Abstract
Application of minimal access surgery in acute care surgery is limited due to various reasons. Laparoscopic omental patch repair (LOPR) for perforated peptic ulcer (PPU) surgery is safe and feasible but not widely implemented. We report our early experience of LOPR with emphasis on strict selection criteria. This is a descriptive study of all patients operated on for PPU at academic university-affiliated institutes from December 2010 to February 2012. All the patients who were operated on for LOPR were included as the study population and their records were studied. Perioperative outcomes, Boey score, Mannheim Peritonitis Index (MPI), and physiologic and operative severity scores for enumeration of mortality and morbidity (POSSUM) scores were calculated. All the data were tabulated in a Microsoft Excel spreadsheet and analyzed using Stata Version 8.x. (StataCorp, College Station, TX, USA). Fourteen patients had LOPR out of a total of 45 patients operated for the PPU. Mean age was 46 years (range 22−87 years). Twelve patients (86%) had a Boey score of 0 and all patients had MPI < 21 (mean MPI = 14). The predicted POSSUM morbidity and mortality were 36% and 7%, respectively. Mean ulcer size was 5 mm (range 2−10 mm), mean operating time was 100 minutes (range 70−123 minutes) and mean length of hospital stay was 4 days (range 3−6 days). There was no morbidity or mortality pertaining to LOPR. LOPR should be offered by acute care surgical teams when local expertise is available. This can optimize patient outcomes when strict selection criteria are applied.
Key words: Laparoscopy, Peptic ulcer, Surgical training
Perforated peptic ulcer (PPU) disease is a common abdominal surgical emergency. Evolution of laparoscopic surgery has affected the operative management of PPU with reports favoring laparoscopic approach.1 While laparoscopic surgery has been shown to be safe and feasible, the application of laparoscopic approach is restricted due to various factors, including availability and experience of local expertise in complex laparoscopic procedures on an emergent basis. From our early experience we would like to propose selection criteria to reduce the effect of learning curve and enhance patient safety.
Materials and Methods
Our hospital is a 550-bed academic center with a university affiliation; the hospital provides a comprehensive range of medical services. All consecutive patients diagnosed with perforated peptic ulcers (PPU) were identified from prospective hospital electronic database using the ICD-9 and ICD-10 codes. Only the patients who underwent laparoscopic omental patch repair (LOPR) of the PPU disease between December 2010 and February 2012 were included for the study purpose. This study was granted an IRB exemption. Patient data was extracted from electronic medical records and case notes. Demographic and clinical data was evaluated with emphasis on the operative details of laparoscopic approach and short-term perioperative outcomes. Boey score,2 Mannheim Peritonitis Index (MPI),3 and physiologic and operative severity score for enumeration of mortality and morbidity (POSSUM)4 scores were calculated for all patients. Peri-operative care was not standardized, and nasogastric tube, abdominal drains, feed commencement, and analgesia regimen were documented from the case notes. All the patients received empirical triple therapy comprising of amoxicillin, 1000,mg; clarithromycin, 500 mg; and omeprazole, 40 mg, twice a day for a duration of 7–14 days, followed by omeprazole, 40 mg, twice a day for 4−6 weeks. All the data were tabulated in a Microsoft Excel spreadsheet and analyzed using Stata Version 8.x. (StataCorp, College Station, TX, USA). Categorical variables were analyzed using the Chi-square test while continuous variables were compared using the Student t test (parametric distribution) or Mann-Whitney test (nonparametric distribution). All tests were two-sided and P < 0.05 was considered to be statistically significant.
