Abstract
Single fraction total body irradiation (SFTBI) as part of a myeloablative preparative regimen in allogeneic hematopoietic stem cell transplantation (HSCT) for hematopoietic malignancies was shown to have similar survival compared with fractionated total body irradiation (FTBI)-containing regimens, with less acute toxicity. The objective of this study was to determine long-term toxicity >2 years following SFTBI-based HSCT. Twenty-one patients were evaluated at a median follow-up of 6.8 years. Thyroid dysfunction was found in 21% of patients, 1 of whom (5.2%) was symptomatic; 23% had gonadal failure; 50% of patients with growth potential had linear growth disturbance; 27% had mild to moderate pulmonary disease; and 25% had cataracts. Intelligence quotient was stable. cGVHD was present in 28%, and 4 patients (19%) were on immune suppression 2 years posttransplant. Overall survival subsequent to 2 years posttransplant was 76% in this cohort of patients. No secondary malignancies were observed. In conclusion, the toxicities of SFTBI occurred at similar or reduced frequency compared with FTBI. SFTBI should be considered for patients who may benefit from a radiation-containing HSCT preparative regimen.
Key Words: pediatric allogeneic hematopoietic stem cell transplant, total body irradiation, hematopoietic malignancy, late effects, toxicity
Myeloablative hematopoietic stem cell transplantation (HSCT) is the treatment of choice for certain very high-risk, relapsed, or refractory hematopoietic malignancies, including acute lymphoblastic leukemia (ALL), acute myelogenous leukemia (AML), myelodysplastic syndrome, chronic leukemias, and lymphomas. Total body irradiation (TBI) is often used in ablative transplant preparative regimens. Early studies of a variety of both lymphoid and myeloid malignancies, including both pediatric and adult age groups, showed that regimens using TBI had superior survival rates to those using chemotherapy alone.1–6 The role of TBI in treating hematopoietic malignancies is evolving. TBI-based regimens remain a preferred treatment for lymphoid malignancies, but they are no longer preferred for AML. The use of intravenous (IV) busulfan, which has replaced the more toxic and less efficacious oral preparation used in early comparative studies, yields better survival in this population.7 The frequent use of TBI, however, warrants its continued investigation.
TBI-based regimens typically consist of a total dose of ≥1000 cGy, fractionated total body irradiation (FTBI), and delivered over several days according to various schemas at a dose rate of 7 to 19 cGy/min. TBI is associated with significant multiorgan toxicities, both acute and chronic. Acute toxicities include interstitial pneumonitis and severe mucositis; chronic toxicities include restrictive pulmonary disease, gonadal dysfunction, hypothyroidism, bone abnormalities such as osteochondroma and avascular necrosis (AVN), cataracts, secondary malignancies, and of particular concern in children, growth hormone deficiency, linear growth deceleration, and neurocognitive dysfunction.8–15
Our institution explored whether the TBI toxicity profile could be improved without compromising outcomes. A novel regimen was developed in which a lower total amount of TBI was administered in a single fraction of 550 cGy (SFTBI), but administered at a high-dose rate of 30 cGy/min to achieve myeloablation.16 This approach, based on preclinical models as well as a single human-based feasibility study by Fyles et al,17 yielded similar efficacy but with lower toxicity relative to regimens using a higher total dose and a lower rate of delivery.18,19 A SFTBI regimen was developed for children by Druley et al,20 and demonstrated a 1-year overall survival (OS) of 60% and event-free survival (EFS) of 47%, which was similar to that seen with FTBI in both children and adults, with less acute toxicity. The utility of this treatment regimen in the pediatric population, however, is contingent not only on effective disease control, but also on the magnitude of long-term toxicities on children’s growth and development. The objective of this study is to examine the long-term effects in children >2 years following SFTBI-based HSCT.
METHODS
Patients and Assessments
Sixty-one consecutive patients between the ages of 1 and 21 years with hematopoietic malignancies underwent transplant while enrolled on an institution-based study at St Louis Children’s Hospital using SFTBI and cyclophosphamide between March 1998 and May 2006. This was a heterogenous population of high-risk patients who had been exposed to a variety of prior treatments.
Cyclophosphamide (60 mg/kg IV) was given on days −3 and −2 and SFTBI (550 cGy) was given on day −1. The protocol allows for additional radiation for CNS or local disease before or as part of the conditioning regimen. Details of TBI administration and stem cell dose and administration were described previously by Druley et al.20
An objective of this institution-based study was to explore toxicity of the novel preparative regimen described above. Early toxicities were described by Druley and colleagues. This work explores the late toxicities experienced by patients enrolled on this clinical trial, which completed accrual in May 2006. Patients included in this analysis met the following criteria: (1) alive at least 2 years following transplant with chart available for review; (2) in remission at the beginning of the late effects period (defined as beginning at 2 y following transplant); and (3) no additional radiation used in subsequent (posttransplant) treatment, that is, for relapse, before late effects period. Approval for this retrospective chart review was granted by the Washington University School of Medicine Institutional Review Board, with waiver of consent.
