Abstract
Progesterone influences central neuronal excitability, a key event in migraine pathophysiology. Progesterone receptor gene (PGR) rs1042838 (G/T - Val660Leu) variant is indicative of PROGINS haplotype and associated to a reduced PGR activity. With the aim of investigating whether any type of association existed between this genetic variant and migraine pathophysiology, genotyping was performed in 380 consecutive migraine patients and 185 age-, sex-, and race-ethnicity-matched healthy controls from Interinstitutional Multidisciplinary BioBank (BioBIM) of IRCCS San Raffaele Pisana, Rome, Italy. rs1042838 genotypes did not correlate with demographics or clinical migraine features. However, TT (Leu) genotype was significantly associated with a later age of migraine onset: Patients affected by migraine with aura showed a linear relationship between copy number of the T allele carried by the individual and the age of migraine onset. Our data suggest that the PROGINS PGR polymorphism does not directly predispose to migraine but significantly delays migraine onset probably via a reduction in brain neuronal excitability.
Introduction
Migraine is the most common disabling neurological disorder involving sensory sensitivity and characterized by recurrent episodes of headache (Barbanti et al., 2011). Despite the prevalence and social consequences of this condition, the molecular and genetic mechanisms underlying the pathophysiology of migraine are still partly unknown (Goadsby et al., 2009).
Various clinical and epidemiological observations indicate a strong correlation between female gender, sex female hormones, and migraine susceptibility: (1) The disease is predominant in women after puberty (Gupta et al., 2007; Corominas et al., 2009); (2) the clinical course is modified by reproductive events such as menarche, pregnancy, and menopause (Mac Gregor, 1997; Brandes, 2006; Martin and Behbehani, 2006a); and (3) the use of hormonal contraceptives and hormone replacement may influence migraine occurrence (Massiou and Mac Gregor, 2000; Martin and Behbehani, 2006b; Nappi et al., 2013).
According to the significant female hormone involvement in migraine, some authors have postulated that genes encoding hormone receptors and causing changes in neurotransmitter systems may play a role in the molecular pathogenesis of migraine headache (Colson et al., 2004, 2005; Martin and Behbehani, 2006a; Lee et al., 2007; Joshi et al., 2010; Schürks et al., 2010; Rodriguez-Acevedo et al., 2013).
However, while the correlation between migraine and estrogen receptors appears to be fairly assessed, the association studies with progesterone receptor are less numerous and more controversial (Schürks et al., 2010).
Progesterone receptor gene (PGR), located on chromosome 11q22 (OMIM# 607311), encodes a steroid receptor that principally mediates the effect of progesterone on the establishment and maintenance of reproductive events (UniProt# P06401).
PGR is characterized by the presence of three common variants consisting of a 306-bp ALU insertion/deletion in intron 7/G, a missense Val660Leu (G/T substitution) (rs1042838) in exon 4, and a silent His770His (C/T substitution) (rs1042839) in exon 5. All these three variants are in linkage disequilibrium; therefore, the variation of a single polymorphism is indicative of the simultaneous variation of the other two (Rockwell et al., 2012).
On the basis of such biological evidence, several authors define these complex variants with the name of “PROGINS haplotype” (Alu insertion and T alleles for the other two single nucleotide polymorphisms [SNPs]) determining a reduced amount of gene transcripts and decreased protein activity (Romano et al., 2007; Rockwell et al., 2012).
Several studies have investigated a possible association between PGR polymorphisms and migraine (Colson et al., 2004, 2005; Lee et al., 2007; Corominas, et al., 2009; Joshi et al., 2010; Schürks et al., 2010; Rodriguez-Acevedo et al., 2013), providing conflicting results due to several biases, including a poor clinical patients' characterization.
Therefore, to shed more light on this topic, we investigated the association between PGR rs1042838 polymorphism and migraine in a large cohort of Mediterranean Caucasian migraine patients from the Interinstitutional Multidisciplinary BioBank (BioBIM) of IRCCS San Raffaele Pisana, Rome, extensively investigating and carefully detailing their demographic and clinical features.