Operative Technique
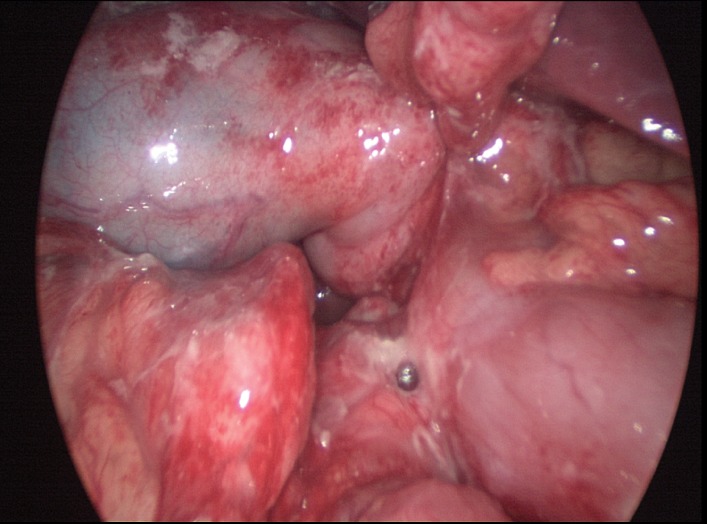
All the surgeries were done in the presence of a consulting surgeon. All the surgeries were actively performed by trainees at various levels of training with consultant surgeon. All the laparoscopic omental patch repairs (LOPR) were done under general anesthesia. The patient was either placed in a supine or Lloyd-Davies position. All the patients received prophylactic 1 gm cefazolin (or 1 gm ceftriaxone) and 500 mg metronidazole intravenously at the induction. Marcaine (0.5%) was infiltrated prior to the skin incisions. Peri-umbilical 10 mm open Hassan's access was used to create pneumoperitoneum of up to 15 mmHg abdominal pressure. Rest of the 5-mm ports were placed in the right upper, right lower, left upper, left lower, and/or epigastric region at the discretion of the operating surgeon. Either a 3-port or a 4-port technique was employed. Figure 1 shows the laparoscopic view of PPU. Diagnostic exploration and warm saline irrigation was done in all cases. Peritoneal fluid was sent for culture at the discretion of the operating surgeon. LOPR was done with the help of polyglactin 910 intracorporeal suturing. Figure 2 shows the intracorporeally-sutured omental patch to repair the perforation. Intraoperative upper gastrointestinal endoscopy to perform an air leak test and/or take biopsies was done at the discretion of the operating surgeon. Drainage tubes were used at the discretion of operating surgeon.
Fig. 1.
Laparoscopic view of the perforated peptic ulcer (PPU).
Fig. 2.
Laparoscopic omental patch repair (LOPR) with intracorporeal suturing.
Results
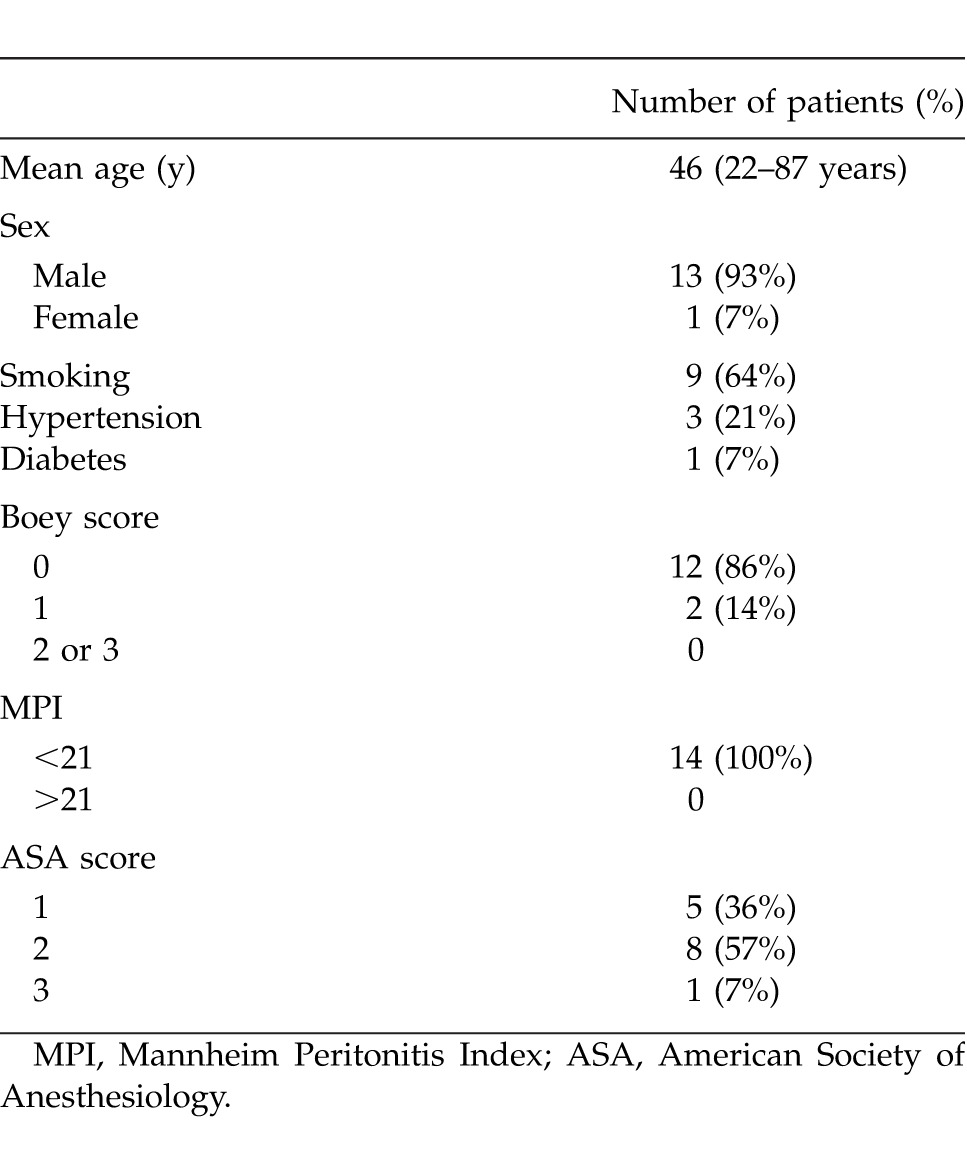