Treatment-related toxicities were graded by a single investigator using the CTCAE v4.0 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf). Patients underwent a standard evaluation annually, which included height and weight measurements, Tanner staging, metabolic panel, and TSH and T4 levels. Pulmonary function tests were performed when developmentally appropriate at frequent intervals in the first year posttransplant (minimally at days +100, +180, 1 y) then annually for 5 years, and as clinically indicated. Echocardiogram and audiology studies were performed 2 years posttransplant and then repeated if clinically indicated. Ophthalmology studies were performed every 6 months posttransplant. Measurement of sex hormone levels (FSH, LH, testosterone, estradiol) of patients who were entering puberty (Tanner stage II), were in puberty (Tanner stages III to IV), or had completed puberty was offered beginning at 2 years posttransplant. Gonadal failure was defined as failure to begin puberty at expected age (standard Tanner criteria: 13 y for girls, 14 y for boys), regression of pubertal development, or depressed testosterone and estradiol levels. Growth hormone (GH) level was measured at the discretion of the treating physician.
Linear growth disturbance was assessed using Centers for Disease Control and Prevention (CDC) standard pediatric growth curves, comparing pretransplant and posttransplant growth velocity for each patient. Growth suppression is defined by the CTCAE as a decrease in expected growth velocity, and is classified as grade 1 (decrease of 10% to 29%; equivalent to 1 line crossed in a CDC standard growth curve), grade 2 (30% to 49%; equivalent to 2 lines crossed), or grade 3 (≥50%; equivalent to ≥3 lines crossed). Patients were tested at the discretion of the treating physician for osteopenia using dual-energy x-ray absorptiometry scan. Magnetic resonance imaging and plain film XR were used to evaluate AVN or osteochondroma if indicated by bone pain, joint signs or symptoms. Graft-versus-host disease (GVHD) was classified according to the NIH Consensus Development Project on Criteria for Clinical Trials in Chronic GVHD working group report.21
Neurocognitive testing was performed by the Pediatric Psychology Department at St Louis Children’s Hospital using age-appropriate standard test batteries pretransplant and typically 1 to 2 years posttransplant. Additional testing may have been performed at the request of the treating physician or family. A change in full-scale intelligence quotient (FSIQ) of 15 points (1 SD) was considered meaningful.
Statistical Analysis
IBM SPSS Statistics version 21 was used for analysis. The χ2 analysis was performed for categorical variables. The level of significance was specified as P<0.05.
RESULTS
Patient characteristics, including indication for transplant, are summarized in Table 1. Eighty-one percent of patients were male. Fifty-two percent of patients had pre-B ALL, 9.5% T-cell ALL, 14% AML, 4.8% had biphenotypic leukemia, and 19% had NHL. Forty-eight percent were in CR1 and 52% in CR2 at transplant. Median follow-up time posttransplant was 6.8 years (range, 2.0 to 11.1 y). Median age at transplant was 13.2 years (range, 1.0 to 20.5 y) and median age at follow-up was 16.3 years (range, 9.1 to 30.5 y). Six patients (28%) received additional radiation therapy before or as part of the preparative regimen: 1 patient received radiation therapy to abdomen, liver, testes at relapse of Burkitt lymphoma; 1 received testicular and craniospinal (CS) radiation for extramedullary relapse of T-cell lymphoblastic lymphoma; 2 received CS radiation for isolated CNS relapse of pre-B ALL and previously unradiated CNS-negative T-cell ALL; 1 received CS radiation for marrow plus CNS relapse of pre-B ALL; and 1 received CS radiation for T-cell ALL in CR1 (induction failure).
TABLE 1.
Patient Characteristics

Survival and follow-up status of the original cohort is shown in Figure 1. Sixty-one patients were transplanted on this regimen between March 1998 and May 2006; 55 patients had records available for evaluation of survival demonstrating a 2-year OS of 49% and 2-year EFS of 44%.
FIGURE 1.
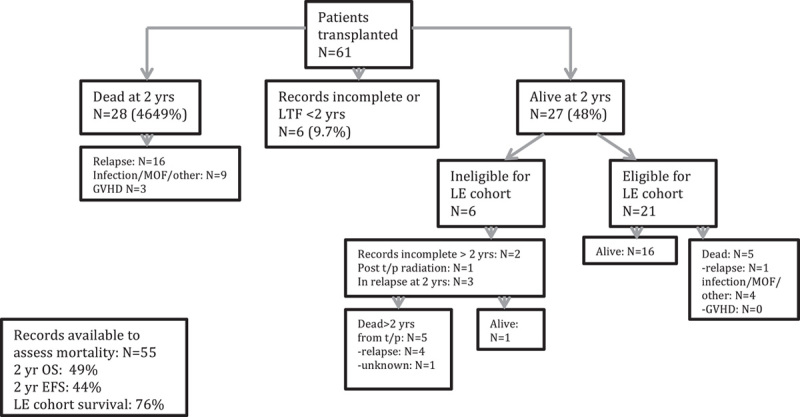
Mortality and cohort eligibility. Schema of mortality, survival, and eligibility for late effects cohort. EFS indicates event-free survival; GVHD, graft-versus-host disease; LE, late effects; LTF, lost to follow-up; MOF, multiorgan failure; OS, overall survival.
