Abstract
While peripheral insulin resistance is common during obesity and aging in mice and people, the progression to type 2 diabetes (T2D) is largely due to loss of β-cell mass and function through apoptosis. We recently reported that genistein, a soy derived isoflavone, can improve glycemic control and β-cell function in insulin-deficient diabetic mice. However, whether it can prevent β-cell loss and diabetes in T2D mice is unknown. Our current study aimed to investigate the effect of dietary supplemented genistein in a nongenetic T2D mouse model. Nongenetic, middle-aged obese diabetic mice were generated by high fat diet and a low dose of streptozotocin injection. The effect of dietary supplementation of genistein on glycemic control and β-cell mass and function was determined. Dietary intake of genistein (250 mg·kg–1 diet) improved hyperglycemia, glucose tolerance, and blood insulin level in obese diabetic mice, whereas it did not affect body weight gain, food intake, fat deposit, plasma lipid profile, and peripheral insulin sensitivity. Genistein increased the number of insulin-positive β-cell in islets, promoted islet β-cell survival, and preserved islet mass. In conclusion, dietary intake of genistein could prevent T2D via a direct protective action on β-cells without alteration of periphery insulin sensitivity.
Keywords: genistein, type 2 diabetes, β-cells, mice, high fat diet, streptozotocin
Introduction
Type 2 diabetes (T2D) is a result of chronic insulin resistance and loss of β-cell mass and function (Stoffers 2004). Both in experimental animals and people, obesity is a leading pathogenic factor for developing insulin resistance. Constant insulin resistance will progress to T2D when β-cells are unable to secret adequate amount of insulin to compensate for decreased insulin sensitivity, which is largely due to insulin secretory dysfunction and significant loss of functional β-cells (Kahn et al. 2006). Indeed, those individuals with T2D always manifest increased β-cell apoptosis and reduced β-cell mass (Butler et al. 2003). As such, the search for novel agents that simultaneously promote insulin sensitivity and β-cell survival may provide a more effective strategy to prevent the onset of diabetes.
Genistein is widely used as a dietary supplement in the United States for various presumed health benefits (Si and Liu 2008), although the research evidence supporting the beneficial effects of genistein consumption on human health is not well established. Genistein intake is considered safe as no toxic effects were observed in monkeys (Anthony et al. 1996) and humans (Bloedon et al. 2002) following pharma cological administration, although genistein may have weak estrogenic or anti-estrogenic effects in some tissues by primarily binding to estrogen receptor-β (Kuiper et al. 1997). Genistein has been previously investigated for its potential beneficial effects on cancer treatment, cognitive function, and cardiovascular and skeletal health, with a primary focus on exploring its potential hypolipidemic, anti-oxidative, and estrogenic effects (Si and Liu 2008). While studies on whether genistein has an effect on diabetes are very limited, available data showed that administration of genistein moderately lowered plasma glucose in diabetic patients (Villa et al. 2009) without affecting insulin sensitivity and fat metabolism. Consistently, dietary supplementation of isoflavones has been shown to improve insulin secretion and reduce serum glucose levels in chemically induced diabetic rats (Lu et al. 2008). However, the mechanism of this genistein action is unknown. There is increasing evidence that shows that oxidative stress and reactive oxygen species (ROS) play a potential role in the initiation of diabetes (Lankin et al. 2005). Genistein has been reported to exhibit antioxidant activity (Ruiz-Larrea et al. 1997). However, this effect of genistein is achieved only at concentrations ranging from 25–100 μmol·L–1, while the achievable levels of plasma genistein in humans through dietary ingestion of genistein or soy-based diet is always less than 10 μmol·L–1 (Xu et al. 1995).
We recently discovered for the first time that genistein at physiologically achievable concentrations (0.1–5 μmol·L–1) activated cAMP/PKA signaling by stimulating adenylate cyclase activity in β-cells and islets (Liu et al. 2006). We further found that dietary intake of genistein improved pancreatic β-cell proliferation and survival and prevented diabetes in insulin-deficient type 1 diabetic (T1D) mice (Fu et al. 2010). Because loss of functional β-cell mass and its progressive dysfunction are hallmarks in the pathogenesis of T2D (Weir and Bonner-Weir 2004), we tested in the present study the hypothesis that genistein can also protect pancreatic islets from apoptosis and thereby prevent T2D. In that regard, we gave genistein to nongenetic, middle-aged diabetic mice that were generated by high fat feeding and a low dose of STZ that did not cause diabetes in chow-fed mice. We show that dietary intake of genistein ameliorated hyperglycemia and improved β-cell mass in middle-aged T2D mice, which were associated with improved pancreatic β-cell survival and circulating insulin levels.
