Abstract
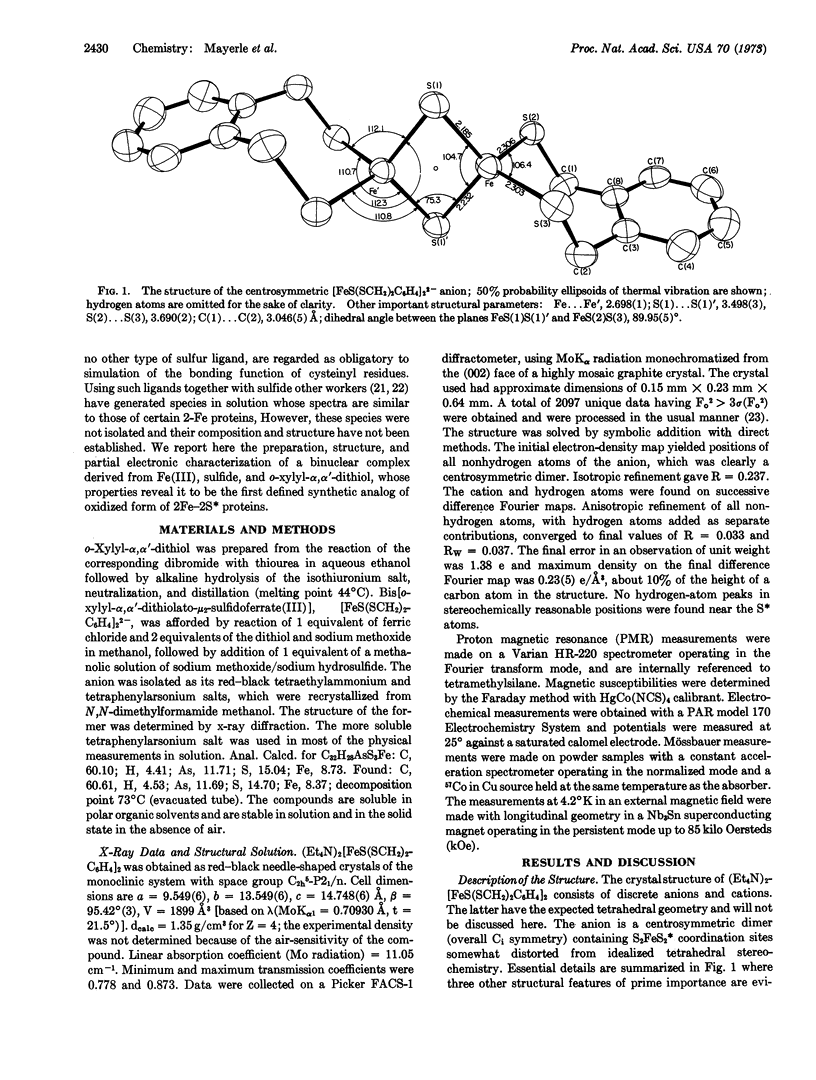
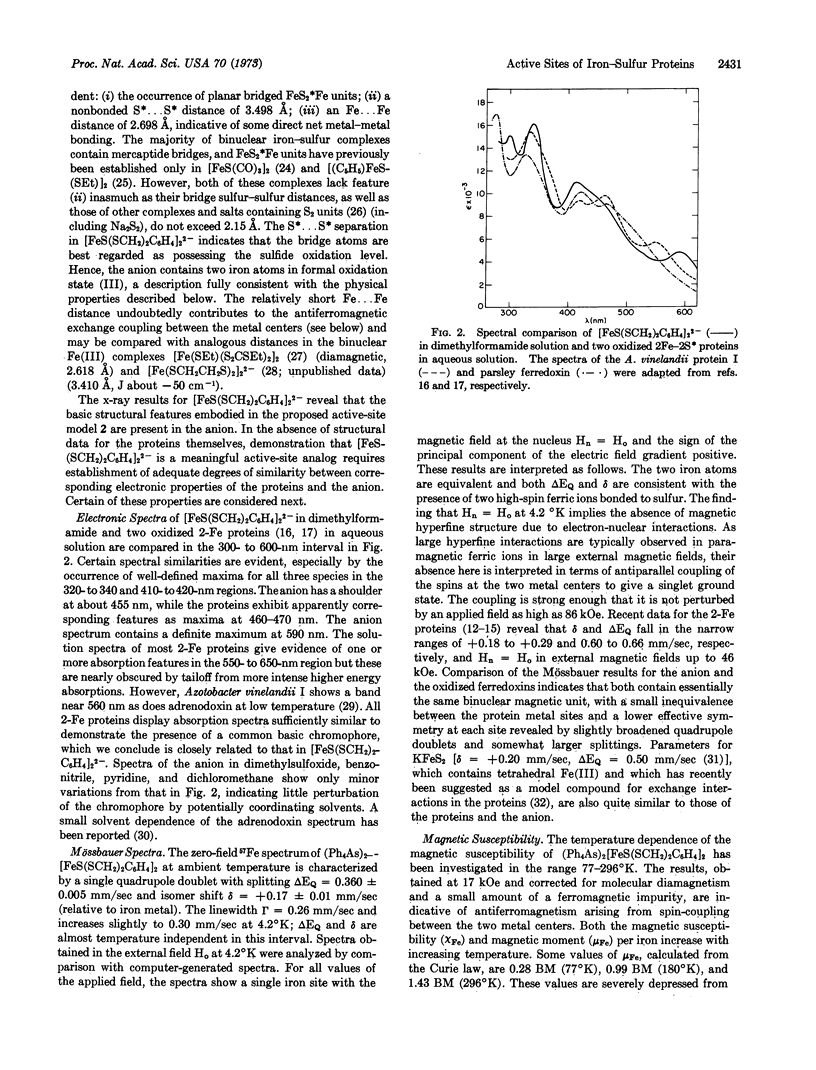
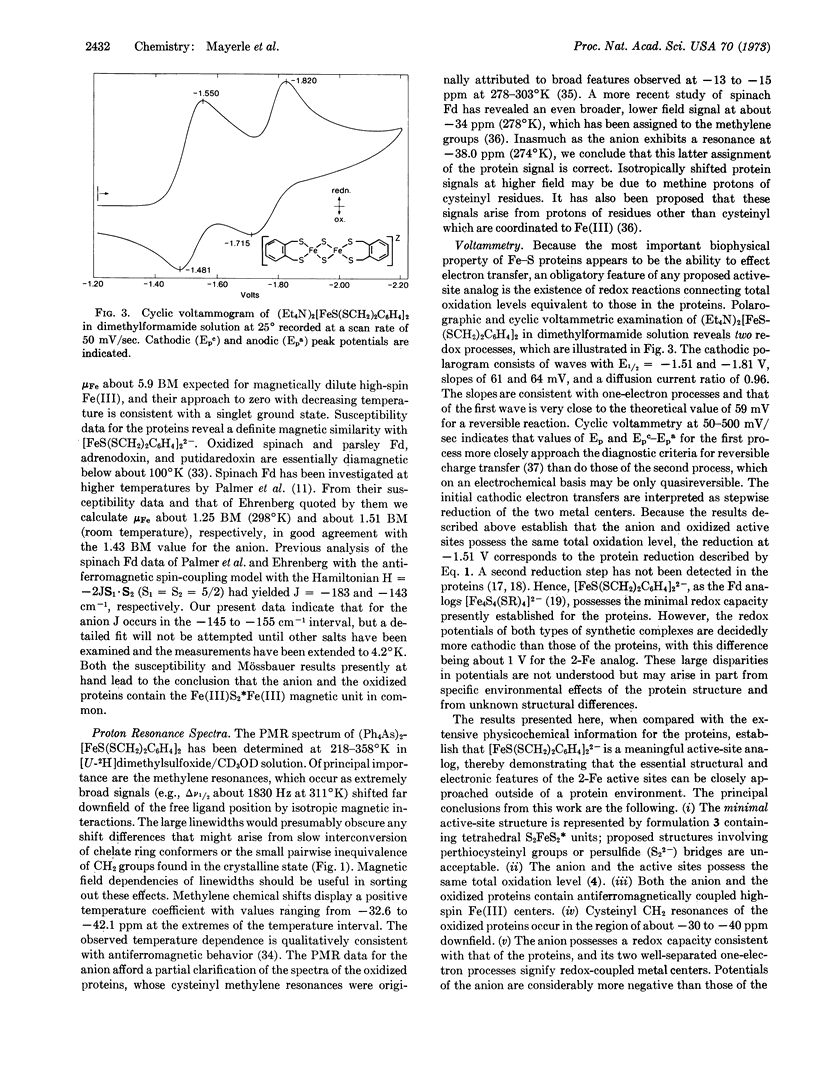
The synthetic analog approach has been applied to a clarification of the active sites of 2Fe-2S* proteins. The compound (Et4N)2[FeS(SCH2)2C6H4]2, derived from o-xylyl-α,α′-dithiol, has been prepared and its structure has been determined by x-ray diffraction. The centrosymmetric anion contains two tetrahedrally coordinated ferric ions bridged by two sulfide ions and separated by 2.70 Å. Comparison of electronic, Mössbauer, and proton magnetic resonance spectra and magnetic susceptibility of the anion with the corresponding properties of the oxidized forms of the proteins reveals significant degrees of similarity. The anion also exhibits the essential redox capacity of the proteins. We conclude that [FeS(SCH2)2C6H4]22- possesses the same total oxidation level and electronic configuration as the active sites of the oxidized proteins, and that its structure provides a feasible representation of the minimal structure of the active site. [FeS(SCH2)2C6H4]22- is thus the first well-defined synthetic analog of the active sites of two-iron ferredoxins.
Keywords: Fe2S2 core, iron-sulfur complexes, x-ray diffraction
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averill B. A., Herskovitz T., Holm R. H., Ibers J. A. Synthetic analogs of the active sites of iron-sulfur proteins. II. Synthesis and structure of the tetra(mercapto-m 3 -sulfido-iron) clusters, (Fe 4 S 4 (SR) 4 ) 2- . J Am Chem Soc. 1973 May 30;95(11):3523–3534. doi: 10.1021/ja00792a013. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O., Johnson C. E. Mössbauer studies of adrenodoxin. The mechanism of electron transfer in a hydroxylase iron-sulphur protein. Biochem J. 1971 Dec;125(3):849–856. doi: 10.1042/bj1250849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. W., Jr, Freer S. T., Xuong N. H., Alden R. A., Kraut J. Structure of the iron-sulfur cluster in the Chromatius iron protein at 2.25 Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:381–385. doi: 10.1101/sqb.1972.036.01.049. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A., Sieker L. C., Adman E., Jensen L. H. A comparison of Fe 4 S 4 clusters in high-potential iron protein and in ferredoxin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3526–3529. doi: 10.1073/pnas.69.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham W. R., Bearden A. J., Salmeen I. T., Palmer G., Sands R. H., Orme-Johnson W. H., Beinert H. The two-iron ferredoxins in spinach, parsley, pig adrenal cortex, Azotobacter vinelandii, and Clostridium pasteurianum: studies by magnetic field Mössbauer spectroscopy. Biochim Biophys Acta. 1971 Nov 2;253(1):134–152. doi: 10.1016/0005-2728(71)90240-4. [DOI] [PubMed] [Google Scholar]
- Dunham W. R., Palmer G., Sands R. H., Bearden A. J. On the structure of the iron-sulfur complex in the two-iron ferredoxins. Biochim Biophys Acta. 1971 Dec 7;253(2):373–384. doi: 10.1016/0005-2728(71)90041-7. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Palmer G., Fee J. A., Kimura T., Lovenberg W. Tetrahedral iron in the active center of plant ferredoxins and beef adrenodoxin. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3015–3020. doi: 10.1073/pnas.68.12.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickson J. D., Phillips W. D., McDonald C. C., Poe M. PMR characterization of alfalfa and soybean ferredoxins: the existence of two ferredoxins in soybean. Biochem Biophys Res Commun. 1971 Jan 22;42(2):271–279. doi: 10.1016/0006-291x(71)90098-2. [DOI] [PubMed] [Google Scholar]
- Herriott J. R., Sieker L. C., Jensen L. H., Lovenberg W. Structure of rubredoxin: an x-ray study to 2.5 A resolution. J Mol Biol. 1970 Jun 14;50(2):391–406. doi: 10.1016/0022-2836(70)90200-7. [DOI] [PubMed] [Google Scholar]