Twenty-one patients from the original cohort met the criteria for analysis of long-term effects. Six patients (9.8%) were lost to follow-up before 2 years posttransplant, 2 (3.3%) had insufficient records for review during the long-term effects period. Three patients relapsed just before the late effects period and were excluded. One patient with multiple local relapses underwent further radiation and was excluded from analysis. Five deaths occurred in the late effects cohort, resulting in a 76% survival for this group. One patient had a late relapse of ALL 5.5 years posttransplant, received salvage chemotherapy, and is alive and in remission >4 years after relapse and is included. One child had a relapse of his Philadelphia chromosome-positive ALL and underwent a second myeloablative HSCT with chemotherapy preparative regimen 1 year following the first HSCT with SFTBI. This patient entered the cohort 2 years following the first transplant with SFTBI.
Posttransplant Complications
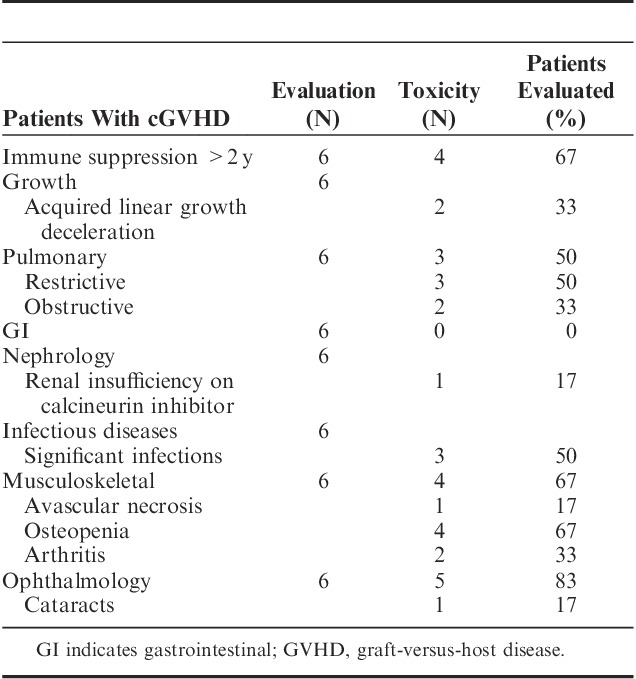
Generalized grading criteria are described in Table 2. Posttransplant complications are shown in Table 3. Because of the inevitable confounding of GVHD, its treatment complications, and TBI-related toxicity, toxicities in the subset of patients with GVHD are shown in Table 4 to clarify the toxicity profile of this group.
TABLE 2.
General Guideline of CTCAE V4.0 Grading

TABLE 3.
Complications Posttransplant
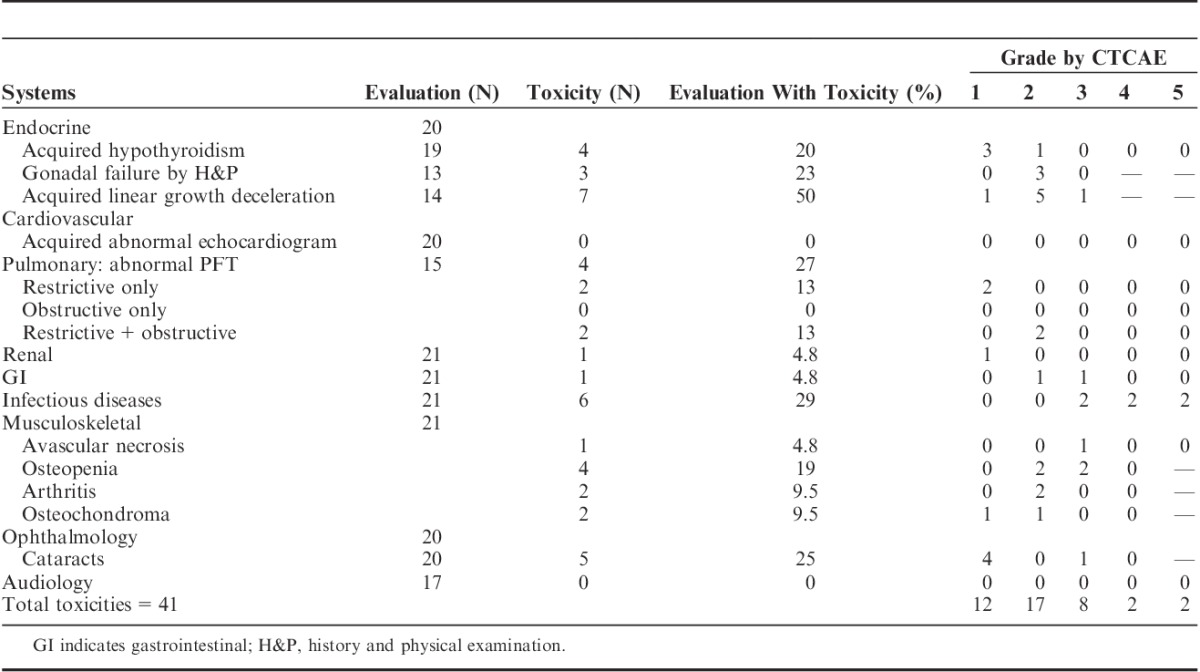
TABLE 4.