Materials and methods
Animals and study design
Ten-month-old male C57BL/6 mice (NCI, Frederick, Md., USA) were housed individually on a 12-h light / 12-h dark cycle with free access to food and water. Mice were divided into 4 groups with 8 mice per group and fed either a standard diet (STD) with 10% of calories derived from fat, or a high fat diet (HF; Research Diets Inc., New Brunswick, N.J., USA) with 60% of calories from fat, or a HF diet containing 250 mg genistein·kg–1 diet (genistein, LC Laboratory Inc., Woburn, Mass., USA; Table 1). After 4 weeks of dietary treatment, mice were intraperitoneally injected with a single dose of streptozotocin (90 mg·kg–1; Sigma–Aldrich, St. Louis, Mo., USA) or vehicle. Fasting blood glucose was measured once every other week. Food intake and body weight were monitored weekly. Kidney, liver, pancreas, heart, spleen, and abdominal subcutaneous fat pad were weighed after mice were euthanized.
Table 1.
Formula for standard diet (STD), high fat diet (HF), and high fat diet supplemented with genistein (HF-GE).
| STD |
HF |
HF-GE |
||||
|---|---|---|---|---|---|---|
| gm% | kcal% | gm% | kcal% | gm% | kcal% | |
| Casein, 80 Mesh | 19.0 | 19.7 | 25.8 | 19.7 | 25.8 | 19.7 |
| l-Cystine | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 |
| Corn starch | 29.9 | 31.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Maltodextrin 10 | 3.3 | 3.5 | 16.2 | 12.3 | 16.1 | 12.3 |
| Sucrose | 33.2 | 34.5 | 8.9 | 6.8 | 8.9 | 6.8 |
| Cellulose, BW200 | 4.7 | 0.0 | 6.5 | 0.0 | 6.5 | 0.0 |
| Corn Oil | 2.4 | 5.5 | 3.2 | 5.5 | 3.2 | 5.5 |
| Lard | 1.9 | 4.4 | 31.7 | 54.4 | 31.7 | 54.4 |
| Mineral Mix, S10026 | 0.9 | 0.0 | 1.3 | 0.0 | 1.3 | 0.0 |
| DiCalcium Phosphate | 1.2 | 0.0 | 1.7 | 0.0 | 1.7 | 0.0 |
| Calcium carbonate | 0.5 | 0.0 | 0.7 | 0.0 | 0.7 | 0.0 |
| Potassium citrate, 1 H2O | 1.6 | 0.0 | 2.1 | 0.0 | 2.1 | 0.0 |
| Vitamin Mix, V10001 | 0.9 | 1.0 | 1.3 | 1.0 | 1.3 | 1.0 |
| Choline Bitartrate | 0.2 | 0.0 | 0.3 | 0.0 | 0.3 | 0.0 |
| Genistein | 0.0 | 0.0 | 0.0 | 0.0 | 0.025 | 0.0 |
| Protein | 19 | 20 | 26 | 20 | 26 | 20 |
| Carbohydrate | 67 | 70 | 26 | 20 | 26 | 20 |
| Fat | 4 | 10 | 35 | 60 | 35 | 60 |
Note: The amount of calories for STD was 3.8 kcal·g−1, HF was 5.2 kcal·g−1, and HF-GE was 5.2 kcal·g−1. The amount genistein in STD and HF was 0 mg·kg−1, and in HF-GE was 250 mg·kg−1.
Animal procedures performed in this study were reviewed and approved by the Institutional Animal Care and Use Committee at Virginia Tech.
Fasting blood glucose, glucose tolerance test (GTT), and insulin tolerance test (ITT)
Fasting blood glucose was measured from whole blood attained via tail vein after overnight fasting using an ultra-sensitive hand-held glucometer (The Kroger Co., Cincinnati, Ohio, USA). For glucose tolerance tests, mice were fasted overnight and intraperitoneally injected with a single bolus of glucose (2 g·kg–1 body weight). Glucose levels were measured at 0, 15, 30, 60, and 120 min after glucose administration. For ITT, mice were intraperitoneally injected with insulin (0.75 units·kg–1 body weight), and blood glucose levels were measured before and at 15, 30, 60, and 120 min after insulin administration as described previously (Fu et al. 2010).
Blood lipid profile and insulin
Blood total cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglycerides were measured using plasma using a CardioChek blood analyzer (Polymer Technology Systems, Indianapolis, Ind., USA). The low-density lipoprotein (LDL)-cholesterol levels were calculated using the Friedewald equation: LDL-cholesterol = total cholesterol – (HDL-cholesterol + triglycerides/5). Insulin levels in plasma were measured using a mouse insulin ELISA kit (Mercodia, Inc., Uppsala, Sweden).
