence setting, a strategy of home-visit for couple HCT more than doubled the number of male partners reached and tested for HIV. The 85% rate of male partner access and couple HIV counseling and testing (HCT) found in this study is the highest reported for male partners of pregnant women and substantially higher than that reported in previous studies that used community mobilization, written invitations, couple-oriented post-test HIV counseling or home based door-to-door HCT [15, 18, 20, 28]. There was high acceptability of home-visits for couple HCT most likely due to couples HCT testing approach which provided for confidentiality and sharing of results between partners, experience and flexibility of the counsellors, and prior door-to-door home based HIV testing in the region[29]. Home-visits may overcome the challenges of accessing male partners and providing couple HCT. In this way it could increase male partner involvement, lead to improved uptake of PMTCT, enhance infant HIV-free survival, increase detection of male HIV infection, accelerate access to treatment, and potentially contribute to HIV prevention among women at risk by identifying HIV concordant negative and discordant couples [30– 32]. In addition, increased male involvement may improve other maternal and child health outcomes such as skilled attendance at delivery and under-five mortality.
Intriguingly, tested couples in the clinic-invitation arm were more likely to be concordant positive than those in the home-visit arm and less likely to be HIV discordant. This suggests that HIV-infected men and couples who suspected or knew their HIV positive status were more likely to come to clinic than those who did not know their status. On the other hand, the home visit captured all couples, including those in which risk was less. This is a key finding since serodiscordant couples are at high risk of heterosexual HIV transmission and maternal seroconversion during pregnancy and breastfeeding substantially increases the risk of MTCT [9, 10, 33]. Early initiation of antiretroviral therapy (ART) among HIV serodiscordant couples where the man is HIV positive can reduce sexual transmission, maternal HIV infection, and MTCT [34]. In contrast to other interventions, such as preexposure antiretroviral prophylaxis that have been minimally evaluated in pregnant/postpartum women due to concerns regarding fetal/infant safety, partner ART is an immediately implementable intervention to prevent maternal HIV infection during this high-risk period [35]. In addition, home-based HCT may also reduce the high female-male heterosexual transmission during pregnancy because the male partner may support maternal uptake of ART and consistent use of condoms [36].
In this study, more men were newly diagnosed as HIV positive at home and referred for HIV care. Studies show that men are less likely to undergo HCT and more likely to present late for ART in part because they are less likely to undergo HCT [37, 38]. Home-visits for couple HCT during pregnancy may accelerate male partner HIV diagnosis, and increase access to HIV care, reduce HIV mortality and morbidity, lower community HIV viral load, and further reduce the risk of HIV transmission [22, 31, 39].
We did not find increased intimate partner violence or relationship instability among couples 6 weeks post-enrollment. This is consistent with other studies that have not found increased risk of partner violence or relationship dissolution following couple HCT [15, 28, 40]. In fact, during follow-up, more women reported improved relationships following home-based than clinic-based couple HCT. Home-visits for couple HCT during pregnancy were safe and did not negatively impact stable relationships in the short-term. Larger and longer studies should monitor any intimate partner violence and relationship outcomes to confirm these findings.
In addition to being a randomized trial, our study had several strengths. The study was conducted in a rural setting where HIV prevalence is high and socioeconomic status is low. Nyanza has an HIV prevalence of 13.9%, which is more than twice the national average of 6.3% [41]. In a recent study, Nyanza had the highest incident maternal HIV infection during pregnancy and breastfeeding compared to other sites (13.8 versus 6.8 per 100 woman-years)[9]. This finding therefore lends generalizability to other parts of sub- Saharan Africa that are hard-hit by the HIV epidemic where pregnant women have similar sexual partnerships. Second, the intervention was conducted after women had received initial antenatal care and did not interfere with other ANC activities. Therefore, implementing home visits for couple HCT may not require major programmatic changes. Another strength is that we achieved high retention rates that enabled a comprehensive evaluation of short-term outcomes. This may have resulted from male partner support, suggesting that home-based approach to couple HCT during pregnancy can improve short-term adherence. Our study limitations included the fact that we excluded women in unstable relationships whose risk of incident sexual and vertical HIV transmission may be higher. This did not markedly limit generalizability because the majority of women screened in this study were in stable relationships. The study did not address long-term outcomes including incident maternal HIV, linkage to care, other maternal and child health outcomes, or cost effectiveness. These should be addressed in other studies in order to provide further information relevant to scaling up this intervention and assessing additional benefits to women, men and children.
