Abstract
Fatty acids and their derivatives play a role in the response to ocular disease. Our current study investigated the effects of dietary mead acid (MA, 5,8,11-eicosatrienoic acid) supplementation on N-methyl-N-nitrosourea (MNU)-induced cataract and retinal degeneration in Sprague-Dawley rats. Experiment 1 was designed to inhibit cataract formation, with the dams fed a 2.4% MA or basal (<0.01% MA) diet during lactational periods. On postnatal day 7, male pups received a single intraperitoneal (ip) injection of 50 mg/kg MNU or vehicle. Lens opacity and morphology were examined 7 and 14 days after the MNU injection. Experiment 2 was designed to inhibit retinal degeneration and was performed with female postweaning rats. In this experiment, dams were fed the 2.4% MA or basal diet during the lactational periods. Thereafter, the female pups were continuously fed the same diets during their postweaning periods. On postnatal day 21 (at weaning), pups received a single ip injection of 50 mg/kg MNU. Retinal morphology was examined 7 days after the MNU injection. In experiment 3, six-week-old female rats were fed the 2.4% MA or basal diet starting at one week before the MNU injection and were then continuously fed the same diets until sacrifice. Rats at 7 weeks of age were given a single ip injection of 40 mg/kg MNU, and the retina was then examined morphologically one week after the MNU injection. In experiment 1, mature cataract was found in all of the MNU-treated groups, with or without MA supplementation. In experiments 2 and 3, atrophy of both the peripheral and central outer retina occurred in all rats exposed to MNU, with or without MA supplementation, respectively. The severities of the cataracts and retinal atrophy in the rats were similar regardless of MA supplementation. Dietary mead acid, which is used as a substitute in essential fatty acid deficiency in the body, does not modify MNU-induced cataract and retinal degeneration in rat models.
Keywords: arachidonic acid, cataract, mead acid, N-methyl-N-nitrosourea, retinal degeneration, rats
Introduction
As of 2012, it has been estimated that 285 million people are visually impaired worldwide, with 39 million blind and 246 million with poor vision. According to estimates of the World Health Organization (WHO), the leading causes of chronic blindness are cataract (51%), glaucoma (8%), age-related macular degeneration (5%), childhood blindness including genetic disorders (4%), diabetic retinopathy (1%), and other causes (31%)1. VISION 2020, which is supported by the WHO Program for the Prevention of Blindness, is currently attempting to eliminate the main causes of blindness by 2020 in order to ensure all people in the world have the right to sight, particularly the millions who will needlessly become blind1, 2. Retinitis pigmentosa (RP) is a human disease characterized by the loss of photoreceptor cells, especially rods, with the loss leading to visual disturbance and eventually to blindness3. The prevalence of RP is about 1 in 4000, with about 2 million people affected worldwide. The majority of these patients will develop central posterior subcapsular cataracts4.
Animal models of cataract and retinal degeneration have been shown to be important tools for elucidating the mechanism of human blindness and exploring potential treatments5,6,7. N-methyl-N-nitrosourea (MNU) is an alkylating agent that targets the lens epithelial and photoreceptor cells, thereby rapidly inducing mature cataract and retinal degeneration in neonatal and adult rats, respectively7, 8. MNU-induced lens and retinal damage is due to selective 7-methyldeoxyguanosine DNA adduct formation in the lens epithelial and photoreceptor cell nuclei, which leads to cell death via an apoptotic mechanism8, 9. The MNU-induced apoptosis cascade involves upregulation of the Bax protein, down-modulation of the Bcl-2 protein, and activation of the caspase families. As one of the early signs of photoreceptor cell damage is disorientation of the outer segments, a decreased existence of PDE6β- and rhodopsin-labeled outer segments has been shown to be indicative of photoreceptor cell dysfunction in MNU-induced retinal degeneration in rats10. Several lens metabolites have been reported to be associated with MNU-induced cataract formation and development. Since α-amino acids, glutathione, and taurine have been shown to exhibit substantial decreases during the formation of cataract, these metabolites might be marker candidates11. Furthermore, since the currently used models can mimic the cell death process in human cataract and RP6, 12, they can be used for screening studies that examine potential new therapeutic interventions.
The effects of dietary supplementation of an n-3 polyunsaturated acid (PUFA) such as docosahexaenoic acid (DHA) and an n-6 PUFA such as arachidonic acid (AA) on retinal disorders have been extensively investigated in human patients4, 6, 13 and in the MNU-induced retinal degeneration models in rats5, 14. Supplementation with a 9.5% DHA-rich diet for 2 weeks prior to and after receiving MNU delayed progression of the MNU-induced photoreceptor damage in parallel with the serum DHA levels15. Administration of a 2.0% AA-rich diet during the gestation, lactation, and postweaning periods rescued rats from the retinal degeneration induced by MNU by inhibiting photoreceptor apoptosis14. However, few studies have investigated the effects of dietary supplementation of fatty acids on cataract. It has been reported that in humans, there is a 42% lower risk of cataract when subjects consume 0.5-1.42 g/day of n-3 PUFA via seafoods16. In contrast, it has been reported that n-6 PUFA appears to lead to significant damage of human lens epithelial cells in culture5.
Saturated and monounsaturated fatty acids can be synthesized de novo in most cells17. These fatty acids are used by the liver or transported as triglycerides, phospholipids and cholesterol esters to extrahepatic cells via very-low-density lipoproteins. Regarding the synthesis of polyunsaturated fatty acids such as DHA and AA, we need n-3 and n-6 precursor fatty acids, namely, α-linolenic acid (18:3n-3) and linoleic acid (18:2n-6), respectively, which must be obtained from our diet. The ocular organ also has a synthesis and transporting system. Futterman et al. (1968) demonstrated a de novo synthetic pathway in animal retinal tissues18. The retina accumulates DHA, which is transported from the liver via albumin19. Similarly, the lens has a de novo synthetic pathway20, and serum albumin works as a carrier of long-chain fatty acids into the lens21.