Materials and Methods
Patients' recruitment, inclusion and exclusion criteria
Since January 2008, 380 Mediterranean Caucasian unrelated individuals affected by migraine without aura (MwoA), migraine with aura (MwA), and chronic migraine (CM) (Headache Classification Committee of the International Headache Society, 2013) were consecutively recruited at the Headache and Pain Unit, IRCCS San Raffaele Pisana, Rome, Italy. Specifically trained neurologists have performed a complete physical examination on migraine patients, including a neurological and funduscopic examination and anthropometric measurements. Detailed information on clinical migraine features, concomitant diseases and treatments, sociodemographics, and lifestyle was gathered with face-to-face interviews using a structured questionnaire, as previously described (Palmirotta et al., 2013a). Comorbidities diagnoses were based on analysis of medical charts and documents, symptoms, treatments, and the patient's self-reported medical history. Patients with significant metabolic, renal, endocrine, hepatic, pulmonary, cardiovascular, or other systemic disease were excluded from the study.
We enrolled in our Biobank 185 healthy controls from subjects in a state of good health with no significant medical diseases and not-pharmaceutical therapies. They were screened for sociodemographics, lifestyle, and concomitant diseases and treatments by a direct interview using a structured questionnaire. Healthy subjects with a family history of migraine or other neurological diseases were excluded.
Informative and consent forms, approved by the institutional Ethics Committee of San Raffaele Scientific Institute, were provided to both groups, along with the permission to obtain blood samples for research purposes (Palmirotta et al., 2013a).
Genotyping
All biological samples were stored at the BioBIM of IRCCS San Raffaele Pisana following the biobanking standard operative procedures (Palmirotta et al., 2011). DNA extraction, PCRs, and sequencing analysis were performed as previously described (Palmirotta et al., 2013b). PGR polymorphism rs1042838 (G/T - Val660Leu) was determined by a 135-bp PCR amplification on the basis of the PGR Ensembl (Ensembl accession number ENSG00000082175) using the following primers: F5′-AGTTGTGAGAGCACTGGATGC-3′ and R5′-AACAGGTTGATCAGTGGTGG-3′.
Statistical analysis
An association between categorical variables was assessed by a generalized Cochran–Mantel–Haenszel test to control for gender as a confounder. For continuous variables, mean differences were evaluated through a univariate general linear model (GLM) for fixed main effects. Particularly, to avoid an excessive stratification, the effects of familiarity for migraine and the kind of migraine were controlled, considering them as factors in the GLM besides the PGR genotype.
Tukey's HSD post hoc test was used for pairwise comparisons that were possible. Normality was assessed through the Kolmogorv–Smirnov test, and homogeneity of variance was assessed through the Levene test. Logistic regression was used to estimate odds ratio (OR). Statistical models were corrected for the most significant covariates found in this study (familiarity for migraine). Statistical significance was assessed for p<0.05 (two-tailed probability). All calculations were done by IBM SPSS 20 (IBM Corp., Armonk, NY), except for power analysis done by G*Power 3.1.3 free software (Heinrich Heine Universitat, Dusseldorf, Germany). Data are represented as mean±standard deviation or mean±standard error of the mean.
Results
Demographic characteristics and the age of migraine onset of the study populations are reported in Table 1. Genotypes and the allele frequencies of the rs1042838 polymorphism between migraineurs and controls did not significantly differ from those predicted by the Hardy–Weinberg equilibrium in controls (chi-squared=3.71, p=0.054) as well as in MwoA (chi-squared=0.03, p=0.820), MwA (chi-squared=0.95, p=0.329), and CM (chi-squared=0.8, p=0.370) patients (Table 2). When controlling for gender, no significant statistical association was found between the PGR genotype and the kind of migraine (p=0.666), laterality (p=0.242), unilateral autonomic symptoms (p=0.229), prophilaxis (p=0.124), abuse of drug (p=0.748), and kind of drug abused (p=0.732).
Table 1.