A total of 45 patients with a median age of 52 years (17−92 years) with PPU were identified. Forty-two patients (93%) underwent omental patch repair and our overall median hospital stay was 6 days with mortality of 8.9%. Three patients underwent a resectional surgery. Fourteen patients underwent LOPR for PPU and the details of these patients are presented. Mean age was 46 years (range 22−87 years) and there was only 1 female patient (7%) in the study group. Nine patients (64%) were smokers, 1 patient (7%) was on nonsteroidal anti-inflammatory medicine and another 1 patient (7%) was on traditional Chinese medicine for longstanding history of epigastric pain.
All the patients had history of sudden onset epigastric pain of less than 24-hour duration. One patient (7%) had previous history of peptic ulcer disease. None of the patients had previous abdominal surgeries. Twelve patients (86%) had a Boey score of 0 and 2 patients (14%) had score of 1. Chest X-ray showed free air under diaphragm in 9 patients (64%) and in other 5 patients, a computerized tomography scan was done to confirm the diagnosis of perforated peptic ulcer. All patients had MPI < 21 and mean index score was 14 (range 10−20). The predicted POSSUM morbidity and mortality were 36% and 7%, respectively. The demographic and clinical patient characteristics are listed in Table 1.
Table 1.
Demographic and clinical data of 14 patients who underwent laparoscopic omental patch repair (LOPR)
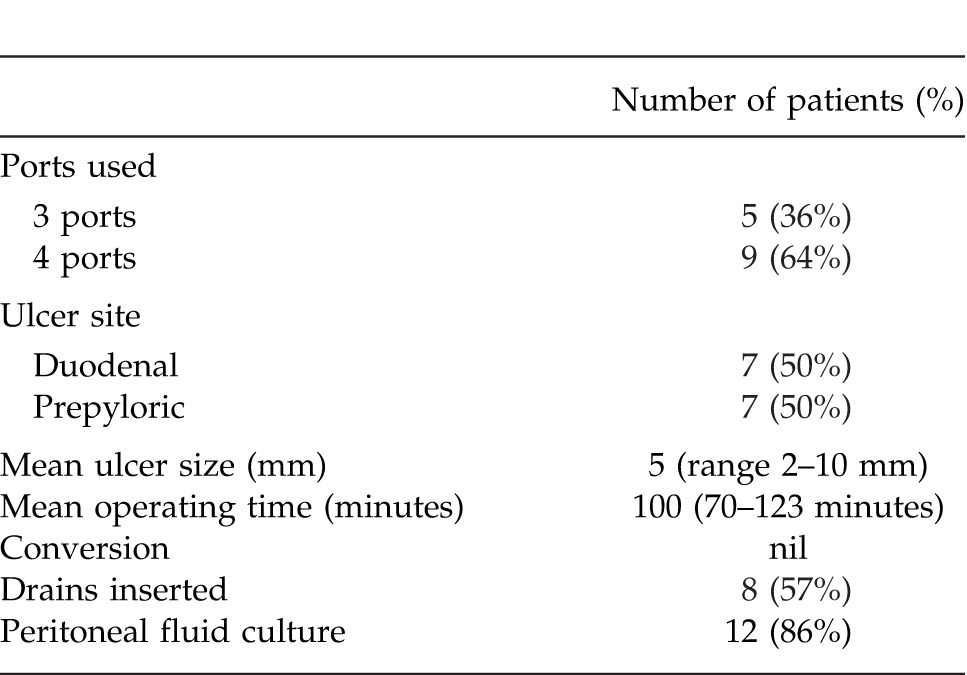
There were 7 gastric and 7 duodenal ulcers each with majority of the gastric ulcers in the prepyloric location. Mean ulcer size was 5 mm (range 2–10 mm). Five patients (36%) were (American Society of Anesthesiology score) ASA 1; additionally, 8 patients (57%) were ASA 2, and 1 patient (7%) had ASA score of 3. Nine patients (64%) had a 4-port technique and 5 patients (36%) had 3-port technique. Mean operating time was 100 minutes (range 70–123 minutes). Mean operating time for 4-port technique was 102 minutes, while for 3-port technique was 97 minutes (P > 0.05). There were no conversions, complications or mortality. Table 2 illustrates operative findings of 14 patients who underwent LOPR.