Mead acid (MA, 20:3n-9; also referred to as 5,8,11-eicosatrienoic acid) is an n-9 PUFA, and in adult animals it is a minor constituent of the plasma and tissue. During a state of essential fatty acid deficiency, adult mammals are able to synthesize MA from oleic acid22, 23. It was difficult to mass-produce MA industrially until recent years, and therefore, only a few studies on pharmacological research have been published24,25,26. Large quantities of MA have been found in the cartilage, where it is responsible for helping to decrease osteoblastic activity, maintain the cartilage, and prevent ossification, in addition to inhibiting vascular endothelial growth factor (VEGF)-stimulated angiogenesis24, 25. MA has been shown to inhibit the growth of human breast cancer cell lines in both in vitro and in vivo models21. However, there have not been any investigations of the effect of the n-9 PUFA, MA, on ocular disease. Therefore, the goal of our present study was to elucidate the effect of dietary MA on MNU-induced cataract and retinal degeneration in rats.
Materials and Methods
Animal procedures
The experimental protocol and all animal procedures used in this study were approved by the Animal Care and Use Committee of Kansai Medical University and were in accordance with the guidelines for animal experimentation at Kansai Medical University. Animals were housed in plastic cages with paper-chip bedding (Paper Clean; SLC, Hamamatsu, Japan) in an air-conditioned room maintained at 22 ± 2°C and a relative humidity of 60 ± 10% with a 12 hr light/dark cycle. The illumination intensity was below 60 lux in the cages. For experiments 1 and 2, 9-week-old pregnant Sprague-Dawley (SD) rats [Crl:CD] (n=10) were purchased from Charles River Laboratories Japan (Yokohama, Japan). Rats were maintained under specific pathogen-free conditions and had free access to water and a commercial pellet diet (CMF 30kGy; Oriental Yeast, Chiba, Japan) during pregnancy. Offspring were culled to a maximum of 10 per dam. Male and female pups were separated at the age of 4 days and used in experiments 1 and 2, respectively. For experiment 3, 6-week-old female rats (n=25) were purchased and used after a 1-week acclimatization period.
Chemical and dose formulation
MNU was obtained from Sigma-Aldrich (St. Louis, MO, USA), and kept at −80ºC in the dark. The MNU solution was dissolved in physiologic saline containing 0.1% acetic acid just before use. MNU at a dose of 40 or 50 mg/kg or vehicle (physiological saline) was administered once by an intraperitoneal (ip) route.
MA-supplemented diet
The MA diet contained 5% SUNTGM33 (a kind gift from Suntory Wellness, Tokyo, Japan), which contains 48.0% MA. SUNTGM33 is a microbial oil obtained by fungal fermentation26. Olive oil purchased from Nacalai Tesque (Kyoto, Japan) was used for the basal diet given to the controls. The fatty acid compositions of SUNTGM33 and olive oil are listed in our previously published study26. Each experimental diet was formulated by Oriental Yeast (Tokyo, Japan) and then stored at 4°C to prevent lipid oxidation before use. The basal and MA diets were given twice a week during the experiments in order to prevent lipid oxidation due to the temperature of the animal room.
Experimental procedures
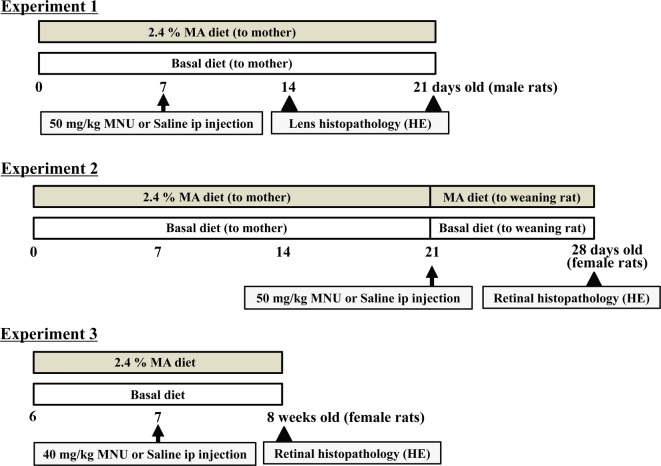
Figure 1 shows the experimental protocol scheme. Experiment 1 was designed to inhibit cataract formation by MA supplementation, with the dams either fed the 2.4% MA or basal (<0.01% MA) diet during the lactational periods (for 21 days) (Fig. 1). The lens is sensitive to MNU in neonatal rats. In our previous report9, cataracts occurred within seven days after 100 mg/kg MNU exposure in rats at 0 and 5 days of age, and some of the rats died. Using a modified method from our previous study9, 48 male pups were given a single ip injection of 50 mg/kg MNU or vehicle on postnatal day 7. From our preliminary study, we selected 50 mg/kg, as it is not a lethal dose for rats at the age of seven days. At 7 and 14 days after the injection, lens opacity and morphology of the rats in the MA diet group were compared with those of the rats in the basal diet group. Six randomly selected rats in each group were used for the analysis.
Fig. 1.
Schema of the experimental protocol. Experiment 1 was designed to inhibit cataract formation in the MNU-induced cataract model using male neonatal rats9. Experiments 2 and 3 were designed to confirm the effects on the MNU-induced retinal degeneration models using female postweaning and adult rats8, 14.
Experiment 2 was designed to inhibit retinal degeneration in postweaning rats. Many RP cases are diagnosed before birth by using genetic methods and/or a pedigree diagram, and clinical symptoms typically start in the early teenage years3, 6, 12. There is a possibility that the initiation of therapy in the infant period prevents or delays retinal damage in young people with RP. The protocol for Experiment 2 mimicked the therapeutic strategy for these RP patients, as described in our previously published study14. The dams were fed the 2.4% MA or basal diet during the lactational periods (for 21 days), with the pups then continuously fed the same diets during the postweaning periods (for 7 days) (Fig. 1). On postnatal day 21 (at weaning), female pups received a single ip injection of 50 mg/kg MNU, in accordance with the method of our previously published study14. Retinal morphology was examined 7 days after the MNU injection. Five rats in each group were used for analysis. Experiment 2 additionally analyzed the concentration of fatty acids in the serum and ocular tissue (lens and retina) in three or four vehicle-treated rats who were also fed either the basal or MA diets.
Experiment 3 was designed to inhibit retinal degeneration in adult rats. Six-week-old rats were fed the 2.4% MA or basal diet starting from one week before the MNU injection, with the same diets continued until sacrifice (total 14 days) (Fig. 1). The female rats were given a single ip injection of 40 mg/kg MNU, and the retina was examined morphologically one week after the injection, in accordance with our previously published report8. The age of the rats and MNU dose were selected in accordance with a retinal degeneration model10, 12. Six or seven rats in each group were used for the analysis.