GVHD-associated Medical Complications in Patients With cGVHD
Endocrine Toxicity
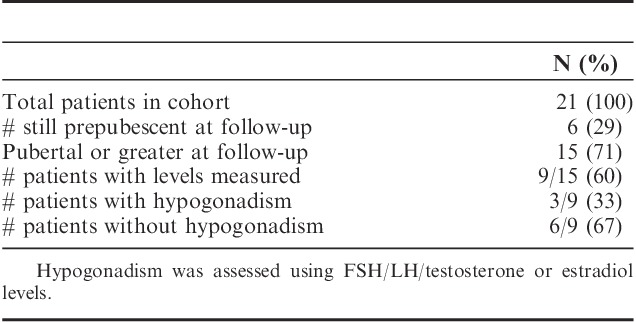
One child had panhypopituitarism as a complication of prior brain surgery during infancy and is not included in the analysis of endocrine toxicity and 1 had no records for endocrine follow-up. Thyroid: 4 of 19 evaluable patients (21%) had hypothyroidism identified with screening labs. Only 1 of 19 (5.2%) had overt (symptomatic) hypothyroidism. Gonadal function: documentation of gonadal dysfunction by history, Tanner stage, and/or menarche was available for 13 of the 15 patients, who were of pubertal age or greater at or after HSCT. By history and physical examination, 3 of the 13 (23%), 2 males and 1 female, had gonadal failure, which was confirmed by laboratory testing. Both males had received testicular radiation, 1 of the males and 1 female received CS radiation. Sex hormone levels were measured in 9 of the 15 patients (Table 5), yielding a 33% rate of hypogonadism in this group. All were treated successfully with hormone replacement. No patients had pubertal regression. One 3-year-old female patient was found to have precocious puberty on physical examination and was found to have elevated FSH and LH levels. GH deficiency is discussed below.
TABLE 5.
Laboratory Measurement of Gonadal Failure
Linear Growth Toxicity
Fourteen patients had linear growth potential at the time of the transplant, and 7 of the 14 (50%) had growth disturbance. One of the 14 (7.1%) had mild (grade 1) failure and history of steroid treatment for GVHD, and his subsequent growth tracked consistently along a new curve. Five of the 14 (36%) had moderate (grade 2) disturbance: 2 had steroid treatment for GVHD, 2 others had CS radiation, and 1 had preexisting panhypopituitarism. One child had severe (grade 3) growth failure and history of CS radiation. GH level was evaluated in 4 of the 6 patients with grade 2 or 3 linear growth impairment, and was found to be abnormally low in 1 patient with grade 2 impairment and history of CS radiation.
Musculoskeletal Toxicity
One patient in the 21-patient cohort (4.8%) had AVN and 4 of 21 patients (19%) had osteopenia. Two of the 21 patients (9.5%) had diagnosis of arthritis without diagnosis of AVN, although arthritis was attributed to steroid treatment in 1 of the 2. Two of 21 (9.5%) had osteochondroma.
Pulmonary, Cardiac, and Renal Toxicity
Fifteen patients had the capability to undergo pulmonary function tests and records available. Four of the 15 (27%) had evidence of lung disease: 2 (13%) had grade 2 obstructive plus restrictive disease and 2 (13%) had grade 1 (asymptomatic) restrictive disease only. Three of the 4 had history of GVHD. There were no long-term cardiac toxicities. One patient of the 21 evaluated (4.8%) on prolonged calcineurin inhibitor therapy had grade 1 renal insufficiency as evidenced by mildly elevated creatinine.
Ophthalmology and Audiology
Results of ophthalmological evaluations were available for 20 patients. Five of 20 (25%) had cataracts: 4 (20%) were asymptomatic (grade 1) and 1 (5.0%) had overt disease, requiring surgery (grade 3). Audiology records were available for 17 patients. There was no transplant-associated hearing impairment.
GVHD, Immune Dysfunction, Recurrence, and Secondary Malignancy
Six patients (28%) had active cGVHD ≥2 years following transplant. Four patients had mild cGVHD (3 with skin disease and 1 with liver and sicca syndrome), 1 had moderate cGVHD (skin), and 1 had severe cGVHD (lung). Four patients were on systemic immune suppression at ≥2 years posttransplant.