Pancreatic β-cell mass and apoptosis
Pancreata were collected and weighed from animals after euthanasia. Pancreas samples were embedded in paraffin and sectioned by AML Laboratories Inc. (Baltimore, Md., USA). A series of tissue sections (5-μm thickness) were prepared, mounted on glass slides, and immunofluorescently stained with an insulin antibody and FITC-conjugated secondary antibody (Abcam, Cambridge, Mass., USA) for determining β-cell mass. Pancreatic β-cell area was measured using images acquired from 5 serial insulin-stained pancreatic sections sampled at 2.5 mm intervals. Pancreatic β-cell mass was calculated by dividing the area of insulin-positive cells by the total area of pancreatic tissue and multiplied by the pancreas weight (Fu at al. 2010, Xu et al. 1999). Average was obtained from 8 mice in each group. Apoptotic β-cells were labeled with an antibody against activated caspase-3 followed by detection with a streptavidin-biotin immunoenzymatic antigen system (Abcam, Cambridge). Five pancreatic sections from each mouse with 8 mice per group were co-stained with activated caspase-3 and insulin.
Statistical analysis
Data were analyzed with 1-way ANOVA using the mixed models procedure of SAS (SAS Inc., Cary, N.C., USA). The statistical model included the main effects of food and water intake, blood glucose, and body weight. Significant treatment differences were subjected to Tukey's test. A p value <0.05 was considered significant.
Results
Food intake, body, and organ weights
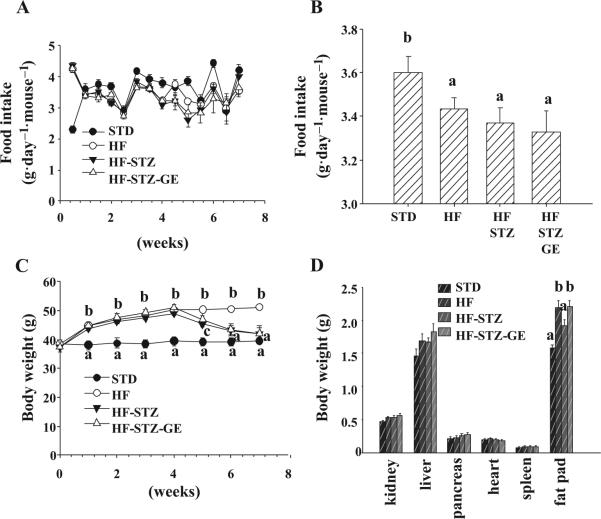
Genistein supplementation (~0.7–0.9 mg·day–1·mouse–1 genistein except for the first adaptation week) did not alter food consumption pattern compared with HF diet-fed mice before or after STZ injection. The high fat diet decreased the accumulative average food intake, though it increased food consumption at the first week (Figs. 1A and 1B). Four weeks of consuming HF diet significantly increased body weight of mice. However, dietary intake of genistein at 250 mg·kg–1 diet had no effect on body weight gain before STZ injection. Injection of STZ resulted in a reduction in body weight. After STZ injection, mice fed genistein supplemented HF diet (HF-STZ-GE mice) were slightly heavier compared with mice on HF diet alone (Fig. 1C).
Fig. 1.
Genistein supplementation had no influence on food consumption, body weight, or major organ weight. Food intake was recorded every 3 or 4 days (A). Cumulative daily food consumption was calculated at the termination of the experiment (B). Body weight was monitored every week (C). Major organs were weighed after mice were sacrificed (D). Data are shown as means ± SE (n = 8 mice per group; groups that are identified by different letters are significantly different, p < 0.05). STD, standard diet; HF, high fat diet; STZ, streptozotocin; GE, genistein.
There was no change in heart, pancreas, and spleen absolute weight. A slight increase in liver and kidney weight was observed in mice on HF diet compared with mice on STD diet but the change was not significant. The abdominal sub-cutaneous fat pad was 50% greater in HF mice, while fat pads of mice in the HF-STZ group was 20% greater than that of STD mice. The fat pad weight of HF-STZ-GE mice was similar to that of HF mice (Fig. 1D).