To evaluate the actual dosages of MA administered to the dams (experiments 1 and 2) and the adult rats (experiment 3), body weight was measured once a week, while food consumption was measured twice a week. This made it possible to estimate the actual dosage of MA. All rats were anesthetized with isoflurane (Forane®; Abbot Japan, Tokyo, Japan) and sacrificed by exsanguination from an aortic transection. All rats were observed daily during the experiments for clinical signs of toxicity in addition to being weighed at the time of MNU treatment and on the day of sacrifice. Both eyes were quickly removed at the time of sacrifice, and complete necropsies were conducted on all animals.
Analysis of fatty acids in the serum, lens, and retina
After collection of the serum, lens, and retina from both eyes of the vehicle-treated rats that had been fed the respective diets, all specimens were stored at −80°C prior to lipid extraction and measurement of the fatty acid composition. Extraction of the total lipids was performed using the method of Bligh and Dyer27. For an internal standard, we added 1,2-diheptadecanoyl-sn-glycero-3 phosphocholine (Avanti Polar Lipids, Inc., Alabaster, AL, USA) to each of the specimens. Total lipids were transmethylated with HCL-methanol, with the fatty acid composition then analyzed by gas chromatography (GC-2014; Shimadzu Corporation, Kyoto, Japan) using a DB-225 capillary column (0.25 mm × 30 m × 0.25 µM; J&M Scientific, Folsom, CA, USA). The entire system was controlled using gas chromatography software (GCsolution; Shimadzu Corporation).
Morphologic analysis of the ocular tissue
Eyes from all of the rats fed each diet were fixed overnight in methacarn. Subsequently, they were embedded in paraffin, sectioned at a thickness of 4 μm, and stained with hematoxylin and eosin (HE). Ocular sections were cut along a line parallel to the optic axis and nerve (including the ora serrata). HE-stained slides were scanned with a high-resolution digital slide scanner (NanoZoomer 2.0 digital slide scanner; Hamamatsu Photonics, Hamamatsu, Japan) in order to prepare digital images that could be opened in color mode by the NDP.view software (Hamamatsu Photonics). In experiments 2 and 3, the total retinal thickness (from the internal limiting membrane to the pigment epithelium), inner retinal thickness (from the internal limiting membrane to the outer plexiform layer) and outer retinal thickness (from the outer nuclear layer to the pigment epithelial cell layer) were individually measured in the HE-stained slides using NDP.view, as described previously14. To further evaluate the photoreceptor cell loss, we calculated the photoreceptor ratio [(outer retinal thickness / total retinal thickness) ×100].
To determine the area of retinal damage, the entire length of the retina and the length of the damaged area in the HE preparations were measured. A damaged retina was defined as the presence of less than four rows of photoreceptor nuclei in the outer nuclear layer14, with the retinal damage ratio calculated as (length of damaged retina/whole retinal length) ×100. Two toxicologic pathologists (K.Y., A.T.) certified by the Japanese Society of Toxicologic Pathology performed histopathological evaluations according to previously defined histopathological terminology and diagnostic criteria9, 14.
Statistical analysis
All discrete values were expressed as the mean ± standard error (SE). After confirming the homogeneity of the variances, data were analyzed using a two-tailed independent Student’s t-test for the unpaired samples (Excel 2007®; Microsoft, Redmond, WA, USA). The results presented below include comparisons between the rats fed the basal diet and the rats fed different doses of the MA-supplemented diet in both the MNU-treated and vehicle-treated groups. P values < 0.05 were considered to show statistical significance.
Results
General remarks
In all experiments, no deaths occurred in any of the diet groups with or without the MNU treatment during the study period. Although the MA diets did not influence body weight gain (the growth rate) with or without MNU treatment, the growth rate in the MNU-treated pups tended to be lower than that in vehicle-treated rats (data not shown). All neonatal rats exposed to MNU with or without the MA diet in experiment 1 developed bilateral lens opacity (Fig. 2a and b) and systemic alopecia at one week after the MNU injection. The MA diets in experiments 1 and 2 did not cause any deaths in the dams, did not evoke any clinical signs or symptoms, and did not affect the body weight changes between the groups.
Fig. 2.
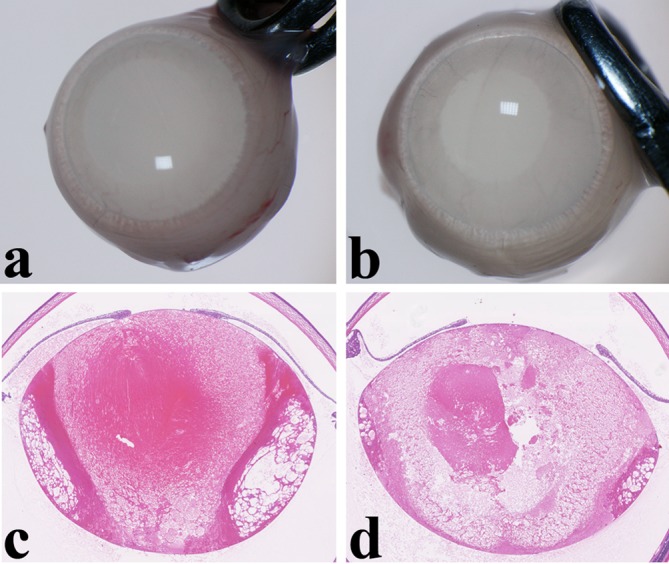
Lens opacity and mature cataract in experiment 1. (a) A severe degree of opacity in the whole lens is seen two weeks after injection of N-methyl-N-nitrosourea (MNU) in a rat fed a basal diet. (b) Similar lens opacity is seen in an MNU-treated rat fed a 2.4% mead acid (MA) diet. (c) Histopathologically, swelling, vacuolation and liquefied fibers occur in the whole lens at two weeks after injection of MNU in a rat fed a basal diet. These changes corresponded to the diagnosis of mature cataract. (d) Mature cataract is similarly seen in the whole lens of a MNU-treated rat fed an MA diet. Hematoxylin and eosin (HE) staining, ×30.