Six of the 21 patients (29%) had moderate to severe single or multiple infections. Five of the 6 had current active, or history of, cGVHD. Two (9.5%) experienced fatal infections with history of prolonged immune suppression for cGVHD. One patient (4.8%) on prolonged immune suppression experienced posttransplant lymphoproliferative disorder. There were no regimen-associated secondary malignancies.
The association between cGVHD and growth disturbance, infection rate, endocrinopathy, pulmonary toxicity, and mortality was not statistically significant.
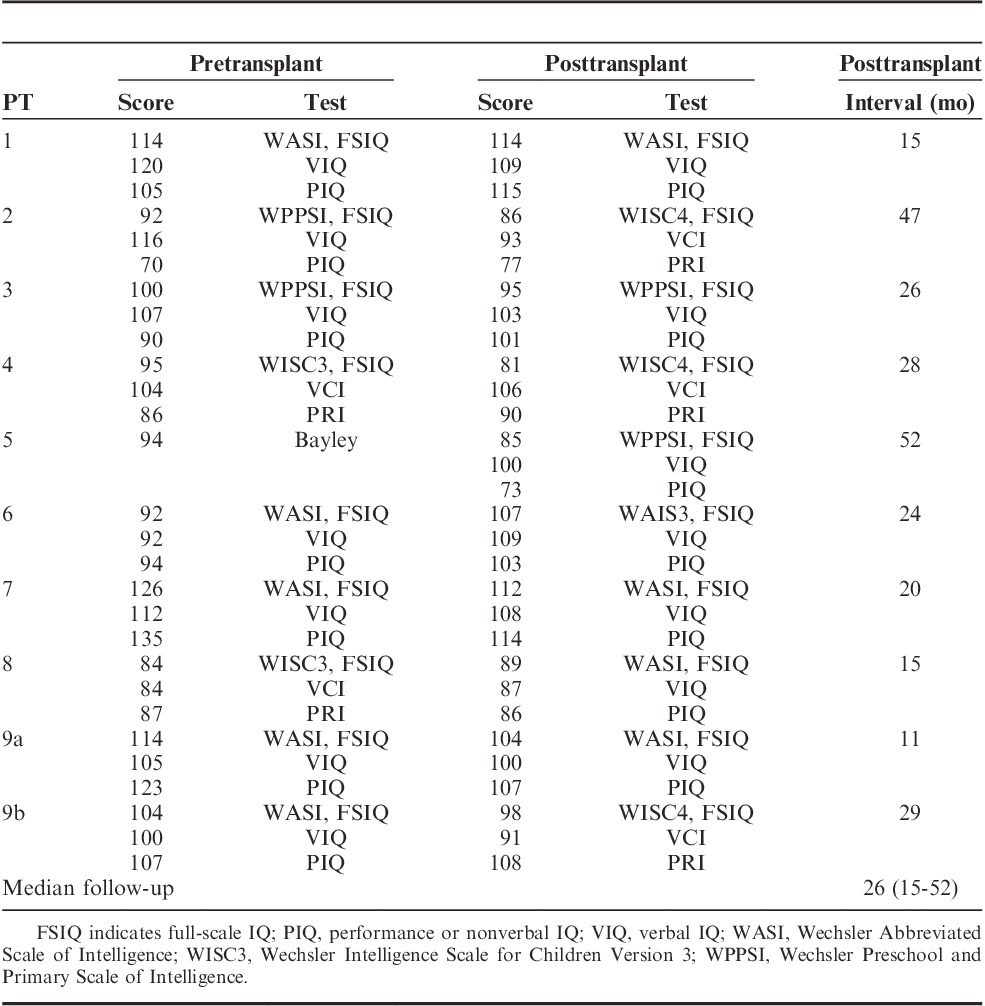
Neurocognitive Effects and Performance Status
Neurocognitive data were available for 9 of the 21 (43%) patients (Table 6). Three patients had follow-up testing at <2 years after transplant. FSIQ, verbal (VIQ), and performance or nonverbal (PIQ) IQs were compared. There was meaningful change in FSIQ in only 1 patient. This patient had stable IQ following transplant with SFTBI. He subsequently underwent a second myeloablative transplant 1 year after the first, and had a decline of 16 points after the second transplant.
TABLE 6.
Neurocognitive Testing Results
Performance status was assessed in all patients. The majority of patients (17 of 21, 81%) had Lansky or Karnofsky performance status of 100%. Two of 21 (9.5%) had performance of 90% and 2 (9.5%) of 80%.
DISCUSSION
Here we report the late toxicities in a cohort of 21 patients followed for ≥2 years after receiving a SFTBI-based HSCT. There were a total of 41 toxicities, the majority of which (71%) were grade ≤2. Known complications of cGVHD and corticosteroid treatment overlap with those of TBI and therefore confound the analysis. These include growth failure, endocrinopathy, infection, bone disease, and restrictive and obstructive lung disease.22 Although we observed that some of the most severe overlapping toxicities occurred in patients with GVHD, χ2 analysis revealed that this association was not statistically significant. It is possible that this finding was due to the small numbers in this cohort, and warrants further study.