Fasting blood glucose
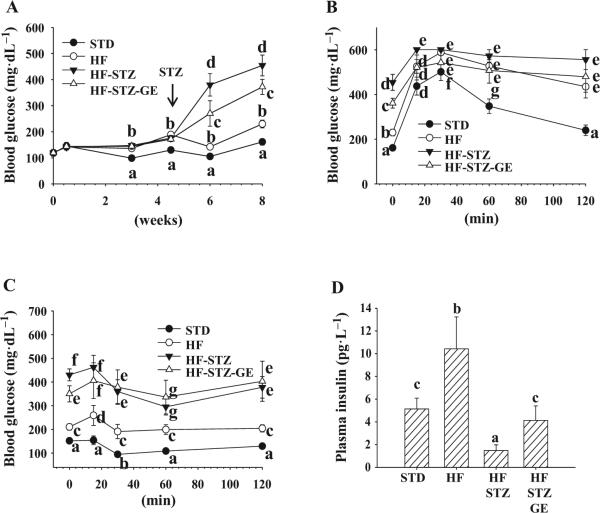
After 4 weeks of HF diet consumption, mice displayed significantly elevated fasting blood glucose concentrations compared with animals that consumed STD diet. However, genistein did not affect HF diet-induced rise in blood glucose. One week after injection of STZ, fasting blood glucose started to rise sharply in HF-STZ mice. In HF-STZ-GE mice blood glucose levels were significantly lower (30% reduction) at this point than in HF-STZ mice (Fig. 2A). Although in the following weeks, fasting blood glucose of animals that received STZ injection continued to rise, blood glucose levels in HF-STZ-GE mice continued to be significantly lower.
Fig. 2.
Genistein supplementation improved glycemic control and insulin levels, but did not affect insulin tolerance. Blood glucose was monitored every other week. Arrow points to the time when STZ injection was started (A). Glucose tolerance (B) and insulin tolerance (C) tests were performed as stated in the Materials and methods section. Plasma insulin levels (D) were measured with an ELISA kit. Data are means ± SE (n = 8 mice per group; groups that are identified by different letters are significantly different, p < 0.05). STD, standard diet; HF, high fat diet; STZ, streptozotocin; GE, genistein.
GTT, ITT, and plasma insulin levels
Two weeks post-STZ-injection, we performed a GTT, and the data showed that blood glucose levels in HF-STZ-GE mice was significantly lower at baseline and 15 min after glucose injection than those in HF-STZ mice; but followed the same pattern after 30 min as HF-STZ mice (Fig. 2B). The results from GTT demonstrated that blood glucose levels of HF-STZ mice at all time points were almost twice that of HF animals (p < 0.05), which were the result of STZ administration. HF-STZ-GE mice had a similar insulin response pattern as HF-STZ mice except a significantly lower (p < 0.05) baseline blood glucose level (Fig. 2C). The plasma insulin levels in HF mice was almost twice as high as mice that received a STD diet (p < 0.05). HF-STZ mice displayed the lowest plasma insulin levels, while HF-STZ-GE mice had plasma insulin concentrations close to those of STD mice and significantly higher than those in HF-STZ mice (p < 0.05) (Fig. 2D).
Blood lipid profile
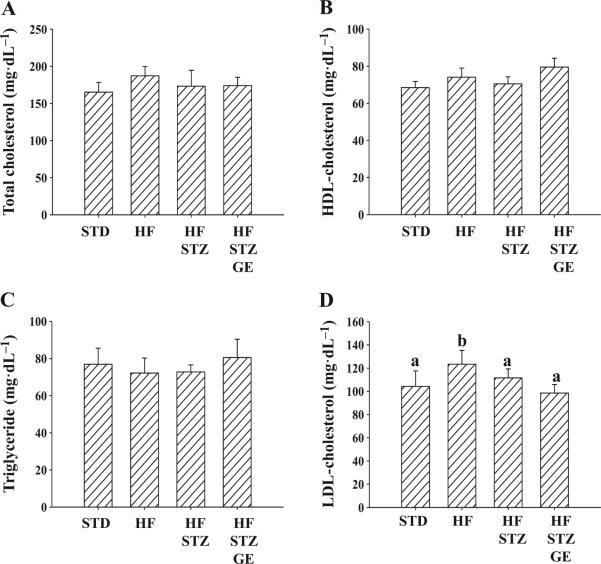
Whole blood was collected from overnight-fasted animals for measurement of total cholesterol, HDL-cholesterol, triglyceride, plasma insulin, and then calculation of LDL-cholesterol. HF mice had slightly higher total cholesterol but not significant and significantly higher LDL-cholesterol. HF-STZ and HF-STZ-GE mice had similar levels of total cholesterol (Fig. 3A), HDL-cholesterol (Fig. 3B), triglyceride (Fig. 3C), and LDL-cholesterol (Fig. 3D) as STD mice.
Fig. 3.
Genistein supplementation did not affect blood lipid profile. At the end of the eighth week of the experiment, fasting plasma total cholesterol (A), HDL-cholesterol (B), and triglyceride (C) were measured. Low-density lipoprotein (LDL)-cholesterol levels (D) were calculated based on total cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglyceride. Data are shown as means ± SE (n = 8 mice per group; groups that are identified by different letters are significantly different, p < 0.05). STD, standard diet; HF, high fat diet; STZ, streptozotocin; GE, genistein.