Estimated intake of MA
During the lactation period, dams in experiments 1 and 2 who consumed the basal and 2.4% MA diets were orally exposed to 0.0 and 3931.2 mg/kg/day MA, respectively. In experiment 2, pups were orally exposed after the weaning periods to 0.0 and 2145.6 mg/kg/day MA, respectively. In experiment 3, the adult rats in the MA diet groups were orally exposed to 1829.3 mg/kg/day MA.
Morphological and morphometric analyses:
Experiment 1: Lenticular lesions were detected microscopically in all of the MNU-treated rats starting from day 7 to day 14 after the 50 mg/kg MNU injection. Characteristics of the lesions included swelling, vacuolation and liquefied fibers in the bilateral whole lens (Fig. 2c and d). These changes corresponded to the diagnosis of mature cataract. No changes in the severity of mature cataract were observed between the rats fed the basal and 2.4% MA diets (Fig. 2c and d). In the vehicle-treated rats, which were fed MA and basal diets, no lenticular changes were seen. There were also no retinal changes detected in any of the rats with or without MNU treatment. In a previous study9, our research group found that there was sensitivity to MNU in the lenticular tissue but not in the retina of 7-day-old rats.
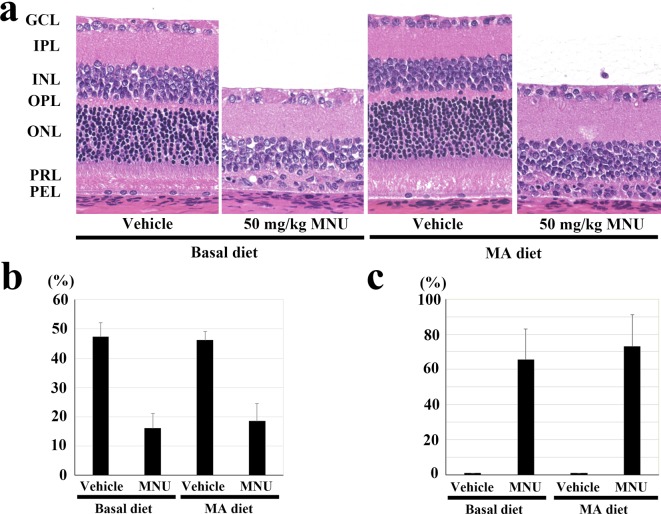
Experiment 2: Retinal histology showed that regardless of the diet consumed by the vehicle-treated rats, there were no abnormal changes in the central and peripheral retina. In all rats, the retinas contained more than ten layers of photoreceptor nuclei in the central retina (Fig. 3a) and more than eight layers of cells in the peripheral retina (data not shown). At 7 days after a single MNU injection in the rats fed the 2.4% MA and basal diets, the outer nuclear layer and the photoreceptor layer in the central and peripheral retina either disappeared or were reduced to a few rows of photoreceptor cell nuclei (Fig. 3a). To further evaluate the effects of the MA diet on retinal thickness at the central retina, the photoreceptor cell ratio was calculated. There were no statistically significant changes observed for the photoreceptor ratios between the MNU-treated rats that received the 2.4% MA diet and those fed a basal diet, with the ratios being 47.4 ± 4.7, 16.0 ± 5.1, 46.2 ± 2.9 and 18.5 ± 6.0% in the vehicle + basal diet, MNU + basal diet, vehicle + MA diet and MNU + MA diet groups, respectively (Fig. 3b). There was also no statistically significant changes found for the photoreceptor cell ratio at the peripheral retina between the MNU-treated rats fed MA or basal diet, with the ratios being 53.9 ± 2.0, 48.9 ± 10.9, 53.2 ± 2.4 and 40.3 ± 14% in the vehicle + basal diet, MNU + basal diet, vehicle + MA diet and MNU + MA diet groups, respectively (data not shown). In addition, the MNU-treated rats that received the 2.4% MA diets did not exhibit any statistically significant change in the retinal damage ratio as compared with the rats fed a basal diet (0, 65.5 ± 17.4, 0 and 73.0 ± 18.1% in the vehicle + basal diet, MNU + basal diet, vehicle + MA diet and MNU + MA diet groups, respectively (Fig. 3c)).
Fig. 3.
Effect of the mead acid (MA) diet on 50 mg/kg N-methyl-N-nitrosourea (MNU)-induced retinal damage in the postweaning rats in experiment 2. (a) Histology of the central retina in rats with or without MNU that were fed 2.4% MA and basal diets. At 7 days after a single ip injection of MNU, the outer nuclear layer and photoreceptor layer disappeared in the rats fed the MA and basal diets. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PRL, photoreceptor cell layer; and PEL, pigment epithelial cell layer. HE staining, ×200. (b) Photoreceptor cell ratio in the central retina 7 days after a single ip injection of MNU in rats fed 2.4% MA and basal diets. Rats treated with 50 mg/kg MNU and fed MA the diet do not have any statistically significant changes in their photoreceptor ratios at the central retina compared with the MNU-treated basal diet group. The index is calculated as [(outer retinal thickness / total retinal thickness) ×100]. (c) Retinal damage ratio in MNU-treated rats fed 2.4% MA and basal diets. Rats fed the MA diet do not show any statistically significant changes in the retinal damage ratio compared with the MNU-treated basal diet group. The index was evaluated as [(length of retina composed of less than four photoreceptor cells / whole retinal length) × 100]. The mean ± SE of the five rats in each treatment group is shown.
