Abstract

Readily available triethylammonium 1-(2-oxoindolin-3-ylidene)-2-aroylethanethiolates are efficiently converted into a variety of fully substituted thiophene derivatives by copper(I)-catalyzed denitrogenative reactions with terminal alkynes and N-sulfonyl azides. This new reaction simultaneously installs C–N, C–S, and C–C bonds, allowing direct formation of highly functionalized thiophenes with a wide diversity in substituents in a one-pot manner. A plausible mechanism for the domino process is proposed.
Thiophene derivatives are among the most ubiquitous structural motifs found not only in valuable medicinally active substances but also in a massive range of natural products.1 A variety of synthetic thiophenes have been shown to exhibit important biological activities including anticancer activity2 and protein inhibition.3 Thiophene derivatives have also played a pivotal role in organic material science for several decades owing to their structural rigidity and unique electronic characteristics.4 They can serve as conducting polymers5 and photochromic molecular switches6 and can be widely utilized in research on dyes, liquid crystals, organic field effect transistors, and plastic solar cells.7 In view of their interesting properties, many efforts have been devoted to efficient synthetic approaches to thiophene derivatives, especially polyfunctionalized thiophenes, which have made them more applicable. A survey of the literature shows that two general strategies have been reported including the direct functionalization of the thiophene ring8 and thienannulation of suitable acyclic precursors.9 The latter allows direct formation of thiophenes and their efficient multifunctionalization in particular and thus represents a highly desirable methodology.
On the other hand, 1-sulfonyl-1,2,3-triazoles generated from copper(I)-catalyzed 1,3-dipolar cycloaddition of N-sulfonyl azides with terminal alkynes are valuable building blocks in many fields of application (Figure 1).10 More recently, many groups have focused on in situ generated 1-sulfonyl-1,2,3-triazoles as synthetic precursors to treat with different substrates possessing both electrophilic and nucleophilic character, and a broad variety of functionalized O,N-heterocycles, including azetidines,11 4,5-dihydrofurans,12 pyrroles,13 imidazoles,14 pyrazoles,15 pyridines,16 pyrimidines,17 and other fused skeletons,18 can be accessed through formal [2 + 2]-, [3 + 2]-, and [4 + 2]-cycloadditions of azaheterocumulenes. To the best of our knowledge, a one-pot synthesis of thiophene derivatives via denitrogenative transannulation of 1-sulfonyl triazoles in situ generated from terminal alkynes and N-sulfonyl azides has not been reported so far. Here, we report this interesting transformation. The present method would enable in situ generation of reactive azaheterocumulenes D, thus allowing efficient synthesis of functionalized thiophenes by a copper(I)-catalyzed formal [3 + 1 + 1]-cycloaddition of a preformed triethylammonium 1-(2-oxoindolin-3-ylidene)-2-aroylethanethiolate 3 with readily available N-sulfonyl azides 4 and terminal alkynes 5 in a one-pot manner.
Figure 1.
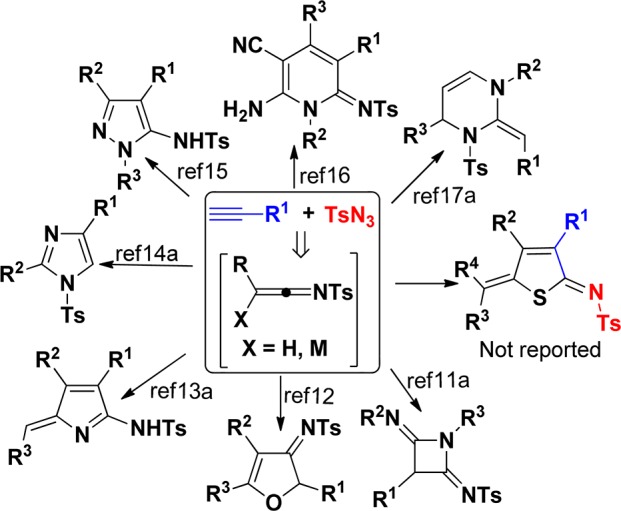
Synthesis of O,N-heterocycles from N-sulfonyl azides with terminal alkynes.