Table 2.
Operative findings
No patient was prescribed patient controlled analgesia and all the patients were able to tolerate soft diet before the fourth postoperative day. Median postoperative Day 1 pain score was 2 on visual analogue scale. Average length of hospital stay was 4 days (range 3–6 days) and 1 patient required high dependency ward admission and monitoring. Twelve patients had peritoneal fluid sent for culture, and 9 patients (75%) had negative fluid culture. One patient each (8%) had E. coli, S. aureus, and Candida albicans in the peritoneal fluid. There was no morbidity or mortality for LOPR.
Discussion
Peptic ulcer perforation (PPU) is a common emergency surgical cause of secondary peritonitis. The majority of our patients fit the profile of a typical patient presentation, i.e., young (mean age, 46 years) male (93%) with history of sudden-onset epigastric pain. Sixty-four percent of our patients were smokers. Although most patients with PPU do not have a history of previous peptic ulcer disease, previous history of ulcer disease does increase the risk. Use of nonsteroidal anti-inflammatory medicines also increase the risk of both upper gastrointestinal bleeding and perforation.5,6 Free air under the diaphragm was revealed in approximately 64% of patients on an erect chest film. This comparative lower proportion of patients with a free air on erect chest film is likely due to early presentation. We do not routinely perform lateral decubitus films or instill air via nasogastric tube and repeat chest films. At our institution, all patients with acute abdomen and with suspicion of perforated peptic ulcer are subjected to a computerized tomography scan of the abdomen if the chest X-ray fails to reveal a free air under the diaphragm.
Once a diagnosis of perforated peptic ulcer is made, prompt treatment within 12 hours is recommended to reduce morbidity and mortality.7 All our patients would receive intravenous omeprazole, antibiotics and fluid resuscitation as per sepsis guidelines and were operated within 6 hours of diagnosis.8 This is achieved as our unit has a policy to prioritize all emergency surgical case listings based on the timing prior to which the surgery must be started.
Boey scoring system has been shown to predict mortality and conversion (to open surgery) risk.9,10 Mannheim Peritonitis Index has also been shown to predict mortality in perforated peptic ulcer disease.11 Our patients had a low Boey score and low MPI. This means earlier presentation, less comorbidities, less deranged physiology and higher likelihood of a successful LOPR with less morbidity. This patient profile is favorable for an inception of a LOPR program. There is no role of nonoperative management of perforated peptic ulcer disease except in a moribund patient.
Emergency repair of PPU is associated with a significant morbidity and mortality.7,12 Simple techniques like omental patch repair have gained wider acceptance and gastro-duodenal resectional procedures are reserved for a minority of selected patients. Only 3 patients in our overall series of 45 patients had to undergo resectional procedures and our overall mortality is 8.9%.
Minimal access surgery has proliferated over last 3 decades and surgery for abdominal emergency is no exception. Laparoscopy for an emergent abdominal condition can serve as an excellent diagnostic tool, facilitate the management of pathology and can potentially avoid a nontherapeutic laparotomy.13 In a recent randomized trial, overall 7% patients had a diagnosis other than PPU and authors have stressed the importance of laparoscopy in treatment planning.14 At our institution we are increasingly offering diagnostic laparoscopy for both trauma related and unrelated abdominal emergencies. We were able to achieve a preoperative diagnosis in all our patients and therefore did not use laparoscopy as a diagnostic tool.