- Herskovitz T., Averill B. A., Holm R. H., Ibers J. A., Phillips W. D., Weiher J. F. Structure and properties of a synthetic analogue of bacterial iron--sulfur proteins. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2437–2441. doi: 10.1073/pnas.69.9.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. J., Kimura T. Studies on adrenal steroid hydroxylases. Oxidation-reduction properties of adrenal iron-sulfur protein (adrenodoxin). Biochemistry. 1973 Jan 30;12(3):406–409. doi: 10.1021/bi00727a007. [DOI] [PubMed] [Google Scholar]
- Kimura T., Huang J. J. Studies on adrenal steroid hydroxylases: optical absorption spectroscopy of adrenal iron-sulfur protein (adrenodoxin) and its apoprotein. Arch Biochem Biophys. 1970 Apr;137(2):357–364. doi: 10.1016/0003-9861(70)90449-2. [DOI] [PubMed] [Google Scholar]
- Kimura T. Organic solvent effects on the chromophore of adrenal 2 iron - 2 labile sulfur protein (adrenodoxin). Biochem Biophys Res Commun. 1971 Jun 4;43(5):1145–1149. doi: 10.1016/0006-291x(71)90582-1. [DOI] [PubMed] [Google Scholar]
- Kubas G. T., Spiro T. G., Terzis A. A novel compound with a planar Fe-S-S-Fe bridge and its possible relation to ferredoxins. J Am Chem Soc. 1973 Jan 10;95(1):273–274. doi: 10.1021/ja00782a065. [DOI] [PubMed] [Google Scholar]
- Mayhew S. G., Petering D., Palmer G., Foust G. P. Spectrophotometric titration of ferredoxins and Chromatium high potential iron protein with sodium dithionite. J Biol Chem. 1969 Jun 10;244(11):2830–2834. [PubMed] [Google Scholar]
- Moleski C., Moss T. H., Orme-Johnson W. H., Tsibris J. C. The magnetic susceptibility of the oxidized and reduced iron-sulfur proteins adrenodoxin and putidaredoxin. Biochim Biophys Acta. 1970 Sep 29;214(3):548–550. doi: 10.1016/0005-2795(70)90315-6. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Petering D., Palmer G. The magnetic susceptibility of oxidized and reduced ferredoxins from spinach and parsley and the high potential protein from Chromatium. J Biol Chem. 1969 May 10;244(9):2275–2277. [PubMed] [Google Scholar]