Scheme 1. Synthesis of Functionalized Thiophenes.
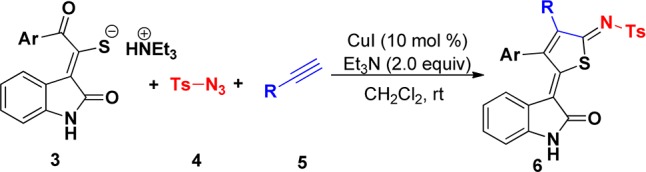
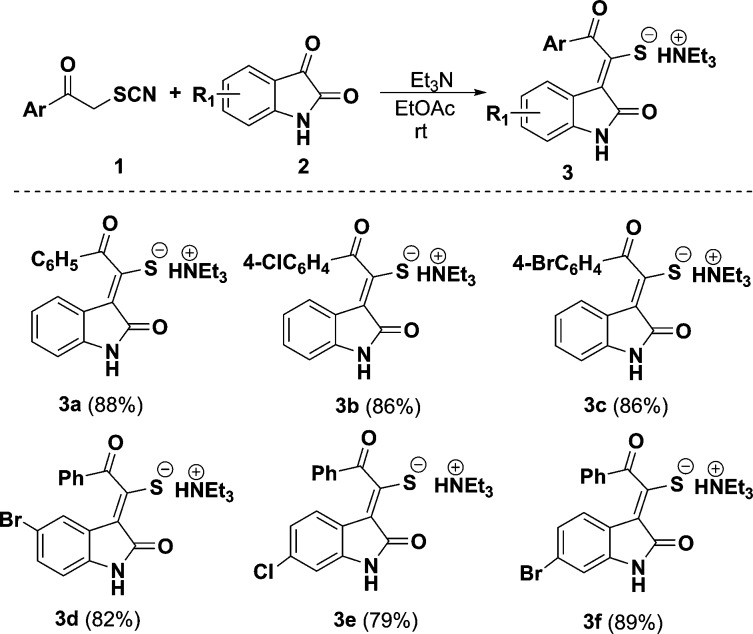
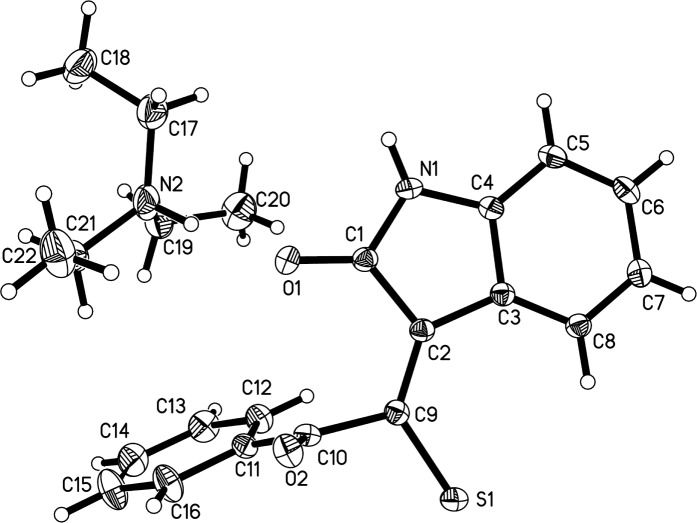
α-Thiocyanate ketones are readily available and highly reactive reagents, which have be applied to highly valuable molecules in recent years.19 In our initial experiments, six examples of triethylammonium 1-(2-oxoindolin-3-ylidene)-2-aroylethanethiolate 3a–f with 79–89% chemical yields were first synthesized through the reaction of α-thiocyanate ketones 1 with indoline-2,3-diones 2 in ethyl acetate using a Et3N base promoter at room temperature (Scheme 2). The structure of 3a was determined by X-ray diffraction analysis (Figure 2).