LOPR is an attractive option for perforated peptic ulcer. Cuschieri and coworkers described laparoscopic management of PPUU in 1990.15 Since the first description of LOPR almost 2 decades ago, there have been an increasing awareness and acceptance to incorporate LOPR in to routine surgical practice. In comparison to open surgical repair, it can reduce the postoperative wound pain, shorten the hospital stay and potentially improve outcomes by facilitating the peritoneal lavage. Various techniques of repair have been described, the simplest of all being sutureless fibrin glue or gelatin plug repair. Fibrin glue application is easy and does not require laparoscopic suturing skills but is associated with unacceptably high leak rates.16 Leakage following a repair is associated with increased morbidity and mortality. Hence sutureless methods of repair for PPU are yet to gain wide acceptance.
LOPR is associated with high conversion rates and may not be feasible in hemodynamically unstable patients, non-juxta pyloric ulcers and ulcers > 10 mm size.17 We believe that during the learning curve, it is safe to exclude patients who meet such criteria. This is evident by majority of our patients with Boey score of 0 and all our patients with MPI < 21. Table 3 summarizes suitable case selection during the learning curve period to ensure safety, enhance competency, promote training, gain proficiency and avoid complications during the learning curve. In our local experience, up to one-third of patients would meet such criteria. We do not have any mortality in our LOPR group due to an obvious selection bias.
Table 3.
Suitable case selection for surgical training

We believe that enormous experience can be acquired by performing LOPR on this highly-selected group of patients and the indications can be liberalized once the competency and experience are gained. Such an approach would ensure patient safety as well as enhance learning/training opportunities. We had no complications, conversions or mortality with such a selective approach. Lau WY and coworkers were the first to conduct and publish a randomized control trial on this topic.18 They reported 24 patients with seven open conversions and a mean operating time of 112.9 minutes. Our mean operating time of 100 minutes is comparable. Siu et al have reported shorter operating times for laparoscopic surgery than for open surgery.1 This may be due to the fact that in their study there were 4 operating surgeons (1 consultant, 2 senior registrars, and 1 registrar) and also 2 trocars (10 mm each) were used. We used only 1 cannula (10 mm) for an optical device and all the working ports were inserted via the 5-mm cannula. This requires removal of a camera and changing to a 5-mm camera system to handle the needle. Interestingly using either a 3-port or a 4-port technique did not result in a difference in operating time. We were able to identify and locate the site of perforation in all cases and there were no technical difficulties encountered. The majority of our patients had a sterile peritoneal fluid culture (75%), indicating early presentation with chemical peritonitis prior to the onset of bacterial contamination. There were no conversions, and all the patients were able to tolerate diet from third day onwards.
In our institution it is a routine to prescribe an opiate-based, patient-controlled analgesia after an open laparotomy. LOPR patients had significant less postoperative pain and reduced analgesia requirements with a median pain score of 2 on the first postoperative day. Mean hospital stay was 4 days (range 3–6 days) and this is consistent with the current literature. A recent Cochrane review suggested that results of laparoscopic approach are not clinically different compared to open surgical repair.19 However the emergent clinical presentation, frequently during the odd hours of the day, restricts the availability of senior expertise, which is necessary to initiate, promote and support the learning the curve of advanced surgical trainees. This is compounded with the fact that many patients have a delayed presentation with deranged physiopathology which precludes laparoscopic approach. Hence modern surgical practice has not yet been able to uniformly advance this minimal access surgical strategy. Our study is limited by a small sample size; however, this is a result of our early experience in reference to an inception of a LOPR training program for acute surgical trainees. There is also a selection bias in favor of laparoscopic group and hence we did not perform a comparison with open surgery group.
If the proposed strict case selection criteria are applied, LOPR may be safely performed on a significant number of PPU patients with minimal perioperative morbidity and mortality. Application of this method should take into consideration the availability of local expertise in advanced laparoscopy to optimize patient outcomes.