- Mukai K., Kimura T., Helbert J., Kevan L. Environment of the iron-sulfur chromophore in adrenodoxin studied by EPR and ENDOR spectroscopy. Biochim Biophys Acta. 1973 Jan 25;295(1):49–56. doi: 10.1016/0005-2795(73)90072-x. [DOI] [PubMed] [Google Scholar]
- Münck E., Debrunner P. G., Tsibris J. C., Gunsalus I. C. Mössbauer parameters of putidaredoxin and its selenium analog. Biochemistry. 1972 Feb 29;11(5):855–863. doi: 10.1021/bi00755a027. [DOI] [PubMed] [Google Scholar]
- Palmer G., Dunham W. R., Fee J. A., Sands R. H., Iizuka T., Yonetani T. The magnetic susceptibility of spinach ferredoxin from 77-250 degrees K: a measurement of the antiferromagnetic coupling between the two iron atoms. Biochim Biophys Acta. 1971 Aug 6;245(1):201–207. doi: 10.1016/0005-2728(71)90022-3. [DOI] [PubMed] [Google Scholar]
- Poe M., Phillips W. D., Glickson J. D., McDonald C. C., Pietro A. S. Proton magnetic resonance studies of the ferredoxins from spinach and parsley. Proc Natl Acad Sci U S A. 1971 Jan;68(1):68–71. doi: 10.1073/pnas.68.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. K., Cammack R., Hall D. O., Johnson C. E. Mössbauer effect in Scenedesmus and spinach ferredoxins. The mechanism of electron transfer in plant-type iron-sulphur proteins. Biochem J. 1971 Apr;122(3):257–265. doi: 10.1042/bj1220257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeen I., Palmer G. Contact-shifted NMR of spinach ferredoxin: additional resonances and partial assignments. Arch Biochem Biophys. 1972 Jun;150(2):767–773. doi: 10.1016/0003-9861(72)90096-3. [DOI] [PubMed] [Google Scholar]