Scheme 2. Synthesis of Compounds 3.
Figure 2.
ORTEP drawing of 3a.
Next, we attempted a three-component reaction of a preformed precursor 3a with N-sulfonyl azides 4 and ethynylbenzene 5a in an equivalent molecular ratio in the presence of various Cu(I) catalysts (10 mol %) and Et3N (2.0 equiv) at ambient temperature using CH3CN as a solvent (Table 1, entries 1–3). It was anticipated that the attack of the thiol anion of precursor 3 on the in situ generated azaheterocumulenes D would form a sulfonamide anion intermediate;13−17 subsequent negative transfer and cyclization would result in thiophenes 6 (see Scheme 5). After workup, the expected thiophene product 6a was indeed isolated in moderate yields under these conditions, although these reactions run rather sluggishly. As shown in Table 1, CuI catalyst worked more efficiently, although the yield did not exceed 60%. Attempts to employ two other Cu catalysts such as CuBr (28%) and CuCl (32%) were unsatisfactory. Subsequently, the dosages of Et3N and CuI were examined. After optimization, the use of 10 mol % of CuI and 2.0 equiv of Et3N gave the most promising results. We then investigated the effect of different solvents including N,N-dimethylformamide (DMF), toluene, CH2Cl2, 1,4-dioxane, EtOH, and CH2Cl2 was found to be the best solvent for this three-component reaction, providing 61% yield of thiophene product 6a. Additionally, the identical reaction catalyzed by CuI was performed in CH2Cl2 at 40 °C, affording a lower yield of 6a (entry 13).
Table 1. Optimization for the Synthesis of 6.

| entry | cat. (mol %) | Et3N (equiv) | solvent | temp (°C) | yielda (%) |
|---|---|---|---|---|---|
| 1 | CuI (10) | 2 | CH3CN | rt | 59 |
| 2 | CuCl (10) | 2 | CH3CN | rt | 32 |
| 3 | CuBr (10) | 2 | CH3CN | rt | 28 |
| 4 | CuI (10) | 1 | CH3CN | rt | 19 |
| 5 | CuI (10) | 3 | CH3CN | rt | 44 |
| 6 | CuI (5) | 2 | CH3CN | rt | 27 |
| 7 | CuI (15) | 2 | CH3CN | rt | 25 |
| 8 | CuI (10) | 2 | DMF | rt | 40 |
| 9 | CuI (10) | 2 | toluene | rt | 55 |
| 10 | CuI (10) | 2 | CH2Cl2 | rt | 61 |
| 11 | CuI (10) | 2 | 1,4-dioxane | rt | 24 |
| 12 | CuI (10) | 2 | EtOH | rt | trace |
| 13 | CuI (10) | 2 | CH2Cl2 | 40 | 39 |
Isolated yield.
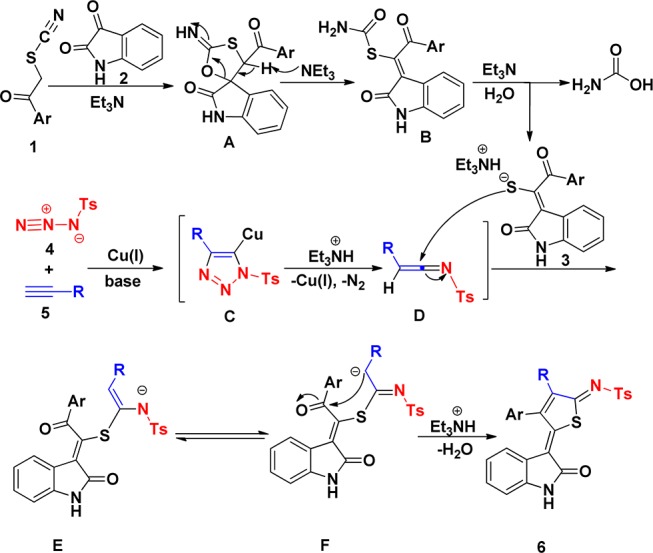
Scheme 5. Mechanism Hypothesis for Forming 6.