Pancreatic β-cell mass and apoptosis
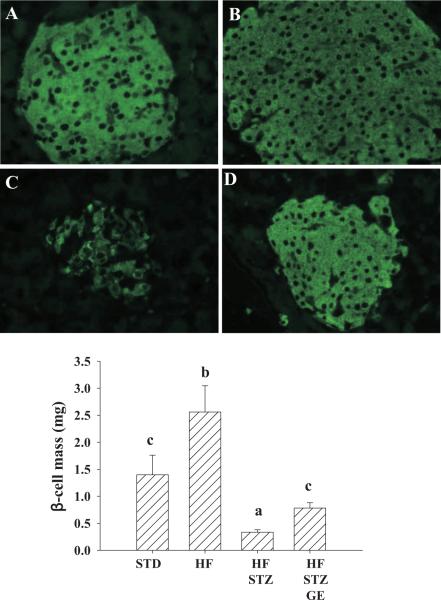
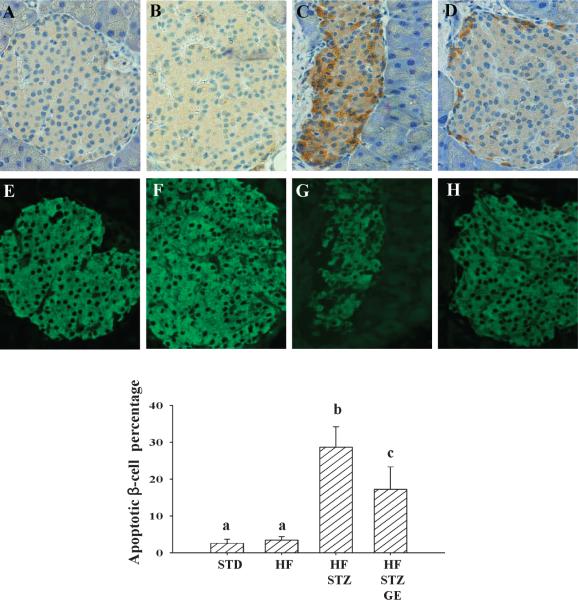
We evaluated pancreatic islet β-cell mass through immunohistochemical technique. We found that consumption of the HF diet dramatically increased β-cell mass, while STZ administration severely decreased β-cell mass compared with mice on STD diet. However, dietary provision of genistein significantly improved islet β-cell mass compared with HFSTZ mice (Fig. 4). In both STD and HF mice, apoptosis was rare. However, almost 30% of β-cells were caspase-3 positive in pancreatic islets of HF-STZ mice. HF-STZ-GE mice have significantly lower (p < 0.05) β-cell apoptosis as compared with that in islets from HF-STZ mice (29% ± 5.6% vs. 17% ± 6.1%) (Fig. 5).
Fig. 4.
Genistein supplementation improved β-cell mass. A, B, C, and D (40× magnification) represent pancreatic sections from mice receive a STD (A), HF (B), HF and STZ administration (C), and genistein supplemented HF and STZ administration (D), respectively. Data are shown as means ± SE. (n = 8 mice per group; groups that are identified by different letters are significantly different, p < 0.05). STD, standard diet; HF, high fat diet; STZ, streptozotocin; GE, genistein.
Fig. 5.
Genistein supplementation reduced apoptosis of pancreatic β-cells. A, B, C, and D (40× magnification) are representative pancreatic sections stained with activated caspase-3 from mice received a STD (A), HF (B), HF and STZ administration (C), and genistein supplemented HF and STZ administration (D); E, F, G, and H (40× magnification) are the same sections fluorescently stained with insulin. Data are means ± SE (n = 8 mice per group; groups that are identified by different letters are significantly different, p < 0.05). STD, standard diet; HF, high fat diet; STZ, streptozotocin; GE, genistein.
Discussion
In humans, insulin resistance is associated with both obesity and T2D. However, most of the individuals with insulin resistance do not develop diabetes because of the compensatory insulin secretion that overcomes the reduced insulin sensitivity in peripheral tissues (Kahn et al. 1993). Therefore, those individuals with constant insulin resistance will progress to T2D only when extensive β-cell destruction occurs and residual β-cells are unable to meet the demands of the increased insulin requirement (Marchetti et al. 2004). Indeed, even though compensatory insulin secretion is capable of maintaining blood glucose homeostasis, β-cell damage reportedly already exists in the individuals at high risk for developing T2D, and those individuals with T2D always manifest increased β-cell apoptosis and reduced β-cell mass (Marchetti et al. 2004). As such, the search for novel agents that promote β-cell survival and thereby preserve functional β-cell mass may provide an effective strategy to prevent the onset of diabetes. In the present study, we determined whether genistein has a protective effect on β-cells in a rodent T2D model.