In summary, in this randomized controlled study, provider initiated home-visits during pregnancy were safe, acceptable, feasible, and significantly increased the number of male partners who underwent couple HCT. As a strategy provider-initiated home-visits for couple HCT during pregnancy could have a major public health impact and contribute to the elimination of MTCT through enhanced uptake of PMTCT especially in high HIV prevalence and low-resource settings. In addition, it may have the potential to improve other maternal, child, and male partner health outcomes.
Acknowledgements
This study was supported by Grant Number D43 TW000007 from the Fogarty International Center of the National Institutes of Health and its’ contents are solely the responsibility of the authors and do not necessarily represent the official views of the Fogarty International Center or of the National Institutes of Health.
Footnotes
Contributions AOO, GJS, BR and CF designed the study and performed the analysis. All investigators contributed to data collection and writing of the manuscript, and all approved the final draft. AOO, GJS, BR and CF wrote the initial manuscript draft and vouch for the data analysis, interpretation and manuscript submission. The primary author is responsible for the completeness and accuracy of the data presented, as well as the adherence of this report to the study protocol.
Financial Disclosures: None
This study was presented at the 7TH IAS Conference on HIV Pathogenesis, Treatment and Prevention in Kuala Lumpur, Malaysia on July 2, 2013
REFERENCES
- 1.UNICEF. State of the world's children. New York: United Nations Children's Fund; [Google Scholar]
- 2.Bajunirwe F, Muzoora M. Barriers to the implementation of programs for the prevention of mother-to-child transmission of HIV: a cross-sectional survey in rural and urban Uganda. AIDS Res Ther. 2005;2:10. doi: 10.1186/1742-6405-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kebaabetswe PM. Barriers to participation in the prevention of mother-to-child HIV transmission program in Gaborone, Botswana a qualitative approach. AIDS Care. 2007;19:355–360. doi: 10.1080/09540120600942407. [DOI] [PubMed] [Google Scholar]
- 4.Sarker M, Sanou A, Snow R, Ganame J, Gondos A. Determinants of HIV counselling and testing participation in a prevention of mother-to-child transmission programme in rural Burkina Faso. Trop Med Int Health. 2007;12:1475–1483. doi: 10.1111/j.1365-3156.2007.01956.x. [DOI] [PubMed] [Google Scholar]
- 5.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Towards the elimination of mother-to-child transmission of HIV : report of a WHO technical consultation. Geneva, Switzerland. Geneva: World Health Organization; 2011. [9–11 November 2010]. UNICEF, UNAIDS, United Nations Population Fund. [Google Scholar]
- 7.Peltzer K, Mlambo M, Phaswana-Mafuya N, Ladzani R. Determinants of adherence to a single-dose nevirapine regimen for the prevention of mother-to-child HIV transmission in Gert Sibande district in South Africa. Acta Paediatr. 2010;99:699–704. doi: 10.1111/j.1651-2227.2010.01699.x. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar C, Kiarie JN, Richardson BA, Kabura MN, John FN, Nduati RW, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37:1620–1626. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinuthia J, Kiarie JN, Farquhar C, Richardson B, Nduati R, Mbori-Ngacha D, et al. Cofactors for HIV-1 incidence during pregnancy and postpartum period. Curr HIV Res. 2010;8:510–514. doi: 10.2174/157016210793499213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moodley D, Esterhuizen TM, Pather T, Chetty V, Ngaleka L. High HIV incidence during pregnancy: compelling reason for repeat HIV testing. AIDS. 2009;23:1255–1259. doi: 10.1097/QAD.0b013e32832a5934. [DOI] [PubMed] [Google Scholar]