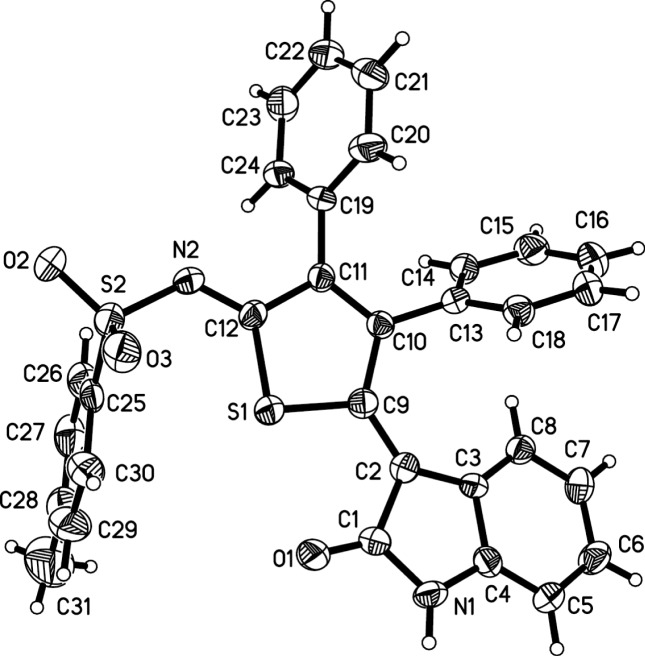
Having identified this acceptable optimization, we began to investigate the scope of the reaction. As shown in Scheme 3, arylacetylenes reacted smoothly with 3a and 5 to afford the corresponding thiophenes in 42–67% yield (6a–e), allowing Cl, methyl, and tert-butyl substituents on the phenyl group to be tolerated. Moreover, hexylalkyne 5f underwent this reaction efficiently with 3a and 4, furnishing the corresponding n-butyl-substituted thiophenes 6f in 45% yield. However, 2-ethynylpyridine, ethynyltrimethylsilane, and methyl propiolate did not work in this reaction system (Scheme 4). To further expand the synthetic utility of this transformation, we next examined the scope of various substituents of triethylammonium 1-(2-oxoindolin-3-ylidene)-2-aroylethanethiolate (Scheme 3). Gratifyingly, substrates possessing electron-neutral and electron-deficient aryl groups showed similar efficiency in this three-component reaction. Similarly, various substituents at the C-5 and C-6 positions of indole ring also participated in this reaction, affording the corresponding thiophenes 6 in moderate to good yields (Scheme 3). The structure of 6a was also determined by X-ray diffraction analysis (Figure 3).
Scheme 3. Domino Synthesis of Functionalized Thiophenes 6.