Baseline Characteristics of the Migraine Patients and Controls
| Variable | Controls (180) | All patients with migraine (380) | MwA (64) | MwoA (191) | CM (125) |
|---|---|---|---|---|---|
| Sex, F/M (F%) | 124/56 (68.9) | 317/63 (83.4) | 49/15 (76.6) | 156/35 (81.7) | 112/13 (89.6) |
| Age at observation, mean (SD) | 38.9 (12.0) | 42.6 (12.9) | 39.3 (12.7) | 40.4 (11.8) | 47.5 (13.3) |
| Age at migraine onset, mean (SD) | — | 20.9 (11.9) | 22.1 (12.7) | 20.5 (11.6) | 21.0 (12.0) |
CM, chronic migraine; F, female; M, male; MwA, migraine with aura; MwoA, migraine without aura; SD, standard deviation.
Table 2.
Distributions of Genotype and Allele Frequencies of PGR rs1042838 Polymorphism Observed in Patients and Controls
| PGR genotypes (%) | PGR alleles (%) | ||||||
|---|---|---|---|---|---|---|---|
| n | GG | GT | TT | G | T | HW (p) | |
| Controls | 180 | 139 (77.2) | 35 (19.4) | 6 (3.3) | 313 (86.9) | 47 (13.1) | 0.054 |
| Male (%) | 56 | 36 (64.3) | 15 (26.8) | 5 (8.9) | 87 (77.7) | 25 (22.3) | 0.089 |
| Female (%) | 124 | 103 (83.1) | 20 (16.1) | 1 (0.8) | 226 (91.1) | 22 (8.9) | 0.978 |
| All patients with migraine (%) | 380 | 274 (72.1) | 95 (25.0) | 11 (2.9) | 643 (84.6) | 117 (15.4) | 0.432 |
| Male (%) | 63 | 46 (73.0) | 16 (25.4) | 1 (1.6) | 108 (85.7) | 18 (14.3) | 0.769 |
| Female (%) | 317 | 228 (71.9) | 79 (24.9) | 10 (3.2) | 535 (84.4) | 99 (15.6) | 0.333 |
| MwA (%) | 64 | 45 (70.3) | 16 (25.0) | 3 (4.7) | 106 (82.8) | 22 (17.2) | 0.330 |
| Male (%) | 15 | 11 (73.3) | 4 (26.7) | 0 (0.0) | 26 (86.7) | 4 (13.3) | 0.551 |
| Female (%) | 49 | 34 (69.4) | 12 (24.5) | 3 (6.1) | 80 (81.6) | 18 (18.4) | 0.199 |
| MwoA (%) | 191 | 137 (71.7) | 50 (26.2) | 4 (2.1) | 324 (84.8) | 58 (15.2) | 0.821 |
| Male (%) | 35 | 27 (77.1) | 8 (22.9) | 0 (0.0) | 62 (88.6) | 8 (11.4) | 0.445 |
| Female (%) | 156 | 110 (70.5) | 42 (26.9) | 4 (2.6) | 262 (84.0) | 50 (16.0) | 0.997 |
| CM (%) | 125 | 92 (73.6) | 29 (23.2) | 4 (3.2) | 213 (85.2) | 37 (14.8) | 0.371 |
| Male (%) | 13 | 8 (61.5) | 4 (30.8) | 1 (7.7) | 20 (76.9) | 6 (23.1) | 0.631 |
| Female (%) | 112 | 84 (75.0) | 25 (22.3) | 3 (2.7) | 193 (86.2) | 31 (13.8) | 0.498 |
Values are given as n (%).
HW, Hardy–Weinberg equilibrium; PGR, progesterone receptor gene.
PGR was not a factor for frequency of attacks per month (males p=0.702, females p=0.773), for the quantity of drug abused (males p=not calculable, females p=0.143), and for the duration of abuse (males p=not calculable, females p=0.896).
The small number of male patients did not allow a reliable analysis of variance to be performed.