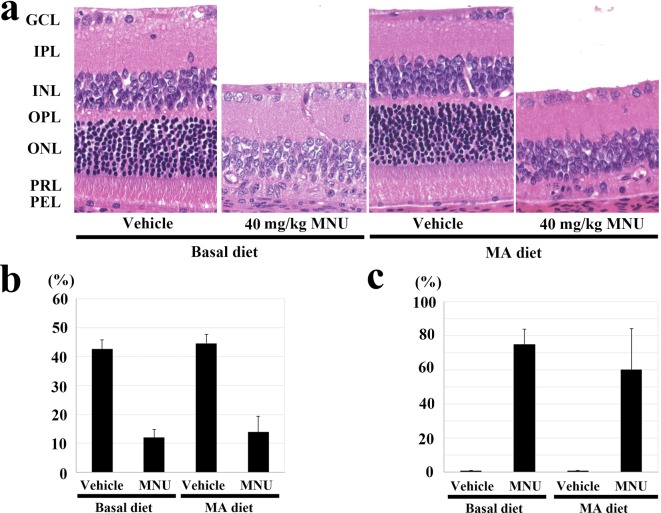
Experiment 3: No abnormal changes were observed in the central and peripheral retina of the adult vehicle-treated rats fed the 2.4% MA or basal diet (Fig. 4a). A severe degree of atrophy was seen in the outer retina in the 40 mg/kg MNU-treated rats fed the 2.4% MA and basal diets, with the outer nuclear layer and the photoreceptor layer in the central and peripheral retina completely disappearing (Fig. 4a). However, there was no statistically significant change in the photoreceptor ratio between the MNU-treated rats that received the 2.4% MA and basal diets, with the ratios being 42.6 ± 3.2, 12.0 ± 2.8, 44.6 ± 3.0 and 13.9 ± 5.4% in the vehicle + basal diet, MNU + basal diet, vehicle + MA diet and MNU + MA diet groups, respectively (Fig. 4b). There was also no significant change for the photoreceptor cell ratio at the peripheral retina between the MNU-treated rats fed the 2.4% MA and basal diets, with the ratios being 51.1 ± 2.1, 39.2 ± 12.4, 50.7 ± 4.2 and 42.1 ± 11.3% in the vehicle + basal diet, MNU + basal diet, vehicle + MA diet and MNU + MA diet groups, respectively (data not shown). Furthermore, there was no statistically significant change in the retinal damage ratio detected between the MNU-treated rats that received the 2.4% MA and basal diets (0, 75.0 ± 8.8, 0 and 60.2 ± 24.2% in the vehicle + basal diet, MNU + basal diet, vehicle + MA diet and MNU + MA diet groups, respectively (Fig. 4c)).
Fig. 4.
Effect of the mead acid (MA) diet on 40 mg/kg N-methyl-N-nitrosourea (MNU)-induced retinal damage in adult rats in experiment 3. (a) Histology of the central retina in rats with or without MNU who were fed the 2.4% MA and basal diets. At 7 days after a single ip injection of MNU, the outer nuclear layer and photoreceptor layer disappeared in the rats fed the MA and basal diets. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PRL, photoreceptor cell layer; and PEL, pigment epithelial cell layer. HE staining, ×200. (b) Photoreceptor cell ratio in the central retina 7 days after a single ip injection of MNU in rats fed the 2.4% MA and basal diets. MNU-treated rats fed the MA diet have a statistically significant decrease in their photoreceptor ratio at the central retina, which is similar to that seen in the MNU-treated basal diet group. The index is calculated as [(outer retinal thickness / total retinal thickness) ×100]. (c) Retinal damage ratio in MNU-treated rats fed the 2.4% MA and basal diets. Rats fed the MA diet do not show any statistically significant changes in the retinal damage ratio, which is similar to that seen in the MNU-treated basal diet group. The index is evaluated as [(length of retina composed of less than four photoreceptor cells / whole retinal length) ×100]. The mean ± SE of the six or seven rats in each treatment group is shown.
No lenticular changes were detected in any of the rats with or without the MNU treatment in experiments 2 and 3.
Fatty acid composition in the serum, lenses, and retinas from vehicle-treated rats
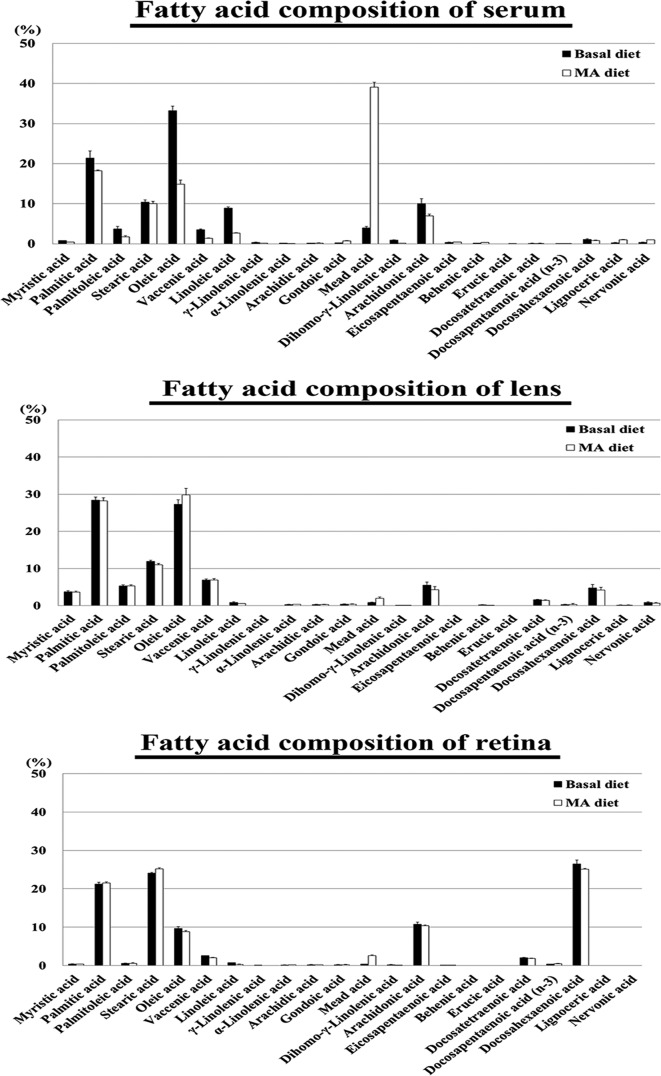
In the serum of vehicle-treated rats that were fed either a basal or 2.4% MA diet, the MA compositions were 3.99 and 39.12% of the total fatty acids, while the MA levels were 121.42 and 730.94 μg/mL, respectively. The MA compositions and levels in the rats fed the 2.4% MA diet were significantly increased compared with those of the rats fed the basal diet (Fig. 5, Table 1). The serum MA/DHA ratio increased in the rats fed the 2.4% MA diet compared with that of the rats fed the basal diet, while the MA/DHA ratios were 3.71 and 50.36% in the rats fed the basal and 2.4% MA diets, respectively. The serum MA/AA ratio increased in the rats fed the 2.4% MA diet compared with that of the rats fed the basal diet, while the MA/AA ratios were 0.40 and 5.60% in the rats fed the basal and 2.4% MA diets, respectively.
