Abstract
Natural IgM inhibits gene transfer by adenovirus type 5 (Ad5) vectors. We show that polyreactive natural IgM antibodies bind to Ad5 and that inhibition of liver transduction by IgM depends on Kupffer cells. By manipulating IgM concentration in vivo, we demonstrate that IgM inhibits liver transduction in a concentration-dependent manner. We further show that differences in natural IgM between BALB/c and C57BL/6 mice contribute to lower efficiency of Ad5 gene transfer in BALB/c mice.
TEXT
When adenovirus type 5 (Ad5) vectors are injected intravenously (i.v.), their biodistribution is heavily influenced by blood proteins that bind to (opsonize) the vector (1). Natural antibodies and complement increase clearance of vector by Kupffer cells (KCs) (2), while coagulation factor X (FX) increases transduction of hepatocytes by vector (3–5).
Natural IgM antibodies can bind to diverse self and non-self-antigens (6, 7), including Ad5 (2). Natural IgM does not directly neutralize Ad5 in vitro (8), but natural IgM nevertheless inhibits liver transduction by Ad5 vectors in vivo (9, 10). KCs are a major sink for Ad5 vectors (11–15). Natural antibody-induced clearance of vector by KCs (2) reduces the amount of vector available for transduction of other cells such as hepatocytes, thereby explaining why natural antibodies inhibit liver transduction.
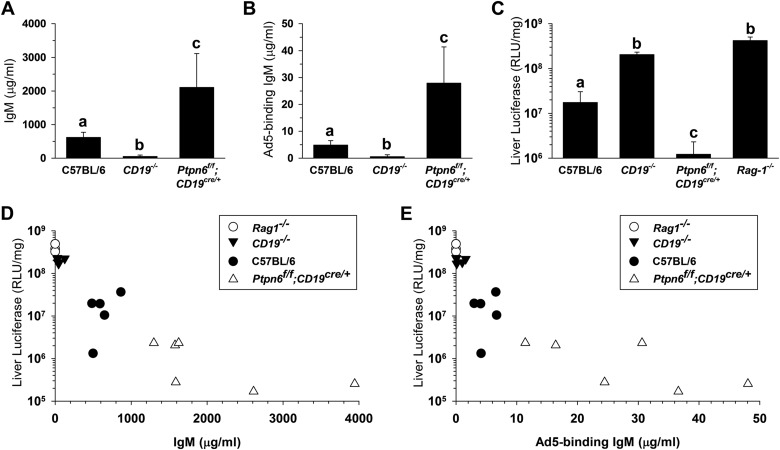
Amounts of natural antibody differ among individual mice and between mouse strains (9, 16). Although the presence of natural antibodies clearly inhibits Ad5 liver transduction (9, 10), the impact of natural antibody concentrations is unclear. To examine the importance of natural IgM concentrations, we identified knockout mouse strains that have abnormal IgM levels (Fig. 1A and B). (Animal experiments were approved by the FDA CBER Institutional Animal Care and Use Committee.) We found a significant negative correlation between the IgM concentration and liver transduction (Fig. 1C to E), suggesting that IgM may inhibit liver transduction in a concentration-dependent manner.
FIG 1.