With regard to female patients, the type of migraine (p=0.749) and the familiarity for migraine (p=0.158) were not factors for mean onset age nor for considering each subgroup of migraineurs patients (interaction term: type of migraine*familiarity p=0.295). Conversely, PGR genotype was a significant factor (p=0.014, effect size: partial η2=0.027), with no difference in each group of migraine type (p=0.092).
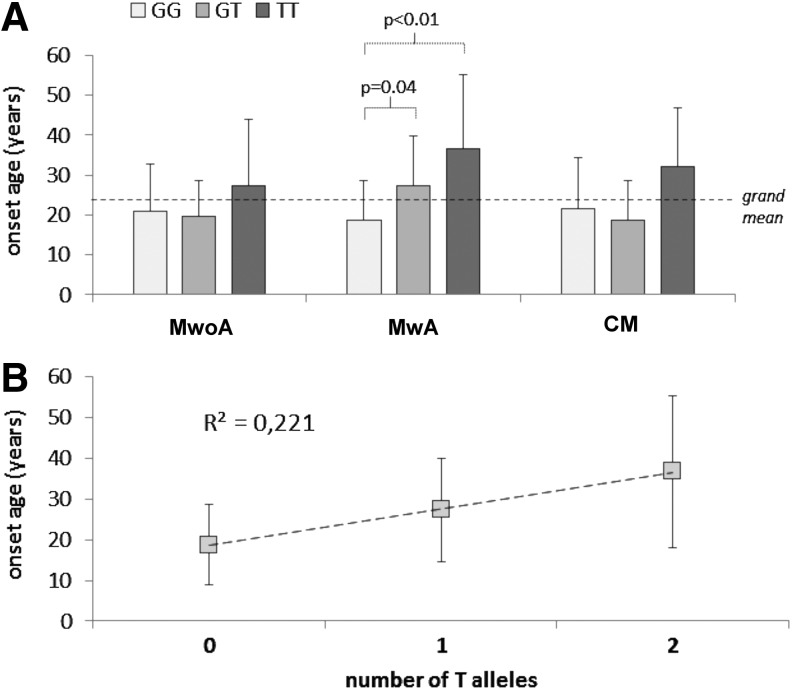
However, in the sole MwA female patients, a significant difference was found between GG carriers and GT carriers (p=0.04), and between GG carriers and TT carriers (p<0.01) (Fig. 1A). Notably, a significantly slight linear relation (r2=0.221; slope=9.08±2.49, p<0.01; intercept=18.34±1.74, p<0.01) was found between the number of T alleles possessed and mean shift in onset age. Hence, it was as follows: Mean onset age in MwA females=18.34 years+9.08*(number of T alleles) years (Fig. 1B).
FIG. 1.
(A) Mean onset age in different migraine kind groups according to the progesterone receptor gene (PGR) genotype; (B) linear relation between mean onset age and number of T alleles in MwA females. CM, chronic migraine; MwA, migraine with aura; MwoA, migraine without aura.
However, in the same group, the PGR TT genotype failed to give a statistically significant OR with regard to an onset age greater than the average for women seen in this study (20.9±11.9 years).
The power reached for the ANOVA model was 92.2% to detect and an effect size like a Cohen's d 1.0; for the logistic regression, it was 82.3% to detect and an effect size like an OR of 1.2.
No correlations were found between mean onset age of migraine and age of menarche (p=0.232), nor were they found between PGR genotype and age of menarche (p=0.84).
Discussion
In this study, we found that the rs1042838 PGR polymorphism, indicative of PROGINS haplotype, is not associated with migraine risk or clinical features but correlates with the age of migraine onset in female patients.
The frequency of this genotype in our study is similar to that observed in normal Caucasian subjects (Schürks et al., 2010). Previous association studies on the link between PROGINS polymorphism and migraine yielded conflicting results: A positive correlation was found in two independent Caucasian populations (Colson et al., 2005): a protective association in an Indian population (Joshi et al., 2010) and no association in a Caucasian and in a mixed population (Corominas et al., 2009; Rodriguez-Acevedo et al., 2013). A meta-analysis study also confirmed the lack of association between PGR gene variants and migraine (Schürks et al., 2010).