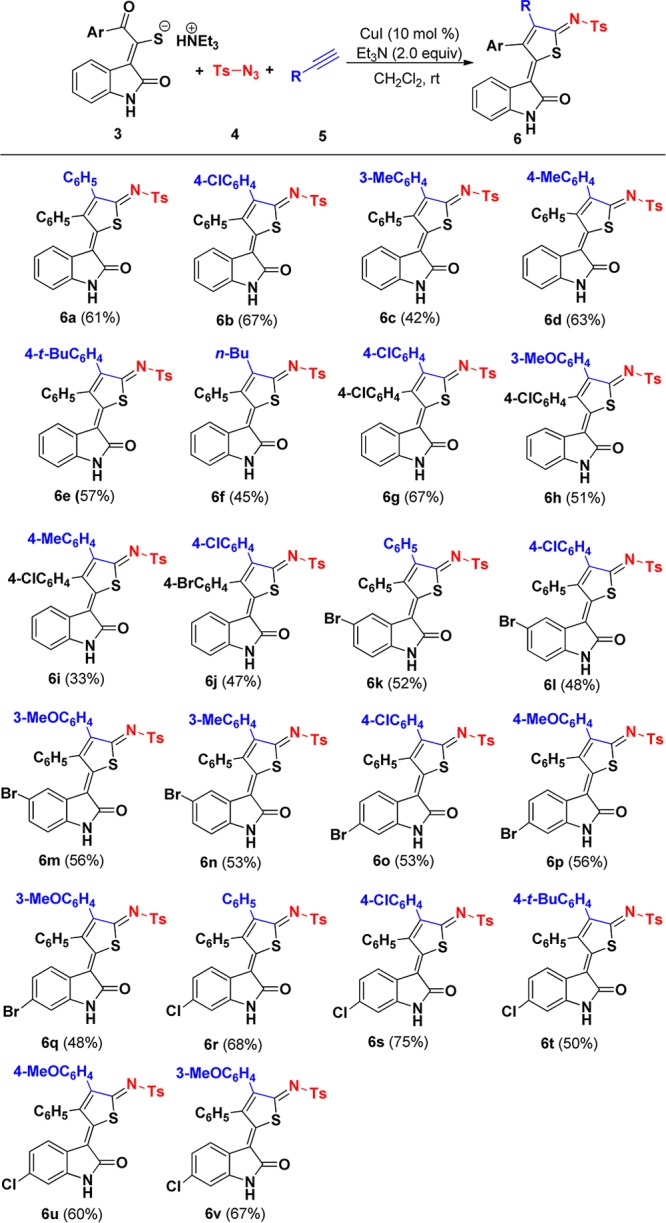
Isolated yield.
Scheme 4. Scope of Multicomponent Reactions.
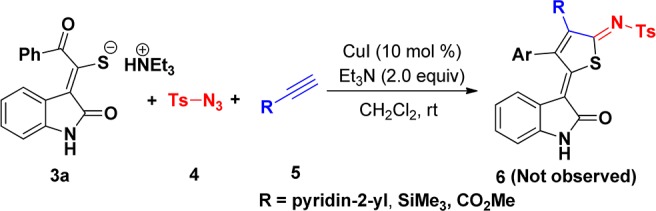
Figure 3.
ORTEP drawing of 6a.
On the basis of literature reports13−18 and our experiments, a plausible mechanism for the formation of thiophenes 6 is shown in Scheme 5. First, Et3N-promoted formal [3 + 2]-cycloaddition between α-thiocyanate ketones 1 and indoline-2,3-diones 2 gives rise to spiro indoles A, followed by ring-opening and elimination of carbamic acid to provide compounds 3. Nucleophilic addition of 3 to the electrophilic center of azaheterocumulenes D, in situ generated from Cu(I)-catalyzed cycloaddition of N-sulfonyl azides with terminal alkynes, gives anionic intermediate E. The subsequent tautomerization, intramolecular nucleophilic addition of F, protonation, and dehydration afford final thiophenes 6.
In conclusion, we have developed a new, practical, and reliable strategy for the construction of thiophenes through a copper-catalyzed formal [3 + 2]-cycloaddition between azaheterocumulenes, generated in situ from sulfonyl azides and terminal alkynes, and triethylammonium 1-(2-oxoindolin-3-ylidene)-2-aroylethanethiolates derived from α-thiocyanate ketones, indoline-2,3-diones, and Et3N. This general and efficient method simultaneously installs C–N, C–S, and C–C bonds, allowing straightforward formation of highly functionalized thiophenes with a wide diversity in substituents. The synthetic applications of this reaction are currently in progress.