We show that genistein (250 mg·kg–1 diet) significantly prevented the development of diabetes and improved pancreatic islet mass in a nongenetic mouse model of T2D, which were generated by HF feeding and a mild dose of STZ administration that did not cause diabetes in chow-fed mice (data not shown). We used C57BL6 mice near 1 year old, the mouse equivalent of middle age in humans, because T2D usually occurs at middle and older age in humans. This mouse model shares the metabolic characteristics of human T2D with peripheral insulin resistance and reduced β-cell mass and function (Mu et al. 2006). The use of a low dose of STZ also minimizes the variability of diet-induced diabetes development and thus provides better experimental controls for evaluating the anti-diabetic effects of this compound. The anti-diabetic effect of genistein observed in this study might be relevant to humans, because this dose of genistein used in the present study (equivalent to the human intake of 75–100 mg·day–1) is within the dose range typically consumed by humans (Teede et al. 2001).
One study reported an improved lipid profile after genistein consumption in obese Zucker rats, which could result in an improvement in insulin sensitivity (Mezei et al. 2003). Thus, we might expect that supplemental genistein in a HF diet may ameliorate lipid profile and therefore influence insulin sensitivity lower HF diet-induced hyperglycemia. However, insulin sensitivity was not modulated by the presence of genistein in the diet for 4 weeks. At the fourth week of consuming HF diet, we did observe a significant increase in fasting plasma cholesterol, blood glucose, and insulin levels in HF mice, which implies the existence of insulin resistance, consistent with the higher body weight observed in these mice. Excess fat deposit in adipocytes plays critical roles in the development of insulin resistance (Steppan et al. 2001). In this study the majority of weight gain in HF diet-fed mice came from adipose tissue as shown by the organ fat pad weight data. However, genistein supplemented in HF diet did not cause any change either in body weight gain, fat mass, or fasting blood glucose after 4 weeks of genistein supplementation. In addition, identical food intake between HF mice and HF-STZ-GE mice suggested unchanged appetite and metabolic rate. These experimental results provide further evidence that genistein had no effect on energy metabolism and insulin sensitivity, which are in line with our recent finding in insulin-deficient diabetic mice (Fu et al. 2010).
We used STZ to induce diabetes by directly causing destruction of β-cells. As expected, STZ-injected mice had a marked increase in fasting blood glucose and started to lose body weight the week after STZ administration. Genistein supplementation significantly lowered fasting blood glucose compared with HF-STZ-treated mice. The results from ITT showed that genistein-supplemented animals had significantly lower baseline blood glucose, while during the time after insulin injection their blood glucose fell to similar levels as HF-STZ mice. These data further confirm that genistein had no significant effect on insulin sensitivity in HF-STZ mice.
To further confirm the effect of genistein on lipid profiles, we measured total cholesterol, triglyceride, LDL-cholesterol, and calculated LDL-cholesterol after mice were sacrificed. All the lipid parameters remained the same between HF-STZ and HF-STZ-GE mice. While mice consumed HF diet had significantly higher LDL-cholesterol and slightly higher total cholesterol. These cholesterol numbers are lowered in all of the mice that received STZ administration regardless of genistein supplementation, which could result from the reduction in fat mass and insulin caused by STZ injection (Maggi et al. 2001).
Fasting blood glucose is derived primarily from hepatic gluconeogenesis. Thus, excessive hepatic glucose output is an important factor contributing to fasting hyperglycemia (Consoli et al. 1989). A recent study demonstrated that dietary provision of genistein elevated blood insulin levels and suppressed hepatic gluconeogenesis enzyme activities in non-obese diabetic (NOD) mice (Choi et al. 2008), suggesting that genistein may improve hyperglycemia partially through inhibition of hepatic gluconeogenesis. However, the effect of genistein on hepatic gluconeogenesis enzymes could be due to a secondary action whereby genistein induces or preserves pancreatic β-cell insulin secretion (Fu et al. 2010; Liu et al. 2006), given that insulin is required for regulating hepatic gluconeogenesis (Moore et al. 1998). In the present study, our results suggest that the improvement in fasting blood glucose by genistein treatment is primarily due to the improved pancreatic insulin secretion as demonstrated by significantly higher levels of circulating insulin levels in genistein-treated diabetic mice, because STZ induces diabetes by directly causing β-destruction and insulin deficiency. We further showed that dietary supplementation of genistein partially prevented islet β-cells from apoptosis, which may primarily contribute to improved β-cell mass and insulin secretion in diabetic mice.