- 11.Auvinen J, Suominen T, Välimäki M. Male participation and prevention of human immunodeficiency virus (HIV) mother-to-child transmission in Africa. Psychol Health Med. 2010;15:288–313. doi: 10.1080/13548501003615290. [DOI] [PubMed] [Google Scholar]
- 12.Byamugisha R, Åstrøm AN, Ndeezi G, Karamagi CA, Tylleskär T, Tumwine JK. Male partner antenatal attendance and HIV testing in eastern Uganda: a randomized facility-based intervention trial. J Int AIDS Soc. 2011;14:43. doi: 10.1186/1758-2652-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Msuya SE, Mbizvo EM, Hussain A, Uriyo J, Sam NE, Stray-Pedersen B. Low male partner participation in antenatal HIV counselling and testing in northern Tanzania: implications for preventive programs. AIDS Care. 2008;20:700–709. doi: 10.1080/09540120701687059. [DOI] [PubMed] [Google Scholar]
- 14.Semrau K, Kuhn L, Vwalika C, Kasonde P, Sinkala M, Kankasa C, et al. Women in couples antenatal HIV counseling and testing are not more likely to report adverse social events. AIDS. 2005;19:603–609. doi: 10.1097/01.aids.0000163937.07026.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohlala BK, Boily MC, Gregson S. The forgotten half of the equation: randomized controlled trial of a male invitation to attend couple voluntary counselling and testing. AIDS. 2011;25:1535–1541. doi: 10.1097/QAD.0b013e328348fb85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nkuoh GN, Meyer DJ, Tih PM, Nkfusai J. Barriers to men's participation in antenatal and prevention of mother-to-child HIV transmission care in Cameroon, Africa. J Midwifery Womens Health. 2010;55:363–369. doi: 10.1016/j.jmwh.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Ditekemena J, Koole O, Engmann C, Matendo R, Tshefu A, Ryder R, et al. Determinants of male involvement in maternal and child health services in sub-Saharan Africa: a review. Reprod Health. 2012;9:32. doi: 10.1186/1742-4755-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bateganya M, Abdulwadud OA, Kiene SM. Home-based HIV voluntary counselling and testing (VCT) for improving uptake of HIV testing. Cochrane Database Syst Rev. 2010:CD006493. doi: 10.1002/14651858.CD006493.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Negin J, Wariero J, Mutuo P, Jan S, Pronyk P. Feasibility, acceptability and cost of home-based HIV testing in rural Kenya. Trop Med Int Health. 2009;14:849–855. doi: 10.1111/j.1365-3156.2009.02304.x. [DOI] [PubMed] [Google Scholar]
- 20.Naik RTH, Doherty T, Zembe W, Jackson D. Client characteristics and acceptability of a home-based HIV counselling and testing intervention in rural South Africa. BMC Public Health. 2012 Sep 25;12:824. doi: 10.1186/1471-2458-12-824. 2012 Sep 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.JN S, H S, J L, MA M, S A, X Y, et al. High acceptance of home-based HIV counseling and testing in an urban community setting in Uganda. BMC Public Health. 2011 Sep 26;11:730. doi: 10.1186/1471-2458-11-730. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulogo EM, Abdulaziz AS, Guerra R, Baine SO. Facility and home based HIV Counseling and Testing: a comparative analysis of uptake of services by rural communities in southwestern Uganda. BMC Health Serv Res. 2011;11:54. doi: 10.1186/1472-6963-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mlay R, Lugina H, Becker S. Couple counselling and testing for HIV at antenatal clinics: views from men, women and counsellors. AIDS Care. 2008;20:356–360. doi: 10.1080/09540120701561304. [DOI] [PubMed] [Google Scholar]