Fig. 5.
Fatty acid composition of the serum, lens and retina of the vehicle-treated rats. In the serum of the rats fed the 2.4% mead acid (MA) diet, the MA composition of the total fatty acids was higher than that seen in the serum of the rats fed the basal diet. In the lens and retina of the rats fed the 2.4% (MA) diet, although the MA composition was higher, the differences were not remarkable when compared with the serum values. The mean ± SE of four rats in the basal group and three rats in the MA diet group are shown.
Table 1. Fatty Acid Composition in the Serum, Lens and Retina Of Rats.
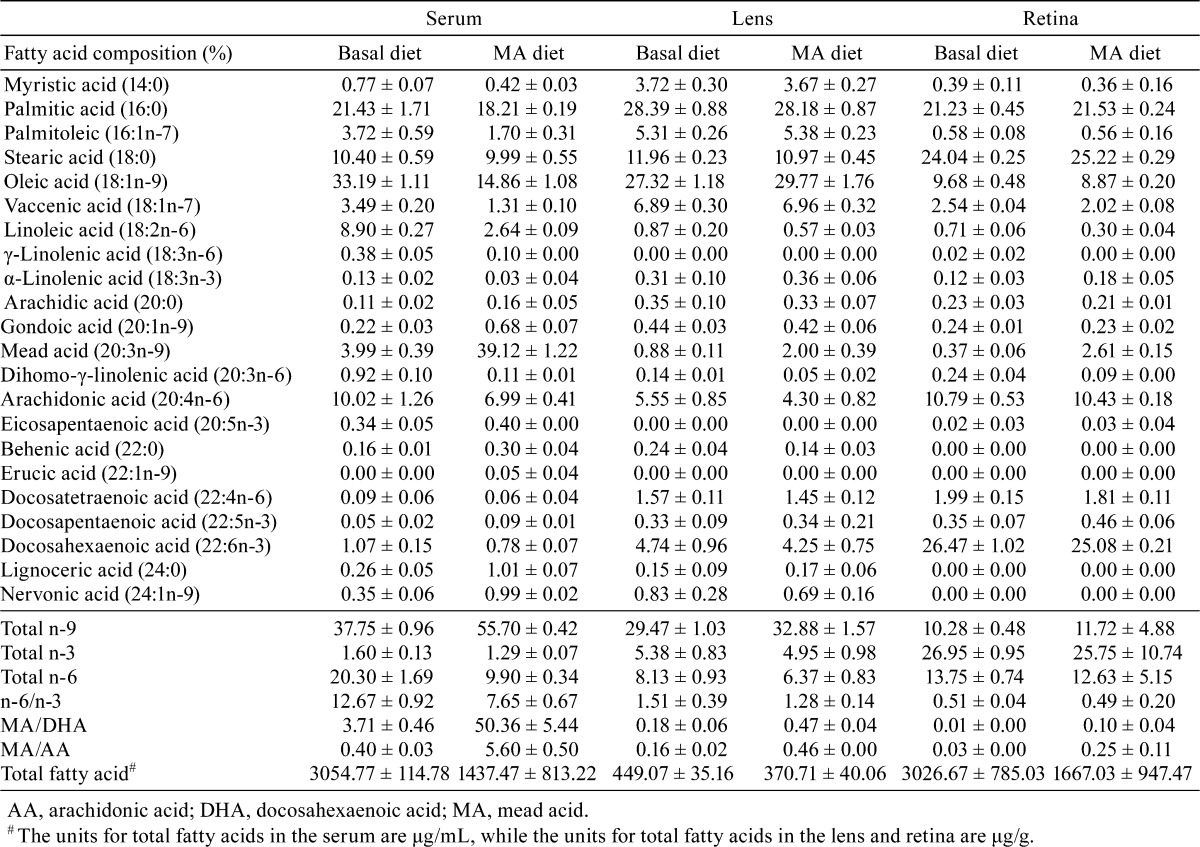
In the lens of the vehicle-treated rats fed the basal and 2.4% MA diets, the MA compositions were 0.88 and 2.00% of the total fatty acids while the MA levels were 3.90 and 7.44 μg/g, respectively (Fig. 5, Table 1). The lenticular MA/DHA ratio was higher in rats fed the 2.4% MA diet compared with those fed the basal diet, with values of 0.18 and 0.47% in the rats fed the basal and 2.4% MA diets, respectively. The MA/AA ratio was also higher in the rats fed the 2.4% MA diet compared with that for the rats fed the basal diet, with values of 0.16 and 0.46% in the rats fed the basal and 2.4% MA diets, respectively.
In the retina of the vehicle-treated rats that were fed the basal and 2.4% MA diets, the MA compositions were 0.37 and 2.61% of the total fatty acids, with the MA levels being 11.26 and 39.51 μg/g, respectively (Fig. 5, Table 1). The retinal MA/DHA ratio increased in the rats fed the 2.4% MA diet compared with those fed the basal diet, with values of 0.01 and 0.10% in the rats fed the basal and 2.4% MA diets, respectively. The MA/AA ratio also increased to 0.25% in the rats fed the 2.4% MA diet compared with 0.03% in the rats fed the basal diet.
In the 2.4% MA diet group, the levels of total fatty acids in the serum and retina were lower than the totals found in the basal diet group (3054.77 and 1437.47 μg/mL in the serum and 3026.67 and 1667.03 μg/g in the retina, respectively) (Table 1). The levels of total fatty acids in the lens were similar between both groups (449.07 and 370.71 μg/g in the basal and MA diet groups, respectively). The eicosapentaenoic acid (EPA) and DHA compositions and/or levels in the rats fed the 2.4% MA diet were not significantly changed compared with those of the rats fed the basal diet (Table 1).