Inverse correlation between natural IgM concentration and liver transduction. Wild-type C57BL/6 mice were compared to knockout mice that have abnormally low or high levels of natural IgM (n = 4 to 6 per group). CD19−/− mice (CD19cre/cre) are deficient in B-1 cells and have low levels of IgM (23). Ptpn6f/f;CD19cre/+ mice have a B cell-specific deletion in the Shp1 tyrosine phosphatase which causes expansion of B-1a cells and elevated IgM (24). Rag-1−/− mice have no immunoglobulins (25). All knockout mice were on the C57BL/6 background and were obtained originally from Jackson Laboratories. (A) Serum IgM concentration measured by enzyme-linked immunosorbent assay (ELISA). Groups that do not share the same letter (a, b, and so on) are significantly different from each other (P ≤ 0.05; analysis of variance [ANOVA] and Holm-Sidak post hoc test). Error bars represent standard deviations (SD). (B) The concentration of Ad5-binding IgM in serum was measured by ELISA on Ad5-coated plates. Monoclonal IgM 2E4 was used to generate a standard curve. (C) Liver transduction (luciferase expression) 48 h after i.v. injection with 4 × 1011 vector particles (vp)/kg of body weight of AdCMV-Luc (an E1-deleted Ad5 vector that expresses luciferase). RLU, relative light units. (D) Negative correlation between serum IgM concentration and liver transduction in individual mice (rs = −0.933, P = 0.0000002; Spearman's rank correlation). (E) Negative correlation between Ad5-binding IgM concentration and liver transduction (rs = −0.952, P = 0.0000002; Spearman's rank correlation).
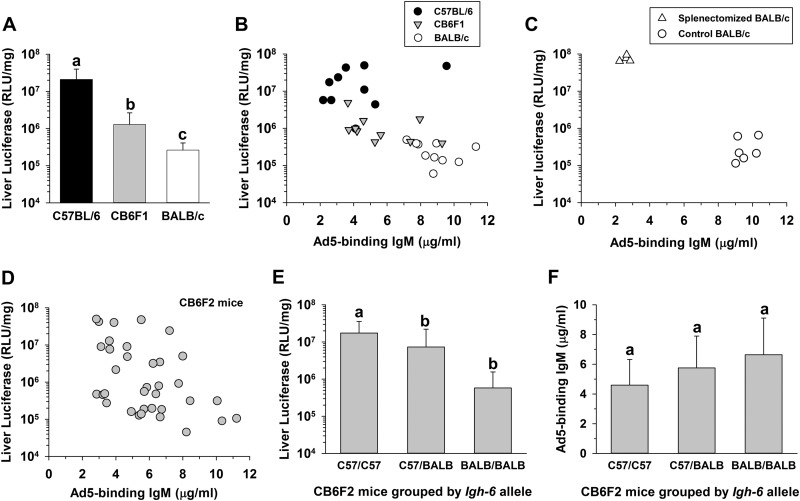
It is known that liver transduction by Ad5 is less effective in BALB/c mice than in C57BL/6 mice (14), but the reasons are unclear. Interestingly, BALB/c mice have higher levels of IgM than C57BL/6 mice (16). We found that F1 hybrid (CB6F1) mice showed levels of IgM and liver transduction that were intermediate between those of the two parental strains (Fig. 2A and B). We also manipulated the IgM concentration in BALB/c mice by splenectomy, which causes a decrease in the numbers of natural antibody-producing B-1a cells (17, 18). Splenectomized BALB/c mice had lower Ad5-binding IgM and much higher liver transduction levels than sham-splenectomized mice (Fig. 2C), again suggesting that high natural IgM concentration contributes to poor liver transduction in BALB/c mice.
FIG 2.
Influence of mouse strain on IgM concentration and liver transduction. C57BL/6, BALB/c, or hybrid mice were pre-bled to determine serum IgM concentrations and then injected i.v. with 4 × 1011 vp/kg of AdCMV-Luc. Liver transduction was assayed at 48 h. (A) Liver transduction in C57BL/6, BALB/c, and F1 hybrid (CB6F1) mice (n = 10 per group). Groups that do not share the same letter (a, b, and so on) are significantly different from each other (P ≤ 0.05; ANOVA and Holm-Sidak post hoc test). Error bars represent SD. (B) Negative correlation between serum Ad5-binding IgM concentration and liver transduction in individual mice (n = 30, rs = −0.683, P = 0.0000194; Spearman's rank correlation). (C) Effect of splenectomy on Ad5-binding IgM concentration and liver transduction in BALB/c mice (n = 4 or 6). The splenectomized and control (sham surgery) groups differed significantly from each other for both Ad5-binding IgM and luciferase expression (P ≤ 0.000001; t tests). Surgery was performed 2 weeks before injection of vector. (D) Negative correlation between serum IgM concentration and liver transduction in CB6F2 (CB6F1 × CB6F1) mice (n = 35, rs = −0.451, P = 0.00682; Spearman's rank correlation). (E) Dependence of liver transduction on Igh-6 alleles in the same set of 35 CB6F2 mice. Mice having only C57BL/6 Igh-6 alleles showed significantly higher liver transduction than mice that had at least one allele of BALB/c origin (n = 7 to 19 per group, P ≤ 0.05; ANOVA and Holm-Sidak post hoc test). The IgM allotypes in serum from each CB6F2 mouse were determined by ELISA, using allotype-specific anti-IgM clones AF6-78 and MA-69 (BioLegend) to identify BALB/c and C57BL/6 IgM (26, 27). (F) No significant relationship between Igh-6 and Ad5-binding IgM concentration in CB6F2 mice (n = 7 to 19 per group, P = 0.777; ANOVA).