Despite the challenges of comparing survival data across studies with variation with respect to disease profile, remission number, age range, and specific preparative and radiation regimens, we feel OS using this SFTBI-based regimen at 2 years remains comparable with that of reasonably similar patient populations undergoing transplant with FTBI-based preparative regimens.23–26 OS and EFS need to be interpreted with the caveat that in the current transplant era, which is subsequent to transplant in this cohort, myeloid malignancies are no longer treated with TBI-based regimens, as discussed above.
Endocrine late effects are among the most common seen following any form of TBI, with hypothyroidism, gonadal failure, and growth hormone deficiency particularly common in this population. We observed a rate of hypothyroidism comparable with the 12% to 21% reported for patients exposed to FTBI, with similar rates of compensated and overt hypothyroidism, and a gonadal failure rate (23% by clinical evaluation) similar to the 22% to 43% reported for patients exposed to FTBI.10,23,27–30 Linear growth disturbance is one of the most common toxicities of TBI and strongly impacts quality of life.31 The etiology of growth disturbance is multifactorial, and includes growth hormone deficiency, sex hormone deficiency, and severe hypothyroidism, as well as nonendocrine causes such as radiation damage to bone and growth plates and protein catabolism due to steroid toxicity. Patients receiving FTBI are reported to have growth disturbances between 24% and 100%, with many patients requiring growth hormone replacement.10,23,30 Our rate (50%) was consistent with these findings. Interestingly, all patients in our cohort with growth disturbance had either a history of prolonged steroid treatment, additional CS radiation, or a preexisting condition (panhypopituitarism). Only 4 of the 7 patients with growth disturbance had GH levels measured, but as these data are limited, it is encouraging that only 1 patient was found to have GH deficiency.
Pulmonary toxicities, including interstitial pneumonitis and pulmonary fibrosis leading predominantly to restrictive lung disease, are among the most serious late effects of TBI-based regimens. Pulmonary disease in the postallogeneic transplant population is commonly confounded by GVHD, which can present both a restrictive and obstructive picture. Although some authors report minimal lung disease in patients receiving FTBI,23 restrictive disease can occur in up to 74% of patients, severe disease in up to 12%, and death in up to 6% in patients receiving FTBI.28,30,32 We saw very little restrictive disease and there was no severe lung disease. Only 1 patient had pulmonary disease (restrictive) outside the setting of history of GVHD. Although not a common toxicity, cardiomyopathies have been reported in patients who have received TBI.23,33 To date, we have not observed any patients with cardiomyopathies in our cohort.
Cataracts are a common complication of TBI, with rates in FTBI ranging from 22% to 78%.23,27,28,30,34 Our rate was similar to the lowest rates reported, with overt cases (5%) consistent with the lowest rates reported for FTBI.
Both steroid use and TBI are risk factors for AVN. In studies including children and adults receiving either a single large dose or FTBI, incidences range from 4% to about 20%, with incidences increasing with increasing doses of radiation.11,27,35–37 Our findings are similar to the lowest levels reported. We saw less osteochondroma than the 24% to 36% reported for FTBI.15,28,38 Our rate of cGVHD at 2 years (28%) was comparable with that seen in a large study by Zecca et al39 at 3 years (27%).
Secondary malignancies are complications of both TBI and chemotherapy, and can occur in up to 20% of patients.13,23,27,28,30 Although none were observed in our cohort, we recognize that longer follow-up is needed for a more meaningful assessment, and most reports of secondary malignancy occur at least 10 years from radiation.
Neurocognitive function is vulnerable, especially in the most formative years of brain growth and development (age below 3), to any form of radiation including TBI, with very young patients exhibiting the most chronic deficits.9,14,40,41 Reducing radiation to the developing brain was a motivation for developing a reduced radiation regimen. Although data are limited, it is reassuring that deficits in our cohort, as measured by IQ, were limited in both quantity and degree.
Limitations of this study include the small number of patients, a variety of underlying diagnoses, and large age range of patients. Chart review design and inconsistent reporting, particularly with respect to neurocognitive function, gonadal function, and GH levels, were also limiting, as was the total of 13% loss to follow-up or incomplete records. Neurocognitive testing posttransplant was performed in only a fraction of patients, and in some cases <2 years from transplant, at a time that may miss the most serious deficits, particularly as younger patients mature. Given scant reporting of neurocognitive effects in the literature, we felt results were worth reporting. We recognize that several toxicities, particularly secondary malignancies, cataracts, and bone toxicity almost certainly require more time than was allotted here to develop. However, this is the first long-term toxicity data reported on patients receiving this novel preparative regimen, and the only report on an exclusively pediatric population. Bearing in mind the limitations, the results are nonetheless encouraging in that some of the more common toxicities of TBI are found to be at worst similar to, and in some cases less than, those seen with FTBI. Study in a larger cohort would be beneficial in exploring these observations further.