Our data support the lack of association between this variant, migraine risk, or migraine clinical features. However, a new clinically useful finding from this study is the evidence of an association between rs1042838 TT genotype, indicative of the “PROGINS variant haplotype,” and a delayed age of migraine onset compared with GT and GG genotypes, with a linear relationship in the MwA female patient group.
Interestingly, in a precedent study performed on 150 migraine patients selected for the presence of concomitant vertigo (migraine-associated vertigo [MAV]), Lee et al. (2007) reported that the PGR PROGINS variant allele T of rs1042838 was significantly associated with MAV. We could not test this effect in our study, as only a few patients had vertigo, a symptom that is more frequent in young migraineurs. It should be emphasized that the mean age of our patients (42±12.9 years) is much higher than that of subjects analyzed in previous studies (Corominas et al., 2009; Joshi et al., 2010).
Our data support the hypothesis that the PGR gene is not a direct migraine risk factor but is more a disease-modifier gene whose own variants result in a more delayed disease onset.
How the PROGINS variants might influence the age of migraine onset is a matter of speculation. We hypothesize that it could alter brain cortical excitability. In fact, the ALU insertion of PROGINS haplotype results in a reduction of gene transcript and the missense Val600Leu leads to different PGR phosphorylation and degradation on ligand binding (Romano et al., 2007). Consequently, the subjects carrying the PROGINS polymorphism show a reduced progesterone responsiveness due to the decreased receptor bioactivity (Romano et al., 2007; D'Amora et al., 2009).
Sex steroids influence neuronal firing through steroid receptors dispersed throughout the brain (Noseda and Burstein, 2013). In the rat, progesterone may enhance neuronal excitability as estrogens (Choudhuri et al., 2002; Chauvel et al., 2013), although its exact molecular mechanisms are unclear. Central neuronal excitability plays a crucial role in the predisposition to develop migraine (Aurora and Wilkinson, 2007) as inferred by cortical spreading depression (CSD), the electrophysiological substrate of MwA and possibly MwoA. Usually, migraine starts in puberty, when sex hormones contribute toward lowering the neuronal response threshold to trigger factors in predisposed individuals. Ovarian hormones have a complex role in CSD susceptibility. In ovariectomized animals, CSD frequency is markedly decreased compared with normal cycling rats. Estrogens can increase the susceptibility to CSD through different mechanisms encompassing effects on glutamate neurotransmission, as well as through an increase of dendritic spines and synaptic density (Eikermann-Haerter et al., 2012). Interestingly, it has been recently demonstrated that progesterone also significantly increases CSD frequency and restores the CSD inhibition by L-kynurenine (Chauvel et al., 2013).
We, therefore, suggest that a lower progesterone receptor functionality in rs1042838 T allele carriers might reduce progesterone excitatory effects on neurons, raising their response threshold to migraine triggers and, hence, leading to a delayed onset of the disease. Considering the correlation between estrogens, CSD susceptibility, and glutamate neurotransmission, it is important to note the linear correlation we found between the copy number of T allele and the age at migraine onset in female patients affected by MwA, the migraine type most clearly characterized by a glutamate-dependent cortical hyperexcitability (Eikermann-Haerter et al., 2012). In addition, it is interesting to report that previous genome-wide analysis and association studies suggested a significant association between “PROGINS” variant and the increase of age for menarche (Guo et al., 2006; Taylor et al., 2010). However, our data did not allow us to find any correlation between age of menarche, PROGINS genotype, and age of migraine onset.
The main strength of this study is the detailed demographic and clinical characterization of migraine patients that allowed for clinical correlations neglected in previous studies (Palmirotta et al., 2013b). Limitation to acknowledge is the presence of possible artifacts, as the number of MwA patients might be too low for sub-analysis.