Liver transduction in F2 hybrid (CB6F2) mice also correlated negatively with IgM concentrations (Fig. 2D). We further examined whether liver transduction was influenced by the Igh-6 gene, which encodes the heavy chain of IgM. Unexpectedly, CB6F2 mice carrying one or two BALB/c Igh-6 alleles had significantly lower liver transduction than CB6F2 mice that were homozygous for the C57BL/6 allele (Fig. 2E). We failed to detect any influence of the Igh-6 locus on the IgM concentration (Fig. 2F), in agreement with a prior study of the genetic control of IgM concentrations (16). Our results therefore suggest that the IgM concentration and the Igh-6 locus may independently influence liver transduction. Our experiments do not define whether the influence of the Igh-6 locus on liver transduction is due to the Igh-6 gene itself or to a closely linked gene, and further investigation will be needed to understand the mechanism for this influence of the Igh-6 locus.
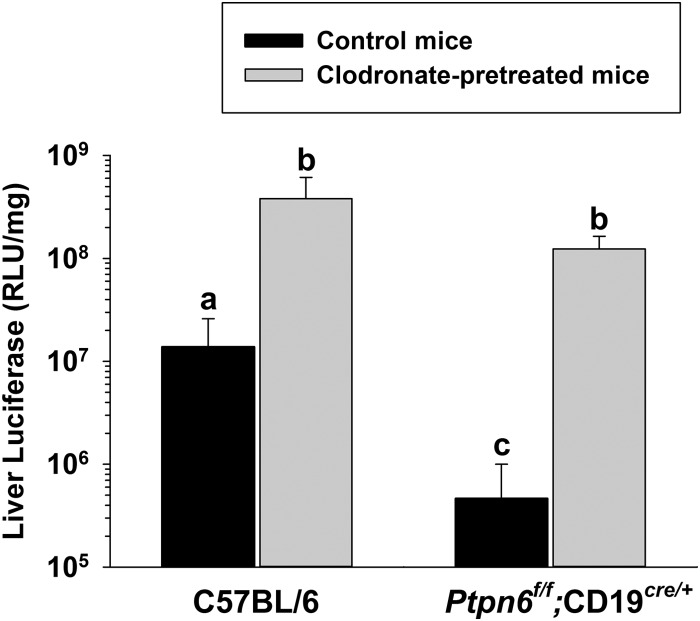
KCs rapidly clear Ad5 vectors from the circulation and have a major negative impact on the amount of vector that is available to transduce hepatocytes (11–14). We found that KC depletion caused a large increase in liver transduction in both C57BL/6 mice and Ptpn6f/f;CD19cre/+ (high IgM) mice; in fact, after KCs were depleted, there was no significant difference between these two strains of mice in the liver transduction levels (Fig. 3). Thus, a high IgM concentration inhibits liver transduction indirectly, through KCs.
FIG 3.