Acknowledgments
We are grateful for financial support from the NSFC (Nos. 21332005, 21232004, 21272095, and 21102124), PAPD of Jiangsu Higher Education Institutions, Jiangsu Science and Technology Support Program (No. BE2011045), the Qing Lan Project (12QLG006), the Robert A. Welch Foundation (D-1361), and the NIH (R33DA031860). We thank Mr. Wei Fan and Mr. Hai-Wei Xu from Jiangsu Normal University for their generous assistance
Supporting Information Available
Experimental procedures and spectroscopic data for all new compounds 3a–f and 6a–v and X-ray crystal data (CIF) for 3a and 6a. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Russell R. K.; Press J. B. In Comprehensive Heterocyclic Chemistry II; Katritzky A. R., Rees C. W., Scriven E. W. F., Padwa A., Eds.; Pergamon: New York, 1996; Vol. 2, pp 679–729. [Google Scholar]; b Goeb S.; De Nicola A.; Ziessel R. Synlett 2005, 1169–1177. [Google Scholar]; c Jesberger M.; Davis T. P.; Barner L. Synthesis 2003, 1929–1958. [Google Scholar]; d Koike K.; Jia Z.; Nikaido T.; Liu Y.; Zhao Y.; Guo D. Org. Lett. 1999, 1, 197–198. [Google Scholar]
- a Medower C.; Wen L.; Johnson W. W. Chem. Res. Toxicol. 2008, 21, 1570. [DOI] [PubMed] [Google Scholar]; b Romagnoli R.; Baraldi P. G.; Kimatrai Salvador M.; Preti D.; Aghazadeh Tabrizi M.; Bassetto M.; Brancale A.; Hamel E.; Castagliuolo I.; Bortolozzi R.; Basso G.; Viola G. J. Med. Chem. 2013, 56, 2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton A. B.; Lee T. T.; Hoffman T. Z.; Wang Y.; Kahraman M.; Cook T. G.; Severance D.; Gahman T. C.; Noble S. A.; Shiau A. K.; Davis R. L. Bioorg. Med. Chem. Lett. 2007, 17, 3562. [DOI] [PubMed] [Google Scholar]
- a Takimiya K.; Nakano M.; Kang M. J.; Miyazaki E.; Osaka I. Eur. J. Org. Chem. 2013, 217. [Google Scholar]; b Perepichka I. F.; Perepichka D. F.. Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics; Wiley-VCH: Weinheim, 2009. [Google Scholar]; c Krebs F. C.Polymer Photovoltaics: A Practical Approach; SPIE: Bellingham, 2008. [Google Scholar]
- a Gueney S.; Becerik I.; Kadirgan F. Bull. Electrochem. 2004, 20, 157. [Google Scholar]; b Ng S. C.; Fu P.; Yu W.-L.; Chan H. S. O.; Tan K. L. Synth. Metal. 1997, 87, 119. [Google Scholar]
- Obydennov K. L.; Klimareva E. L.; Kosterina M. F.; Slepukhin P. A.; Morzherin Y. Yu. Tetrahedron Lett. 2013, 54, 4876. [Google Scholar]