- Shethna Y. I., DerVartanian D. V., Beinert H. Non heme (iron-sulfur) proteins of Azotobacter vinelandii. Biochem Biophys Res Commun. 1968 Jun 28;31(6):862–868. doi: 10.1016/0006-291x(68)90531-7. [DOI] [PubMed] [Google Scholar]
- Sieker L. C., Adman E., Jensen L. H. Structure of the Fe-S complex in a bacterial ferredoxin. Nature. 1972 Jan 7;235(5332):40–42. doi: 10.1038/235040a0. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Kunishima M., Tanaka H. Some factors controlling incorporation of labile sulfur into thiol-iron(3) complexes. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1518–1524. doi: 10.1016/0006-291x(72)90512-8. [DOI] [PubMed] [Google Scholar]
- Sugiura Y., Tanaka H. Iron-sulfide chelates of some sulfur-containing peptides as model complex of non-heme iron proteins. Biochem Biophys Res Commun. 1972 Jan 31;46(2):335–340. doi: 10.1016/s0006-291x(72)80143-8. [DOI] [PubMed] [Google Scholar]
- Sweeney W. V., Coffman R. E. Magnetic properties of potassium dithioferrate: a linear chain antiferromagnet and model compound for the exchange interactions in two-iron ferredoxins. Biochim Biophys Acta. 1972 Nov 24;286(1):26–35. doi: 10.1016/0304-4165(72)90085-2. [DOI] [PubMed] [Google Scholar]
- Watenpaugh K. D., Sieker L. C., Herriott J. R., Jensen L. H. The structure of a non-heme iron protein: rubredoxin at 1.5 Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:359–367. doi: 10.1101/sqb.1972.036.01.047. [DOI] [PubMed] [Google Scholar]
- Wilson D. F. The near infra-red electronic spectra of non-heme iron proteins at minus 196 degrees. Arch Biochem Biophys. 1967 Oct;122(1):254–256. doi: 10.1016/0003-9861(67)90149-x. [DOI] [PubMed] [Google Scholar]
- Yang C. S., Huennekens F. M. Iron-mercaptoethanol-inorganic sulfide complex. Possible model for the chromophore of nonheme iron proteins. Biochemistry. 1970 May 12;9(10):2127–2133. doi: 10.1021/bi00812a015. [DOI] [PubMed] [Google Scholar]