- 24.Emusu D, Ivankova N, Jolly P, Kirby R, Foushee H, Wabwire-Mangen F, et al. Experience of sexual violence among women in HIV discordant unions after voluntary HIV counselling and testing: a qualitative critical incident study in Uganda. AIDS Care. 2009;21:1363–1370. doi: 10.1080/09540120902883077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harling G, Msisha W, Subramanian SV. No association between HIV and intimate partner violence among women in 10 developing countries. PLoS One. 2010;5:e14257. doi: 10.1371/journal.pone.0014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunnell RE, Nassozi J, Marum E, Mubangizi J, Malamba S, Dillon B, et al. Living with discordance: knowledge, challenges, and prevention strategies of HIV-discordant couples in Uganda. AIDS Care. 2005;17:999–1012. doi: 10.1080/09540120500100718. [DOI] [PubMed] [Google Scholar]
- 27.Grinstead OA, Gregorich SE, Choi KH, Coates T Group VH-CaTES. Positive and negative life events after counselling and testing: the Voluntary HIV-1 Counselling and Testing Efficacy Study. AIDS. 2001;15:1045–1052. doi: 10.1097/00002030-200105250-00013. [DOI] [PubMed] [Google Scholar]
- 28.Orne-Gliemann J, Balestre E, Tchendjou P, Miric M, Darak S, Butsashvili M, et al. Increasing HIV testing among male partners. The Prenahtest ANRS 12127 multi-country randomised trial. AIDS. 2013 [Google Scholar]
- 29.Dalal W, Feikin DR, Amolloh M, Ransom R, Burke H, Lugalia F, et al. Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr. 2013;62:e47–e54. doi: 10.1097/QAI.0b013e318276bea0. [DOI] [PubMed] [Google Scholar]
- 30.Aluisio A, Richardson BA, Bosire R, John-Stewart G, Mbori-Ngacha D, Farquhar C. Male antenatal attendance and HIV testing are associated with decreased infant HIV infection and increased HIV-free survival. J Acquir Immune Defic Syndr. 2011;56:76–82. doi: 10.1097/QAI.0b013e3181fdb4c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Rooyen H, Barnabas RV, Baeten JM, Phakathi Z, Joseph P, Krows M, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumwebaze H, Tumwesigye E, Baeten JM, Kurth AE, Revall J, Murnane PM, et al. Household-based HIV counseling and testing as a platform for referral to HIV care and medical male circumcision in Uganda: a pilot evaluation. PLoS One. 2012;7:e51620. doi: 10.1371/journal.pone.0051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munjoma MW, Mhlanga FG, Mapingure MP, Kurewa EN, Mashavave GV, Chirenje MZ, et al. The incidence of HIV among women recruited during late pregnancy and followed up for six years after childbirth in Zimbabwe. BMC Public Health. 2010;10:668. doi: 10.1186/1471-2458-10-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baeten J, Celum C. Oral antiretroviral chemoprophylaxis: current status. Curr Opin HIV AIDS. 2012;7:514–519. doi: 10.1097/COH.0b013e3283582d30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25:1887–1895. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahuerta M, Lima J, Nuwagaba-Biribonwoha H, Okamura M, Alvim MF, Fernandes R, et al. Factors associated with late antiretroviral therapy initiation among adults in Mozambique. PLoS One. 2012;7:e37125. doi: 10.1371/journal.pone.0037125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ndawinz JD, Chaix B, Koulla-Shiro S, Delaporte E, Okouda B, Abanda A, et al. Factors associated with late antiretroviral therapy initiation in Cameroon: a representative multilevel analysis. J Antimicrob Chemother. 2013 doi: 10.1093/jac/dkt011. [DOI] [PubMed] [Google Scholar]
- 39.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiarie JN, Farquhar C, Richardson BA, Kabura MN, John FN, Nduati RW, et al. Domestic violence and prevention of mother-to-child transmission of HIV-1. AIDS. 2006;20:1763–1769. doi: 10.1097/01.aids.0000242823.51754.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenya Demographic and Health Survey 2008/09. Calverton, Maryland: Kenya National Bureau of Statistics (KNBS) and ICF Macro; 2010. Macro. KNBoSKaI. In; 2010. [Google Scholar]