Discussion
The types of long-chain polyunsaturated fatty acid include an n-3, n-6 and n-9 series fatty acids, with the first double bond located at the 3rd, 6th or 9th carbon from the terminal methyl group of the fatty acid. These series of fatty acids may have different effects on ocular diseases. We have researched the effects on MNU-induced cataracts and retinal lesions using n-3 PUFAs such as DHA5, 15 and/or n-6 PUFAs such as AA14. In the present study, we focused on the effects on MNU-induced ocular lesions using n-9 PUFAs, mainly MA, because the effects of MA on ocular disease have not been investigated in detail. Based on our previous report on the therapeutic effect of AA against MNU-induced retinal degeneration14, MA was also expected to have an inhibitory effect on MNU-induced ocular degenerative diseases, because MA has similar biochemical structures and has been proposed as a substitute for AA22, 23. However, the MNU-induced cataract model used in experiment 1 demonstrated that mature cataract was found in MNU-treated rats with or without MA supplementation. Furthermore, use of the MNU-induced retinal degeneration models in postweaning and adult rats showed that there was atrophy of the outer retina in all of the rats exposed to MNU with or without MA supplementation. These findings indicate that a diet containing 2.4% MA could not counteract and protect against the MNU-induced cataract and retinal degeneration that was seen morphologically. Cell loss via apoptosis has been increasingly recognized as being involved in both the clinical course of MNU-induced cataract and retinal degeneration in rats8, 9, as well as the final common pathway of lenticular and photoreceptor cell death in human disease. In the present study, 2.4% MA supplementation may have had no effect on the occurrence of apoptotic lenticular and photoreceptor cell death during the early phase of the lesions that occurred after a single systemic MNU exposure.
Fatty acid amounts in the serum and ocular tissue can be modified by the composition of the dietary fatty acid intake. Because of similar biochemical structures, MA has been proposed as a substitute for AA in biological membranes, with the MA/AA ratio used as an index of essential fatty acid deficiency22, 23. Our previous study examined the therapeutic effect of a 2.0% AA-rich diet on MNU-induced retinal degeneration in weaning rats and demonstrated that AA rescued rats from retinal damage via inhibition of photoreceptor apoptosis, along with an increased AA/DHA ratio in the serum and retina14. Based on the present dietary dose regimen of MA, we found that higher levels in the diets did not have any further effect on MNU-induced cataract and retinal degeneration in rats. In the present study, the concentration of MA was higher in the 2.4% MA diet group as compared with the controls that were fed the basal diet: 730.94 μg/mL (27.76 mol%) vs. 121.42 μg/mL (3.63 mol%) in the serum, 7.44 μg/g (1.37 mol%) vs. 3.90 μg/g (0.79 mol%) in the lens and 39.51 μg/g (0.17 mol%) vs. 11.26 μg/g (0.04 mol%) in the retina (Table 1). In addition, the MA/AA ratios were higher in the 2.4% MA diet group as compared with the controls fed the basal diet: 5.60% vs. 0.40% in the serum, 0.46% vs. 0.16% in the lens and 0.25% vs. 0.03% in the retina (Table 1). MA/DHA ratios were extremely higher in the 2.4% MA diet group as compared with the controls fed the basal diet: 50.36% vs. 3.71% in the serum, 0.47% vs. 0.18% in the lens and 0.10% vs. 0.01% in the retina (Table 1). In humans, the average value of MA in the plasma was 16.6 µmol/L (5.09 μg/mL), which was 0.15% of the total fatty acids, in a group of 250 healthy people28. The plasma concentration of MA in mothers during the lactation period was shown to be 0.22 mol%, while it was 0.16 mol% in plasma from infants at 40 weeks after birth, suggesting that efficient transfer occurs via milk23. In our studies, which included MA exposures during the lactation period (experiment 1), during the postweaning period (experiment 2) and during the adult period (experiment 3), we found that the MA exposure levels were extremely higher than those normally found in healthy humans.
The toxic effects of MA have yet to be investigated in detail. In a previous study in mice, a significantly lighter body weight was found for mice fed an MA diet26. The authors of the study suggested that this finding was most likely due to the animals consuming a lower amount of the MA diet due to its lower palatability in mice. In the present study, MA supplementation had no effect on food consumption and body weight in rats. Additionally, no macroscopic lesions were detected during the complete necropsies performed in the vehicle-treated rats, which suggests that MA has no effects on the health of rats.
In conclusion, an MA-rich diet induced an increased level of lenticular and retinal MA. However, use of dietary MA does not modify MNU-induced cataract and retinal degeneration in postweaning and adult rat models. The fatty acid composition of the diet may affect the response to ocular tissue in human cataract and RP. Further studies will need to be undertaken in other animal ocular disease models in order to completely understand the rescue effect of MA.
Acknowledgments
This research was supported in part by Grant-in Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (24592661 and 25462740) and Research Grant D2 from Kansai Medical University. The author’s responsibilities were as follows: Y.E. and K.Y. designed the study and provided technical expertise; Y.E., Y.K., K.Y., M.Y. and T.Y. conducted the study and pathological analysis; K.H. and H.K. analyzed and/or estimated the levels of fatty acids; and Y.E., A.T. and K.Y. analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declare that they have no competing interests.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/3.0/>.
References
- 1.World Health Organization (WHO), Prevention of Blindness and Deafness Programme Global Data on Visual Impairments 2010. 2012. Retrieved July 15, 2014, from World Health Organization Web site: http://www.who.int/blindness/GLOBALDATAFINALforweb.pdf.