The glucose analogue STZ is reported to be transported into β-cells by the glucose transporter 2 (GLUT2) for exerting its apoptotic effect (Wang and Gleichmann 1998). While we did not study the effect of genistein on GLUT2 protein expression in mouse islets, our recent studies found that genistein had no such an effect in cultured β-cells (Fu and Liu 2009), suggesting that improvement of islet β-cell mass and survival by genistein may not be due to modulation of GLUT2 expression, thereby preventing STZ influx in β-cells. Some studies showed that STZ increased peripheral lymphocytic infiltration into islets by stimulating the production of several pro-inflammatory cytokines, thereby producing insulitis, which may contribute to STZ-induced β-cell apoptosis and diabetes. However, we showed that genistein had no effect on mononuclear cell infiltration into the islets (Fu et al. 2010). Indeed, the mouse strain used in this study is reportedly resistant to STZ-induced insulitis as STZ-caused infiltration of immune cells into islets is very rare in these mice (Leiter 1982)
We recently discovered for the first time that genistein at physiologically achievable concentrations (0.1–5 μmol·L–1) activated cAMP/PKA signaling by stimulating adenylate cyclase activity in β-cells and islets (Liu et al. 2006). Several factors protect pancreatic β-cells from apoptosis by activating the cAMP/PKA pathway (Granata et al. 2008). While we did not measure whether dietary intake of genistein affects the cAMP signaling pathway in the islets in vivo because it is difficult to isolate adequate amount of islets for this study in STZ-induced diabetic mice, it is tempting to speculate that genistein might protect islets from apoptosis through activation of this signaling pathway.
In summary, using a middle-aged T2D mouse model, we provide evidence that genistein as the form of dietary supplement ameliorates hyperglycemia. This anti-diabetic action of genistein is not mediated through improving insulin sensitivity, but rather was due to protecting pancreatic β-cell from apoptosis and preserving functional β-cell mass. Loss of functional β-cell mass is the key for deterioration of glycemic control in both T1D and T2D. In this context, genistein may be a naturally occurring, low-cost agent that can be used as an alternative or complementary treatment for diabetes. However, more studies are needed to further characterize the potential anti-diabetic effect of this compound and to define the cellular and molecular mechanisms underlying this effect.
Acknowledgements
This work was supported by grants from the American Diabetes Association Awards (1-08-JF-30, 7-11-BS-84 to D.L.), National Center for Complementary and Alternative Medicine of National Institute of Health (1R21AT004694 to D.L.), Virginia Commonwealth Health Research Board (to D.L.), and a predoctoral fellowship award (to Z.F.) from the American Heart Associate Mid-Atlantic Affiliate. The sponsors played no role in this study or the decision to submit the manuscript for publication.
References
- Anthony MS, Clarkson TB, Hughes CL, Jr, Morgan TM, Burke GL. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys. J. Nutr. 1996;126(1):43–50. doi: 10.1093/jn/126.1.43. PMID: 8558324. [DOI] [PubMed] [Google Scholar]
- Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM, Dix KJ, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am. J. Clin. Nutr. 2002;76(5):1126–1137. doi: 10.1093/ajcn/76.5.1126. PMID: 12399289. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. doi:10.2337/diabetes.52.1.102. PMID:12502499. [DOI] [PubMed] [Google Scholar]
- Choi MS, Jung UJ, Yeo J, Kim MJ, Lee MK. Genistein and daidzein prevent diabetes onset by elevating insulin level and altering hepatic gluconeogenic and lipogenic enzyme activities in non-obese diabetic (NOD) mice. Diabetes Metab. Res. Rev. 2008;24(1):74–81. doi: 10.1002/dmrr.780. doi:10.1002/dmrr.780. PMID:17932873. [DOI] [PubMed] [Google Scholar]
- Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38(5):550–557. doi: 10.2337/diab.38.5.550. doi:10.2337/diabetes.38.5.550. PMID:2653926. [DOI] [PubMed] [Google Scholar]
- Fu Z, Liu D. Long-term exposure to genistein improves insulin secretory function of pancreatic beta-cells. Eur. J. Pharmacol. 2009;616(1–3):321–327. doi: 10.1016/j.ejphar.2009.06.005. doi:10.1016/j.ejphar.2009.06.005. PMID:19540219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, et al. Genistein induces pancreatic beta-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151(7):3026–3037. doi: 10.1210/en.2009-1294. doi:10.1210/en.2009-1294. PMID:20484465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata R, Settanni F, Gallo D, Trovato L, Biancone L, Cantaluppi V, et al. Obestatin promotes survival of pancreatic beta-cells and human islets and induces expression of genes involved in the regulation of beta-cell mass and function. Diabetes. 2008;57(4):967–979. doi: 10.2337/db07-1104. doi:10.2337/db07-1104. PMID: 18162507. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. doi:10.2337/diabetes.42.11.1663. PMID:8405710. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. doi:10.1038/nature05482. PMID:17167471. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. doi:10. 1210/en.138.3.863. PMID:9048584. [DOI] [PubMed] [Google Scholar]