Kupffer cells mediate the negative effect of natural antibodies on liver transduction. C57BL/6 mice and Ptpn6f/f;CD19cre/+ mice (high IgM) were injected with clodronate liposomes via the tail vein to deplete KCs as previously described (28). Control mice were injected with phosphate-buffered saline (PBS). The next day, mice were injected i.v. with 4.0 × 1011 vp/kg of AdCMV-Luc. Liver transduction was assayed at 72 h. Groups that do not share the same letter (a, b, and so on) are significantly different from each other (P ≤ 0.05; ANOVA and Holm-Sidak post hoc test, n = 5 or 6 per group). Error bars represent SD.
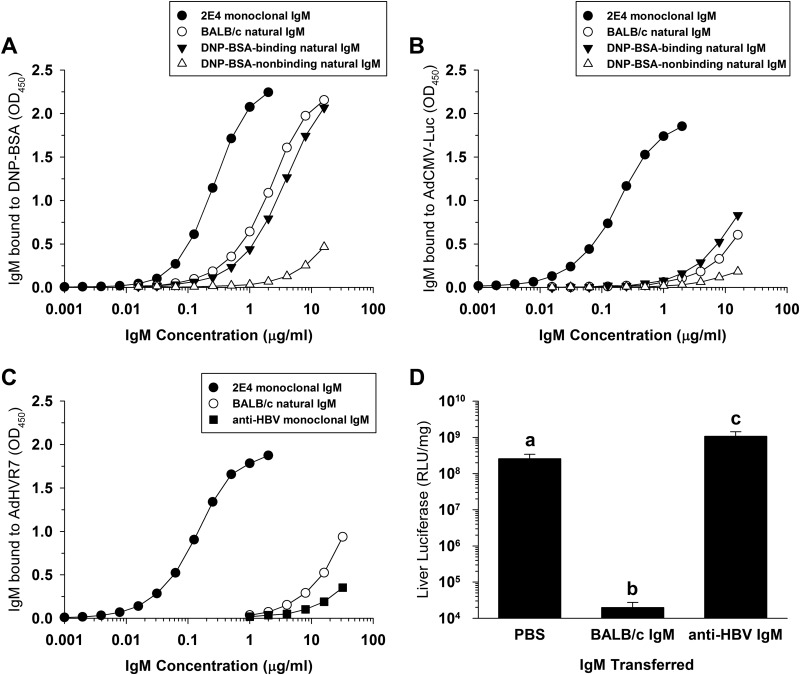
Natural antibodies are often polyreactive: a single antibody clone may bind to diverse unrelated antigens (19, 20). One example of a polyreactive IgM is monoclonal antibody 2E4, which recognizes multiple distinct antigens (21). Interestingly, we found that both 2E4 and BALB/c natural IgM could bind both to Ad5 and to an unrelated antigen: dinitrophenol coupled to bovine serum albumin (DNP-BSA) (Fig. 4A and B). When BALB/c natural IgM was separated by affinity chromatography on DNP-BSA, the DNP-BSA-binding pool of natural IgM was able to bind to Ad5, but DNP-BSA-nonbinding natural IgM showed decreased reactivity to Ad5 (Fig. 4B). Thus, at least some of the Ad5-binding natural IgM antibodies in mouse serum are polyreactive, because these antibodies can bind to both DNP-BSA and Ad5.
FIG 4.
Polyreactivity and specificity of natural IgM. (A) Binding of IgM to DNP-BSA determined by ELISA. BALB/c IgM was purified from serum as previously described (8, 9). DNP-BSA-binding and DNP-BSA-nonbinding pools of IgM were purified from BALB/c IgM by affinity chromatography. Monoclonal antibody 2E4 is a polyreactive IgM that can bind to a variety of antigens (21). (B) Binding of IgM to AdCMV-Luc by ELISA. (C) Binding of IgM to AdHVR7 by ELISA. A monoclonal IgM against HBV (H21F8-1; ATCC CRL-8018) showed poor reactivity to AdHVR7. Similar results were obtained on AdCMV-Luc-coated plates (not shown). (D) Influence of IgM transfer on liver transduction in Rag-1−/− mice. Mice were injected i.v. with PBS or IgM (500 μg) 2 h before i.v. injection with 4 × 1011 vp/kg of AdHVR7. Liver transduction was assayed at 48 h. Groups that do not share the same letter (a, b, and so on) are significantly different from each other (n = 4 or 5 per group, P ≤ 0.05; ANOVA and Holm-Sidak post hoc test). Error bars represent SD. All antibodies were of BALB/c genetic origin.