- 2.Resnikoff S, and Pararajasegaram R. Blindness prevention programmes: past, present, and future. Bull World Health Organ. 79: 222–226 2001. [PMC free article] [PubMed] [Google Scholar]
- 3.Hartong DT, Berson EL, and Dryja TP. Retinitis pigmentosa. Lancet. 368: 1795–1809 2006. [DOI] [PubMed] [Google Scholar]
- 4.Berson EL, Rosner B, Sandberg MA, Weigel-DiFranco C, and Willett WC. ω-3 intake and visual acuity in patients with retinitis pigmentosa receiving vitamin A. Arch Ophthalmol. 130: 707–711 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsubura A, Yuri T, Yoshizawa K, Uehara N, and Takada H. Role of fatty acids in malignancy and visual impairment: epidemiological evidence and experimental studies. Histol Histopathol. 24: 223–234 2009. [DOI] [PubMed] [Google Scholar]
- 6.Shintani K, Shechtman DL, and Gurwood AS. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 80: 384–401 2009. [DOI] [PubMed] [Google Scholar]
- 7.Tsubura A, Yoshizawa K, Miki K, Oishi Y, and Kiuchi K. Animal models for human cataract with special emphasis on N-methyl-N-nitrosourea-induced rat cataractogenesis. Anim Eye Res. 24: 1–9 2005. [Google Scholar]
- 8.Yoshizawa K, and Tsubura A. Characteristics of N-methyl-N-nitrosourea-induced retinal degeneration in animals and application for the therapy of human retinitis pigmentosa. Nippon Ganka Gakkai Zasshi. 109: 327–337 2005; (Abstract in English and text in Japanese). [PubMed] [Google Scholar]
- 9.Yoshizawa K, Oishi Y, Nambu H, Yamamoto D, Yang J, Senzaki H, Miki H, and Tsubura A. Cataractogenesis in neonatal Sprague-Dawley rats by N-methyl-N-nitrosourea. Toxicol Pathol. 28: 555–564 2000. [DOI] [PubMed] [Google Scholar]
- 10.Emoto Y, Yoshizawa K, Kinoshita Y, Yuri T, Yuki M, Sayama K, Shikata N, and Tsubura A. Green tea extract suppresses N-methyl-N-nitrosourea-induced photoreceptor apoptosis in Sprague-Dawley rats. Graefes Arch Clin Exp Ophthalmol. 252: 1377–1384 2014. [DOI] [PubMed] [Google Scholar]
- 11.Miyazono Y, Harada K, Sugiyama K, Ueno M, Torii M, Kato I, Matsuura H, and Hirata K. Toxicological characterization of N-methyl-N-nitrosourea-induced cataract in rats by LC/MS-based metabonomic analysis. J Appl Toxicol. 31: 655–662 2011. [DOI] [PubMed] [Google Scholar]
- 12.Tsubura A, Yoshizawa K, and Kuro M. N-Methyl-N-nitrosourea animal models for retinitis pigmentosa. In: Animal Models for the Study of Human Diseases, PM Conn (ed). Elsevier Inc., Academic Press, London. 117-142. 2014. [Google Scholar]
- 13.Semba RD. Essential Fatty Acids and Visual Development in Infants. In: Handbook of Nutrition and Ophthalmology, RD Semba (ed). Humana Press, New Jersey. 415-441. 2007. [Google Scholar]
- 14.Yoshizawa K, Sasaki T, Kuro M, Uehara N, Takada H, Harauma A, Ohara N, Moriguchi T, and Tsubura A. Arachidonic acid supplementation during gestational, lactational and post-weaning periods prevents retinal degeneration induced in a rodent model. Br J Nutr. 109: 1424–1432 2013. [DOI] [PubMed] [Google Scholar]
- 15.Moriguchi K, Yoshizawa K, Shikata N, Yuri T, Takada H, Hada T, and Tsubura A. Suppression of N-methyl-N-nitrosourea-induced photoreceptor apoptosis in rats by docosahexaenoic acid. Ophthalmic Res. 36: 98–105 2004. [DOI] [PubMed] [Google Scholar]
- 16.Weikel KA, Garber C, Baburins A, and Taylor A. Nutritional modulation of cataract. Nutr Rev. 72: 30–47 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jump DB, Botolin D, Wang Y, Xu J, Christian B, and Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 135: 2503–2506 2005. [DOI] [PubMed] [Google Scholar]
- 18.Futterman S, Rollins MH, and Vacano E. The effect of alloxan diabetes on polyenoic fatty acid synthesis by retinal tissue. Biochim Biophys Acta. 164: 433–434 1968. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Wetzel MG, and O’Brien PJ. Transport of n-3 fatty acids from the intestine to the retina in rats. J Lipid Res. 33: 539–548 1992. [PubMed] [Google Scholar]
- 20.Albers-Jackson B, and Bunch FT. Incorporation of 14C stearic acid in lens organ culture. Curr Eye Res. 2: 233–237 1982-1983. [DOI] [PubMed] [Google Scholar]
- 21.Sabah J, McConkey E, Welti R, Albin K, and Takemoto LJ. Role of albumin as a fatty acid carrier for biosynthesis of lens lipids. Exp Eye Res. 80: 31–36 2005. [DOI] [PubMed] [Google Scholar]
- 22.Ichi I, Kono N, Arita Y, Haga S, Arisawa K, Yamano M, Nagase M, Fujiwara Y, and Arai H. Identification of genes and pathways involved in the synthesis of Mead acid (20:3n-9), an indicator of essential fatty acid deficiency. Biochim Biophys Acta. 1841: 204–213 2014. [DOI] [PubMed] [Google Scholar]
- 23.Sabel KG, Lundqvist-Persson C, Bona E, Petzold M, and Strandvik B. Fatty acid patterns early after premature birth, simultaneously analysed in mothers’ food, breast milk and serum phospholipids of mothers and infants. Lipids Health Dis. 8: 20 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamazaki T, Suzuki N, Widyowati R, Miyahara T, Kadota S, Ochiai H, and Hamazaki K. The depressive effects of 5,8,11-eicosatrienoic Acid (20:3n-9) on osteoblasts. Lipids. 44: 97–102 2009. [DOI] [PubMed] [Google Scholar]
- 25.Hamazaki T, Nagasawa T, Hamazaki K, and Itomura M. Inhibitory effect of 5,8,11-eicosatrienoic acid on angiogenesis. Prostaglandins Leukot Essent Fatty Acids. 86: 221–224 2012. [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita Y, Yoshizawa K, Hamazaki K, Emoto Y, Yuri T, Yuki M, Shikata N, Kawashima H, and Tsubura A. Mead acid inhibits the growth of KPL-1 human breast cancer cells in vitro and in vivo. Oncol Rep. 32: 1385–1394 2014. [DOI] [PubMed] [Google Scholar]
- 27.Bligh EG, and Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37: 911–917 1959. [DOI] [PubMed] [Google Scholar]
- 28.Pouchieu C, Chajès V, Laporte F, Kesse-Guyot E, Galan P, Hercberg S, Latino-Martel P, and Touvier M. Prospective associations between plasma saturated, monounsaturated and polyunsaturated fatty acids and overall and breast cancer risk - modulation by antioxidants: a nested case-control study. PLoS ONE. 9: e90442 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]