- a Roncali J. Chem. Rev. 1992, 92, 711–738. [Google Scholar]; b Su Y. Z.; Lin J. T.; Tao Y.-T.; Ko C.-W.; Lin S.-C.; Sun S.-S. Chem. Mater. 2002, 14, 1884. [Google Scholar]; c Shirota Y.; Kinoshita M.; Noda T.; Okumoto K.; Ohara T. J. Am. Chem. Soc. 2000, 122, 11021. [Google Scholar]; d Thomas K. R. J.; Hsu Y.-C.; Lin J. T.; Lee K.-M.; Ho K.-C.; Lai C.-H.; Cheng Y.-M.; Chou P.-T. Chem. Mater. 2008, 20, 1830. [Google Scholar]; e Ong B. S.; Wu Y.; Li Y.; Liu P.; Pan H. Chem.—Eur. J. 2008, 14, 4766. [DOI] [PubMed] [Google Scholar]; f Osaka I.; McCullough R. D. Acc. Chem. Res. 2008, 41, 1202. [DOI] [PubMed] [Google Scholar]; g Murphy A. R.; Fréchet J. M. J. Chem. Rev. 2007, 107, 1066. [DOI] [PubMed] [Google Scholar]; h Thompson B. C.; Fréchet J. M. J. Angew. Chem., Int. Ed. 2008, 47, 58. [DOI] [PubMed] [Google Scholar]; i Zhang F.; Wu D.; Xu Y.; Feng X. J. Mater. Chem. 2011, 21, 17590. [Google Scholar]; j Wang S.; Kiersnowski A.; Pisula W.; Mullen K. J. Am. Chem. Soc. 2012, 134, 4015. [DOI] [PubMed] [Google Scholar]
- For books, see:; a Blicke F. F.In The Chemistry of Heterocyclic Compounds: Thiophenes and its Derivatives; Hartough H. D., Ed.; Interscience Publishers: New York, 1952; Chapter 2. [Google Scholar]; b Gronowitz S. In The Chemistry of Heterocyclic Compounds: Thiophene and its Derivatives; Gronowitz S. J., Ed.; Wiley and Sons: New York, 1991; Vol. 44, Part 3, Chapter 2. For a representative paper, see: [Google Scholar]; c Tsai C.-H.; Chirdon D. N.; Maurer A. B.; Bernhard S.; Noonan K. J. T. Org. Lett. 2013, 15, 5230. [DOI] [PubMed] [Google Scholar]; d Junker A.; Yamaguchi J.; Itami K.; Wuensch B. J. Org. Chem. 2013, 78, 5579. [DOI] [PubMed] [Google Scholar]
- For representative reports on the synthesis of substituted thiophenes from open chain precursors, see:; a Bartolo G.; Giuseppe S.; Alessia F. Org. Lett. 2000, 2, 351–352.10814320 [Google Scholar]; b Liang F.; Li D.; Zhang L.; Gao J.; Liu Q. Org. Lett. 2007, 9, 4845–4848. [DOI] [PubMed] [Google Scholar]; c Gabriele B.; Mancuso R.; Salerno G.; Larock R. C. J. Org. Chem. 2012, 77, 7640. [DOI] [PubMed] [Google Scholar]; d Gabriele B.; Mancuso R.; Veltri L.; Maltese V.; Salerno G. J. Org. Chem. 2012, 77, 9905. [DOI] [PubMed] [Google Scholar]; e Reddy C. R.; Valleti R. R.; Reddy M. D. J. Org. Chem. 2013, 78, 6495. [DOI] [PubMed] [Google Scholar]; f Robertson F. J.; Wu J. J. Am. Chem. Soc. 2012, 134, 2775. [DOI] [PubMed] [Google Scholar]; g Fang G.; Li J.; Wang Y.; Gou M.; Liu Q.; Li X.; Bi X. Org. Lett. 2013, 15, 4126. [DOI] [PubMed] [Google Scholar]; h Wen L.-R.; He T.; Lan M.-C.; Li M. J. Org. Chem. 2013, 78, 10617. [DOI] [PubMed] [Google Scholar]
- a Chuprakov S.; Worrell B. T.; Selander N.; Sit R. K.; Fokin V. V. J. Am. Chem. Soc. 2014, 136, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Alford J. S.; Spangler J. E.; Davies H. M. L. J. Am. Chem. Soc. 2013, 135, 11712. [DOI] [PubMed] [Google Scholar]; c Chuprakov S.; Kwok S. W.; Fokin V. V. J. Am. Chem. Soc. 2013, 135, 4652. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Spangler J. E.; Davies H. M. L. J. Am. Chem. Soc. 2013, 135, 6802. [DOI] [PubMed] [Google Scholar]; e Zibinsky M.; Fokin Valery. V. Angew. Chem., Int. Ed. 2013, 52, 1507. [DOI] [PubMed] [Google Scholar]