Conclusion
PROGINS PGR polymorphism does not influence migraine risk and features but is positively correlated with a later age of migraine. Further genetic association studies are imperative to understand the role of PGR in migraine physiopathology that may be useful as a biomarker for detecting asymptomatic individuals at an increased risk for migraine and for developing effective treatment strategies as well (Eikermann-Haerter et al., 2012; Nappi et al., 2013).
Acknowledgments
This study was partially supported by the Grant MERIT RBNE08NKH7 to San Raffaele Foundation Ceglie Messapica (www.fondazionesanraffaele.com/) and partially by the grant PO FESR 2007/2013 Linea di Intervento 4.1.1.1–SIASOP.
Disclosure Statement
No competing financial interests exist.
References
- Aurora S.K., and Wilkinson F. (2007). The brain is hyperexcitable in migraine. Cephalalgia 27,1442–1453 [DOI] [PubMed] [Google Scholar]
- Barbanti P., Aurilia C., Egeo G., and Fofi L. (2011). Migraine prophylaxis: what is new and what we need? Neurol Sci 32Suppl 1,S111–S115 [DOI] [PubMed] [Google Scholar]
- Brandes J.L. (2006). The influence of estrogen on migraine: a systematic review. JAMA 295,1824–1830 [DOI] [PubMed] [Google Scholar]
- Chauvel V., Schoenen J., and Multon S. (2013). Influence of ovarian hormones on cortical spreading depression and its suppression by L-kynurenine in rat. PLoS One 8,e82279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri R., Cui L., Yong C., Bowyer S., Klein R.M., Welch K.M., and Berman N.E. (2002). Cortical spreading depression and gene regulation: relevance to migraine. Ann Neurol 51,499–506 [DOI] [PubMed] [Google Scholar]
- Colson N.J., Lea R.A., Quinlan S., Mac Millan J., and Griffiths L.R. (2004). The estrogen receptor 1 G594A polymorphism is associated with migraine susceptibility in two independent case/control groups. Neurogenetics 5,129–133 [DOI] [PubMed] [Google Scholar]
- Colson N.J., Lea R.A., Quinlan S., Mac Millan J., and Griffiths L.R. (2005). Investigation of hormone receptor genes in migraine. Neurogenetics 6,17–23 [DOI] [PubMed] [Google Scholar]
- Corominas R., Ribasés M., Cuenca-León E., Cormand B., and Macaya A. (2009). Lack of association of hormone receptor polymorphisms with migraine. Eur J Neurol 16,413–415 [DOI] [PubMed] [Google Scholar]
- D'Amora P., Maciel T.T., Tambellini R., Mori M.A., Pesquero J.B., Sato H., Girão M.J., Guerreiro da Silva I.D., and Schor E. (2009). Disrupted cell cycle control in cultured endometrial cells from patients with endometriosis harboring the progesterone receptor polymorphism PROGINS. Am J Pathol 175,215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikermann-Haerter K., Can A., and Ayata C. (2012). Pharmacological targeting of spreading depression in migraine. Expert Rev Neurother 12,297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby P.J., Charbit A.R., Andreou A.P., Akerman S., and Holland P.R. (2009). Neurobiology of migraine. Neuroscience 161,327–341 [DOI] [PubMed] [Google Scholar]
- Guo Y., Shen H., Xiao P., Xiong D.H., Yang T.L., Guo Y.F., Long J.R., Recker R.R., and Deng H.W. (2006). Genomewide linkage scan for quantitative trait loci underlying variation in age at menarche. J Clin Endocrinol Metab 91,1009–1014 [DOI] [PubMed] [Google Scholar]
- Gupta S., Mehrotra S., Villalon C.M., Perusquia M., Saxena P.R., and Maassen Van Den Brink A. (2007). Potential role of female sex hormones in the pathophysiology of migraine. Pharmacol Ther 113,321–340 [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (IHS). (2013). The International Classification of Headache Disorders, 3rd Edition (beta version). Cephalalgia 33,629–808 [DOI] [PubMed] [Google Scholar]