In summary, SFTBI as part of an ablative transplant regimen delivers less total radiation than a FTBI regimen. This study was limited by the small number of patients and a large range of ages and underlying diagnoses, hence comparison with FTBI regimen is challenging. Further study directly comparing SFTBI to a FTBI regimen is warranted. We feel this study does demonstrate the feasibility of this preparative regimen, and clinicians might consider this therapy as an alternative to FTBI if committed to a TBI-based regimen.
ACKNOWLEDGMENTS
The authors thank Dr Nicole Cruz for the help in interpreting neurocognitive testing results and Susan Hayashi for the help in interpreting audiology data.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Blaise D, Maraninchi D, Archimbaud E, et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79:2578–2582. [PubMed] [Google Scholar]
- 2.Blume KG, Kopecky KJ, Henslee-Downey JP, et al. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: a Southwest Oncology Group study. Blood. 1993;81:2187–2193. [PubMed] [Google Scholar]
- 3.Devergie A, Blaise D, Attal M, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM). Blood. 1995;85:2263–2268. [PubMed] [Google Scholar]
- 4.Granados E, de La Camara R, Madero L, et al. Hematopoietic cell transplantation in acute lymphoblastic leukemia: better long term event-free survival with conditioning regimens containing total body irradiation. Haematologica. 2000;85:1060–1067. [PubMed] [Google Scholar]
- 5.Davies SM, Ramsay NK, Klein JP, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18:340–347. [DOI] [PubMed] [Google Scholar]
- 6.Socie G, Clift RA, Blaise D, et al. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: long-term follow-up of 4 randomized studies. Blood. 2001;98:3569–3574. [DOI] [PubMed] [Google Scholar]
- 7.Copelan EA, Hamilton BK, Avalos B, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faraci M, Bekassy AN, De Fazio V, et al. Non-endocrine late complications in children after allogeneic haematopoietic SCT. Bone Marrow Transplant. 2008;41suppl 2S49–S57. [DOI] [PubMed] [Google Scholar]
- 9.Smedler AC, Winiarski J. Neuropsychological outcome in very young hematopoietic SCT recipients in relation to pretransplant conditioning. Bone Marrow Transplant. 2008;42:515–522. [DOI] [PubMed] [Google Scholar]
- 10.Sanders JE. Growth and development after hematopoietic cell transplant in children. Bone Marrow Transplant. 2008;41:223–227. [DOI] [PubMed] [Google Scholar]
- 11.Socie G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. [DOI] [PubMed] [Google Scholar]
- 12.Nandagopal R, Laverdiere C, Mulrooney D, et al. Endocrine late effects of childhood cancer therapy: a report from the Children’s Oncology Group. Horm Res. 2008;69:65–74. [DOI] [PubMed] [Google Scholar]
- 13.Felicetti F, Manicone R, Corrias A, et al. Endocrine late effects after total body irradiation in patients who received hematopoietic cell transplantation during childhood: a retrospective study from a single institution. J Cancer Res Clin Oncol. 2011;137:1343–1348. [DOI] [PubMed] [Google Scholar]
- 14.Perkins JL, Kunin-Batson AS, Youngren NM, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatr Blood Cancer. 2007;49:958–963. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki O, Nishimura G, Okamoto R, et al. Induction of systemic bone changes by preconditioning total body irradiation for bone marrow transplantation. Pediatr Radiol. 2009;39:23–29. [DOI] [PubMed] [Google Scholar]
- 16.Blum W, Brown R, Lin HS, et al. Low-dose (550 cGy), single-exposure total body irradiation and cyclophosphamide: consistent, durable engraftment of related-donor peripheral blood stem cells with low treatment-related mortality and fatal organ toxicity. Biol Blood Marrow Transplant. 2002;8:608–618. [DOI] [PubMed] [Google Scholar]
- 17.Fyles GM, Messner HA, Lockwood G, et al. Long-term results of bone marrow transplantation for patients with AML, ALL and CML prepared with single dose total body irradiation of 500 cGy delivered with a high dose rate. Bone Marrow Transplant. 1991;8:453–463. [PubMed] [Google Scholar]
- 18.Girgis M, Hallemeier C, Blum W, et al. Chimerism and clinical outcomes of 110 recipients of unrelated donor bone marrow transplants who underwent conditioning with low-dose, single-exposure total body irradiation and cyclophosphamide. Blood. 2005;105:3035–3041. [DOI] [PubMed] [Google Scholar]
- 19.Adkins DR, DiPersio JF. Total body irradiation before an allogeneic stem cell transplantation: is there a magic dose?Curr Opin Hematol. 2008;15:555–560. [DOI] [PubMed] [Google Scholar]