- Lankin VZ, Lisina MO, Arzamastseva NE, Konovalova GG, Nedosugova LV, Kaminnyi AI, et al. Oxidative stress in atherosclerosis and diabetes. Bull. Exp. Biol. Med. 2005;140(1):41–43. doi: 10.1007/s10517-005-0406-z. doi:10.1007/s10517-005-0406-z. PMID:16254616. [DOI] [PubMed] [Google Scholar]
- Leiter EH. Multiple low-dose streptozotocin-induced hyper-glycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc. Natl. Acad. Sci. U.S.A. 1982;79(2):630–634. doi: 10.1073/pnas.79.2.630. doi:10.1073/pnas.79.2.630. PMID:6210909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes. 2006;55(4):1043–1050. doi: 10.2337/diabetes.55.04.06.db05-1089. doi:10.2337/diabetes.55.04.06.db05-1089. PMID:16567527. [DOI] [PubMed] [Google Scholar]
- Lu MP, Wang R, Song X, Chibbar R, Wang X, Wu L, Meng QH. Dietary soy isoflavones increase insulin secretion and prevent the development of diabetic cataracts in streptozotocin-induced diabetic rats. Nutr. Res. 2008;28(7):464–471. doi: 10.1016/j.nutres.2008.03.009. doi:10.1016/j.nutres.2008.03.009. PMID:19083447. [DOI] [PubMed] [Google Scholar]
- Maggi S, Minicuci N, Harris T, Motta L, Baldereschi M, Di Carlo A, et al. High plasma insulin and lipids profile in older individuals: the Italian longitudinal study on aging. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56(4):M236–M242. doi: 10.1093/gerona/56.4.m236. doi:10.1093/gerona/56.4.M236. PMID:11283197. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J. Clin. Endocrinol. Metab. 2004;89(11):5535–5541. doi: 10.1210/jc.2004-0150. doi:10.1210/jc.2004-0150. PMID:15531508. [DOI] [PubMed] [Google Scholar]
- Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003;133(5):1238–1243. doi: 10.1093/jn/133.5.1238. PMID: 12730403. [DOI] [PubMed] [Google Scholar]
- Moore MC, Connolly CC, Cherrington AD. Autoregulation of hepatic glucose production. Eur. J. Endocrinol. 1998;138(3):240–248. doi: 10.1530/eje.0.1380240. doi:10.1530/eje.0.1380240. PMID:9539293. [DOI] [PubMed] [Google Scholar]
- Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, et al. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes. 2006;55(6):1695–1704. doi: 10.2337/db05-1602. doi:10.2337/db05-1602. PMID:16731832. [DOI] [PubMed] [Google Scholar]
- Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997;26(1):63–70. doi: 10.3109/10715769709097785. doi:10.3109/10715769709097785. PMID:9018473. [DOI] [PubMed] [Google Scholar]
- Si H, Liu D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J. Nutr. 2008;138(2):297–304. doi: 10.1093/jn/138.2.297. PMID:18203895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. doi:10.1038/35053000. PMID:11201732. [DOI] [PubMed] [Google Scholar]
- Stoffers DA. The development of beta-cell mass: recent progress and potential role of GLP-1. Horm. Metab. Res. 2004;36(11–12):811–821. doi: 10.1055/s-2004-826168. doi:10.1055/s-2004-826168. PMID:15655713. [DOI] [PubMed] [Google Scholar]
- Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J. Clin. Endocrinol. Metab. 2001;86(7):3053–3060. doi: 10.1210/jcem.86.7.7645. doi:10.1210/jc.86.7.3053. PMID: 11443167. [DOI] [PubMed] [Google Scholar]
- Villa P, Costantini B, Suriano R, Perri C, Macri F, Ricciardi L, et al. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: relationship with the metabolic status. J. Clin. Endocrinol. Metab. 2009;94(2):552–558. doi: 10.1210/jc.2008-0735. doi:10.1210/jc.2008-0735. PMID:19017760. [DOI] [PubMed] [Google Scholar]
- Wang Z, Gleichmann H. GLUT2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47(1):50–56. doi: 10.2337/diab.47.1.50. doi:10. 2337/diabetes.47.1.50. PMID:9421374. [DOI] [PubMed] [Google Scholar]
- Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(Suppl. 3):S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. doi:10.2337/diabetes.53.suppl_3.S16. PMID:15561905. [DOI] [PubMed] [Google Scholar]
- Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. Bioavailability of soybean isoflavones depends upon gut microflora in women. J. Nutr. 1995;125(9):2307–2315. doi: 10.1093/jn/125.9.2307. PMID: 7666247. [DOI] [PubMed] [Google Scholar]
- Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–2276. doi: 10.2337/diabetes.48.12.2270. doi:10. 2337/diabetes.48.12.2270. PMID:10580413. [DOI] [PubMed] [Google Scholar]