Transferring natural IgM to antibody-deficient mice decreases Ad5 liver transduction, while transfer of natural IgG has no effect (9, 10). Recently, Unzu et al. (10) found that liver transduction was suppressed by transfer of a monoclonal IgM antibody against the human AE2 anion exchanger, suggesting that IgM may nonspecifically suppress liver transduction by Ad5. However, the monoclonal antibody used by Unzu et al. also exhibited strong binding with Ad5 (10), suggesting that their antibody was actually a polyreactive monoclonal antibody with reactivity to both the AE2 anion exchanger and Ad5.
We found that a monoclonal IgM against hepatitis B virus (HBV) (22) showed very weak binding to Ad5 (Fig. 4C), which fails to support the hypothesis of Unzu et al. that IgM interacts with Ad5 in a non-antigen-specific manner. To determine whether the in vivo effect of IgM is specific or nonspecific, we tested whether liver transduction was inhibited by anti-HBV monoclonal IgM. To provide the most stringent test of the effect of IgM on liver transduction, we used the AdHVR7 Ad5 mutant vector, which is unable to bind FX and is extremely sensitive to inhibition by natural antibodies and complement (8). BALB/c natural IgM bound similarly both to the normal AdCMV-Luc Ad5 vector and to the AdHVR7 mutant vector (Fig. 4B and C). As expected, transfer of BALB/c natural IgM to antibody-deficient mice strongly suppressed liver transduction (Fig. 4D). However, the anti-HBV monoclonal IgM did not inhibit liver transduction. Thus, nonspecific IgM has no detectable inhibitory effect on liver transduction.
In summary, we found that natural IgM contains polyreactive antibodies that recognize both Ad5 and an unrelated antigen. We demonstrated that natural IgM impairs the efficiency of gene transfer in a concentration-dependent manner. Interestingly, we found that the efficiency of gene transfer in BALB/c and C57BL/6 mice is influenced not only by the IgM concentration but also by the Igh-6 locus. Finally, we showed that suppression of liver transduction by IgM depends on KCs and requires that IgM be able to bind to the vector.
ACKNOWLEDGMENTS
Funding was provided by the U.S. Food and Drug Administration (FDA), including the FDA's Critical Path program. This project was supported in part by fellowships administered by the Oak Ridge Institute for Science and Education.
We thank Andrew Harmon, Michael Havert, and Suzanne Epstein for helpful comments on the manuscript, and we thank the Center for Biologics Evaluation and Research animal facility staff for outstanding support.