- 20.Druley TE, Hayashi R, Mansur DB, et al. Early outcomes after allogeneic hematopoietic SCT in pediatric patients with hematologic malignancies following single fraction TBI. Bone Marrow Transplant. 2009;43:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. [DOI] [PubMed] [Google Scholar]
- 22.Bresters D, van Gils IC, Kollen WJ, et al. High burden of late effects after haematopoietic stem cell transplantation in childhood: a single-centre study. Bone Marrow Transplant. 2010;45:79–85. [DOI] [PubMed] [Google Scholar]
- 23.Linsenmeier C, Thoennessen D, Negretti L, et al. Total Body Irradiation (TBI) in pediatric patients. A single-center experience after 30 years of low-dose rate irradiation. Strahlenther Onkol. 2010;186:614–620. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki J, Nagatoshi Y, Sakiyama M, et al. TBI and melphalan followed by allogeneic hematopoietic SCT in children with advanced hematological malignancies. Bone Marrow Transplant. 2011;46:1057–1062. [DOI] [PubMed] [Google Scholar]
- 25.Schneider RA, Schultze J, Jensen JM, et al. Long-term outcome after static intensity-modulated total body radiotherapy using compensators stratified by pediatric and adult cohorts. Int J Radiat Oncol Biol Phys. 2008;70:194–202. [DOI] [PubMed] [Google Scholar]
- 26.Petropoulos D, Worth LL, Mullen CA, et al. Total body irradiation, fludarabine, melphalan, and allogeneic hematopoietic stem cell transplantation for advanced pediatric hematologic malignancies. Bone Marrow Transplant. 2006;37:463–467. [DOI] [PubMed] [Google Scholar]
- 27.Bieri S, Roosnek E, Ozsahin H, et al. Outcome and risk factors for late-onset complications 24 months beyond allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2011;87:138–147. [DOI] [PubMed] [Google Scholar]
- 28.Faraci M, Barra S, Cohen A, et al. Very late nonfatal consequences of fractionated TBI in children undergoing bone marrow transplant. Int J Radiat Oncol. 2005;63:1568–1575. [DOI] [PubMed] [Google Scholar]
- 29.Boulad F, Bromley M, Black P, et al. Thyroid dysfunction following bone marrow transplantation using hyperfractionated radiation. Bone Marrow Transplant. 1995;15:71–76. [PubMed] [Google Scholar]
- 30.Ricardi U, Filippi AR, Biasin E, et al. Late toxicity in children undergoing hematopoietic stem cell transplantation with TBI-containing conditioning regimens for hematological malignancies. Strahlenther Onkol. 2009;185suppl 217–20. [DOI] [PubMed] [Google Scholar]
- 31.Ishida Y, Sakamoto N, Kamibeppu K, et al. Late effects and quality of life of childhood cancer survivors: Part 2. Impact of radiotherapy. Int J Hematol. 2010;92:95–104. [DOI] [PubMed] [Google Scholar]
- 32.Latini P, Aristei C, Aversa F, et al. Interstitial pneumonitis after hyperfractionated total body irradiation in HLA-matched T-depleted bone marrow transplantation. Int J Radiat Oncol Biol Phys. 1992;23:401–405. [DOI] [PubMed] [Google Scholar]
- 33.Uderzo C, Pillon M, Corti P, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: a prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant. 2007;39:667–675. [DOI] [PubMed] [Google Scholar]
- 34.Tichelli A, Gratwohl A, Egger T, et al. Cataract formation after bone marrow transplantation. Ann Intern Med. 1993;119:1175–1180. [DOI] [PubMed] [Google Scholar]
- 35.Socie G, Cahn JY, Carmelo J, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM). Br J Haematol. 1997;97:865–870. [DOI] [PubMed] [Google Scholar]
- 36.Faraci M, Calevo MG, Lanino E, et al. Osteonecrosis after allogeneic stem cell transplantation in childhood. A case-control study in Italy. Haematologica. 2006;91:1096–1099. [PubMed] [Google Scholar]
- 37.Gordon BG, Warkentin PI, Strandjord SE, et al. Allogeneic bone marrow transplantation for children with acute leukemia: long-term follow-up of patients prepared with high-dose cytosine arabinoside and fractionated total body irradiation. Bone Marrow Transplant. 1997;20:5–10. [DOI] [PubMed] [Google Scholar]
- 38.Taitz J, Cohn RJ, White L, et al. Osteochondroma after total body irradiation: an age-related complication. Pediatr Blood Cancer. 2004;42:225–229. [DOI] [PubMed] [Google Scholar]
- 39.Zecca M, Prete A, Rondelli R, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100:1192–1200. [DOI] [PubMed] [Google Scholar]
- 40.Shah AJ, Epport K, Azen C, et al. Progressive declines in neurocognitive function among survivors of hematopoietic stem cell transplantation for pediatric hematologic malignancies. J Pediatr Hematol Oncol. 2008;30:411–418. [DOI] [PubMed] [Google Scholar]
- 41.Phipps S, Rai SN, Leung WH, et al. Cognitive and academic consequences of stem-cell transplantation in children. J Clin Oncol. 2008;26:2027–2033. [DOI] [PubMed] [Google Scholar]