- a Xu X.; Cheng D.; Li J.; Guo H.; Yan J. Org. Lett. 2007, 9, 1585. [DOI] [PubMed] [Google Scholar]; b Yavari I.; Nematpour M. Synlett 2012, 2215. [Google Scholar]; c Whiting M.; Fokin V. V. Angew. Chem., Int. Ed. 2006, 45, 3157. [DOI] [PubMed] [Google Scholar]
- Shang Y.; Ju K.; He X.; Hu J.; Yu S.; Zhang M.; Liao K.; Wang L.; Zhang P. J. Org. Chem. 2010, 75, 5743. [DOI] [PubMed] [Google Scholar]
- a Cui S.-L.; Wang J.; Wang Y.-G. Org. Lett. 2007, 9, 5023. [DOI] [PubMed] [Google Scholar]; b Kim C.-E.; Park S.; Eom D.; Seo B.; Lee P. H. Org. Lett. 2014, 16, 1900. [DOI] [PubMed] [Google Scholar]; c Miura T.; Hiraga K.; Biyajima T.; Nakamuro T.; Murakami M. Org. Lett. 2013, 15, 3298. [DOI] [PubMed] [Google Scholar]
- a Horneff T.; Chuprakov S.; Chernyak N.; Gevorgyan V.; Fokin V. V. J. Am. Chem. Soc. 2008, 130, 14972. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jiang Z.; Lu P.; Wang Y. Org. Lett. 2012, 14, 6266. [DOI] [PubMed] [Google Scholar]; c Namitharan K.; Pitchumani K. Org. Lett. 2011, 13, 5728. [DOI] [PubMed] [Google Scholar]
- Li Y.; Hong D.; Zhu Y.-X.; Lu P.; Wang Y. Tetrahedron 2011, 67, 8086. [Google Scholar]
- Zhou F.; Liu X.; Zhang N.; Liang Y.; Zhang R.; Xin X.; Dong D. Org. Lett. 2013, 15, 5786. [DOI] [PubMed] [Google Scholar]
- a Lu W.; Song W.; Hong D.; Lu P.; Wang Y. Adv. Synth. Catal. 2009, 351, 1768. [Google Scholar]; b Yavari I.; Nematpour M.; Ghazanfarpour-Darjani M. Tetrahedron Lett. 2012, 53, 942–943. [Google Scholar]; c Wang Y.; Chi Y.; Zhang W.-X.; Xi Z. J. Am. Chem. Soc. 2012, 134, 2926. [DOI] [PubMed] [Google Scholar]
- a Cui S.-L.; Lin X.-F.; Wang Y.-G. Org. Lett. 2006, 8, 4517. [DOI] [PubMed] [Google Scholar]; b Jin H.; Xu X.; Gao J.; Zhong J.; Wang Y. Adv. Synth. Catal. 2010, 352, 347. [Google Scholar]; c Sun L.; Zhu Y.; Lu P.; Wang Y. Org. Lett. 2013, 15, 5894. [DOI] [PubMed] [Google Scholar]; d Chen Zh.; Zheng D.; Wu J. Org. Lett. 2011, 13, 848. [DOI] [PubMed] [Google Scholar]; e Shang Y.; He X.; Hu J.; Wu J.; Zhang M.; Yu S.; Zhang Q. Adv. Synth. Catal. 2009, 351, 2709. [Google Scholar]
- a Xu H.-W.; Ma G.-H.; Jiang B.; Tu S.-J. Synthesis 2013, 45, 3392. [Google Scholar]; b Wang X.; Wu Q.; Jiang B.; Fan W.; Tu S.-J. Tetrahedron Lett. 2014, 55, 21. [Google Scholar]; c Wu F.-Y.; Li Y.; Feng H.; Wu Q.; Jiang B.; Shi F.; Tu S.-J. Synthesis 2011, 43, 2459. [Google Scholar]; d Bisogno F. R.; Cuetos A. Green Chem. 2009, 11, 452. [Google Scholar]; e El-Din A. S. Sulfur Lett. 2003, 26, 35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.