REFERENCES
- 1.Lopez-Gordo E, Denby L, Nicklin SA, Baker AH. 18July2014, posting date The importance of coagulation factors binding to adenovirus: historical perspectives and implications for gene delivery. Expert Opin Drug Deliv doi: 10.1517/17425247.2014.938637. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z, Tian J, Smith JS, Byrnes AP. 2008. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies and complement. J Virol 82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, Shayakhmetov DM. 2008. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A 105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vigant F, Descamps D, Jullienne B, Esselin S, Connault E, Opolon P, Tordjmann T, Vigne E, Perricaudet M, Benihoud K. 2008. Substitution of hexon hypervariable region 5 of adenovirus serotype 5 abrogates blood factor binding and limits gene transfer to liver. Mol Ther 16:1474–1480. doi: 10.1038/mt.2008.132. [DOI] [PubMed] [Google Scholar]
- 5.Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, Pink R, Buckley SM, Greig JA, Denby L, Custers J, Morita T, Francischetti IM, Monteiro RQ, Barouch DH, van Rooijen N, Napoli C, Havenga MJ, Nicklin SA, Baker AH. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Racine R, Winslow GM. 2009. IgM in microbial infections: taken for granted? Immunol Lett 125:79–85. doi: 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrenstein MR, Notley CA. 2010. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 8.Xu Z, Qiu Q, Tian J, Smith JS, Conenello GM, Morita T, Byrnes AP. 2013. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat Med 19:452–457. doi: 10.1038/nm.3107. [DOI] [PubMed] [Google Scholar]
- 9.Khare R, Hillestad ML, Xu Z, Byrnes AP, Barry MA. 2013. Circulating antibodies and macrophages as modulators of adenovirus pharmacology. J Virol 87:3678–3686. doi: 10.1128/JVI.01392-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unzu C, Melero I, Morales-Kastresana A, Sampedro A, Serrano-Mendioroz I, Azpilikueta A, Ochoa MC, Dubrot J, Martinez-Anso E, Fontanellas A. 2014. Innate functions of immunoglobulin M lessen liver gene transfer with helper-dependent adenovirus. PLoS One 9:e85432. doi: 10.1371/journal.pone.0085432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff G, Worgall S, van Rooijen N, Song WR, Harvey BG, Crystal RG. 1997. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol 71:624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alemany R, Suzuki K, Curiel DT. 2000. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol 81:2605–2609. [DOI] [PubMed] [Google Scholar]
- 13.Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. 2001. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther 3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 14.Snoeys J, Mertens G, Lievens J, van Berkel T, Collen D, Biessen EA, De Geest B. 2006. Lipid emulsions potently increase transgene expression in hepatocytes after adenoviral transfer. Mol Ther 13:98–107. doi: 10.1016/j.ymthe.2005.06.477. [DOI] [PubMed] [Google Scholar]
- 15.Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, Muller J, Byrnes AP. 2006. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol Ther 13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Côrte-Real J, Rodo J, Almeida P, Garcia J, Coutinho A, Demengeot J, Penha-Gonçalves C. 2009. Irf4 is a positional and functional candidate gene for the control of serum IgM levels in the mouse. Genes Immun 10:93–99. doi: 10.1038/gene.2008.73. [DOI] [PubMed] [Google Scholar]
- 17.Rozing J, Brons NH, Benner R. 1978. Effects of splenectomy on the humoral immune system. A study in neonatally and adult splenectomized mice. Immunology 34:909–917. [PMC free article] [PubMed] [Google Scholar]
- 18.Wardemann H, Boehm T, Dear N, Carsetti R. 2002. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med 195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Notkins AL. 2004. Polyreactivity of antibody molecules. Trends Immunol 25:174–179. doi: 10.1016/j.it.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhou ZH, Tzioufas AG, Notkins AL. 2007. Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun 29:219–228. doi: 10.1016/j.jaut.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. 2007. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe 1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wands JR, Zurawski VR Jr. June1981. Process for producing antibodies to hepatitis virus and cell lines therefor. US Patent 4,271,145.
- 23.Rickert RC, Rajewsky K, Roes J. 1995. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 24.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. 2007. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity 27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877. doi: 10.1016/0092-8674(92)90030-G. [DOI] [PubMed] [Google Scholar]
- 26.Stall AM, Loken MR. 1984. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol 132:787–795. [PubMed] [Google Scholar]
- 27.Schreier PH, Quester S, Bothwell A. 1986. Allotypic differences in murine mu genes. Nucleic Acids Res 14:2381–2389. doi: 10.1093/nar/14.5.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian J, Xu Z, Smith JS, Hofherr SE, Barry MA, Byrnes AP. 2009. Adenovirus activates complement by distinctly different mechanisms in vitro and in vivo: indirect complement activation by virions in vivo. J Virol 83:5648–5658. doi: 10.1128/JVI.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]