- Joshi G., Pradhan S., and Mittal B. (2010). Role of the oestrogen receptor (ESR1 PvuII and ESR1 325 C->G) and progesterone receptor (PROGINS) polymorphisms in genetic susceptibility to migraine in a North Indian population. Cephalalgia 30,311–320 [DOI] [PubMed] [Google Scholar]
- Lee H., Sininger L., Jen J.C., Cha Y.H., Baloh R.W., and Nelson S.F. (2007). Association of progesterone receptor with migraine-associated vertigo. Neurogenetics 8,195–200 [DOI] [PubMed] [Google Scholar]
- Mac Gregor E.A. (1997). Menstruation, sex hormones, and migraine. Neurol Clin 15,125–141 [DOI] [PubMed] [Google Scholar]
- Martin V.T., and Behbehani M. (2006a). Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis-part I. Headache 46,3–23 [DOI] [PubMed] [Google Scholar]
- Martin V.T., and Behbehani M. (2006b). Ovarian hormones and migraine headache: understanding mechanisms and pathogenesis-part 2. Headache 46,365–386 [DOI] [PubMed] [Google Scholar]
- Massiou H., and Mac Gregor E.A. (2000). Evolution and treatment of migraine with oral contraceptives. Cephalalgia 20,170–174 [DOI] [PubMed] [Google Scholar]
- Nappi R.E., Merki-Feld G.S., Terreno E., Pellegrinelli A., and Viana M. (2013). Hormonal contraception in women with migraine: is progestogen-only contraception a better choice? J Headache Pain 14,66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R., and Burstein R. (2013). Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain 154Suppl 1,S44–S53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmirotta R., Barbanti P., Ludovici G., Egeo G., Aurilia C., Fofi L., De Marchis M.L., Spila A., Ferroni P., Della-Morte D., and Guadagni F. (2013a). Establishment of a biorepository for migraine research: the experience of Interinstitutional Multidisciplinary BioBank (BioBIM). Neurol Sci 34,1659–1663 [DOI] [PubMed] [Google Scholar]
- Palmirotta R., Ludovici G., De Marchis M.L., Savonarola A., Leone B., Spila A., De Angelis F., Della-Morte D., Ferroni P., and Guadagni F. (2011). Pre-Analytical procedures for DNA studies: the experience of the Interinstitutional Multidisciplinary BioBank (BioBIM). Biopreserv Biobank 9,35–45 [DOI] [PubMed] [Google Scholar]
- Palmirotta R., Ludovici G., Egeo G., Ialongo C., Aurilia C., Fofi L., De Marchis M.L., Della-Morte D., Barbanti P., and Guadagni F. (2013b). Prion protein gene M129V polymorphism and variability in age at migraine onset. Headache 53,540–545 [DOI] [PubMed] [Google Scholar]
- Rockwell L.C., Rowe E.J., Arnson K., Jackson F., Froment A., Ndumbe P., Seck B., Jackson R., and Lorenz J.G. (2012). Worldwide distribution of allelic variation at the progesterone receptor locus and the incidence of female reproductive cancers. Am J Hum Biol 24,42–51 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Acevedo A.J., Maher B.H., Lea R.A., Benton M., and Griffiths L.R. (2013). Association of oestrogen-receptor gene (ESR1) polymorphisms with migraine in the large Norfolk Island pedigree. Cephalalgia 33,1139–1147 [DOI] [PubMed] [Google Scholar]
- Romano A., Delvoux B., Fischer D.C., and Groothuis P. (2007). The PROGINS polymorphism of the human progesterone receptor diminishes the response to progesterone. J Mol Endocrinol 38,331–350 [DOI] [PubMed] [Google Scholar]
- Schürks M., Rist P.M., and Kurth T. (2010). Sex hormone receptor gene polymorphisms and migraine: a systematic review and meta-analysis. Cephalalgia 30,1306–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K.C., Small C.M., Epstein M.P., Sherman S.L., Tang W., Wilson M.M., Bouzyk M., and Marcus M. (2010). Associations of progesterone receptor polymorphisms with age at menarche and menstrual cycle length. Horm Res Paediatr 74,421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]