References
- 1.Siu WT, Leong HT, Law BK, Chau CH, Li AC, Fung KH, et al. Laparoscopic repair for perforated peptic ulcer: a randomized controlled trial. Ann Surg. 2002;235(3):313–319. doi: 10.1097/00000658-200203000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boey J, Wong J. Perforated duodenal ulcers. World J Surg. 1987;11(3):319–324. doi: 10.1007/BF01658109. [DOI] [PubMed] [Google Scholar]
- 3.Wacha H, Linder MM, Feldman U, Wesch G, Gundlach E, Steifensand RA. Mannheim peritonitis index – prediction of risk of death from peritonitis: construction of a statistical and validation of an empirically based index. Theoretical Surg. 1987;1:169–177. [Google Scholar]
- 4.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78(3):355–360. doi: 10.1002/bjs.1800780327. [DOI] [PubMed] [Google Scholar]
- 5.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann Intern Med. 1991;115(10):787–796. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- 6.Lanas A, Serrano P, Bajador E, Esteva F, Benito R, Sáinz R. Evidence of aspirin use in both upper and lower gastrointestinal perforation. Gastroenterology. 1997;112(3):683–689. doi: 10.1053/gast.1997.v112.pm9041228. [DOI] [PubMed] [Google Scholar]
- 7.Svanes C, Lie RT, Svanes K, Lie SA, Soreide O. Adverse effects of delayed treatment for perforated peptic ulcer. Ann Surg. 1994;220(2):168–175. doi: 10.1097/00000658-199408000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 9.Boey J, Choi SK, Poon A, Alagaratnam TT. Risk stratification in perforated duodenal ulcer: a prospective validation of predictive factors. Ann Surg. 1987;205(1):22–26. doi: 10.1097/00000658-198701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau H. Laparoscopic repair of perforated duodenal ulcer: a meta analysis. Surg Endosc. 2004;18(7):1013–1021. doi: 10.1007/s00464-003-8266-y. [DOI] [PubMed] [Google Scholar]
- 11.Uccheddu A, Floris G, Altana ML, Pisanu A, Cois A, Farci SL. Surgery for perforated peptic ulcer in the elderly. Evaluation of factors influencing prognosis. Hepatogastroenterology. 2003;50(54):1956–1958. [PubMed] [Google Scholar]
- 12.Moller MH, Adamsen S, Wojdemann M, Moller M. Perforated peptic ulcer: how to improve outcome? Scand J Gastroenterol. 2009;44(1):15–22. doi: 10.1080/00365520802307997. [DOI] [PubMed] [Google Scholar]
- 13.Kirshtein B, Roy-Shapira A, Lantsberg L, Mandel S, Avinoach E, Mizrahi S. The use of laparoscopy in abdominal emergencies. Surg Endosc. 2003;17(7):1118–1124. doi: 10.1007/s00464-002-9114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertleff M, Halm JA, Bemelman WA, van der Ham AC, van der Harst E, Oei HI, et al. Randomized clinical trial of laparoscopic versus open repair of the perforated peptic ulcer: The LAMA trial. World J Surg. 2009;33(7):1368–1373. doi: 10.1007/s00268-009-0054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathanson LK, Easter DW, Cuschieri A. Laparoscopic repair/peritoneal toilet of perforated duodenal ulcer. Surg Endosc. 1990;4(4):232–233. doi: 10.1007/BF00316801. [DOI] [PubMed] [Google Scholar]
- 16.Lee FYJ, Leung KL, Lai PBS, Lau JWY. Selection of patients for laparoscopic repair of perforated peptic ulcer. Br J Surg. 2001;88(1):133–136. doi: 10.1046/j.1365-2168.2001.01642.x. [DOI] [PubMed] [Google Scholar]
- 17.Siu WT, Chau CH, Law BKB, Tang CN, Ha PY, Li MKW. Routine use of laparoscopic repair for perforated peptic ulcer. Br J Surg. 2004;91(4):481–484. doi: 10.1002/bjs.4452. [DOI] [PubMed] [Google Scholar]
- 18.Lau WY, Leung KL, Kwong KH, Davey IC, Robertson C, Dawson JWJ, et al. A randomized study comparing laparoscopic versus open repair of perforated peptic ulcer using suture or sutureless technique. Ann Surg. 1996;224(2):131–138. doi: 10.1097/00000658-199608000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanabria A, Villegas MI, Morales Uribe CH. Laparoscopic repair for perforated peptic ulcer disease. Cochrane Database Syst Rev. 2005;19(4) doi: 10.1002/14651858.CD004778.pub2. CD004778. [DOI] [PubMed] [Google Scholar]