ABSTRACT
Chikungunya virus (CHIKV) (genus Alphavirus) has a positive-sense RNA genome. CHIKV nonstructural protein 2 (nsP2) proteolytically processes the viral nonstructural polyprotein, possesses nucleoside triphosphatase (NTPase), RNA triphosphatase, and RNA helicase activities, and induces cytopathic effects in vertebrate cells. Although alphaviral nsP2 mutations can result in a noncytotoxic phenotype, the effects of such mutations on nsP2 enzymatic activities are not well understood. In this study, we introduced a P718G (PG) mutation and selected for additional mutations in CHIKV nsP2 that resulted in a CHIKV replicon with a noncytotoxic phenotype in BHK-21 cells. Combinations of PG and either an E116K (EK) substitution or a GEEGS sequence insertion after residue T648 (5A) markedly reduced RNA synthesis; however, neither PG nor 5A prevented nsP2 nuclear translocation. Introducing PG into recombinant nsP2 inhibited proteolytic cleavage of nsP1/nsP2 and nsP3/nsP4 sites, reduced GTPase and RNA helicase activities, and abolished RNA stimulation of GTPase activity. 5A and EK modulated the effects of PG. However, only the RNA helicase activity of nsP2 was reduced by both of these mutations, suggesting that defects in this activity may be linked to a noncytotoxic phenotype. These results increase our understanding of the molecular basis for the cytotoxicity that accompanies alphaviral replication. Furthermore, adaptation of the CHIKV replicon containing both 5A and PG allowed the selection of a CHIKV replicon with adaptive mutations in nsP1 and nsP3 that enable persistence in human cell line. Such cell lines represent valuable experimental systems for discovering host factors and for screening inhibitors of CHIKV replication at lower biosafety levels.
IMPORTANCE CHIKV is a medically important pathogen that causes febrile illness and can cause chronic arthritis. No approved vaccines or antivirals are available for CHIKV. The attenuation of CHIKV is critical to the establishment of experimental systems that can be used to conduct virus replication studies at a lower biosafety level. We applied a functional selection approach to develop, for the first time, a noncytotoxic CHIKV replicon capable of persisting in human cell lines. We anticipate that this safe and efficient research tool will be valuable for screening CHIKV replication inhibitors and for identifying and analyzing host factors involved in viral replication. We also analyzed, from virological and protein biochemistry perspectives, the functional defects caused by mutations conferring noncytotoxic phenotypes; we found that all known enzymatic activities of CHIKV nsP2, as well as its RNA-binding capability, were compromised by these mutations, which led to a reduced capacity for replication.
INTRODUCTION
Chikungunya virus (CHIKV) is a mosquito-transmitted Old World alphavirus belonging to the Togaviridae family. CHIKV infection is characterized by acute illness associated with fever, skin rash, and arthralgia. Symptoms typically resolve within weeks, but frequently CHIKV infection can result in chronic joint pain. Three different genotypes of CHIKV have been identified: the West African (WA), Asian, and East/Central/South African (ECSA) genotypes (1). The recent massive outbreak in the Indian Ocean region was caused by CHIKV of the ECSA genotype (2). In contrast, the ongoing outbreak in the Americas is being caused by CHIKV of the Asian genotype (3). No vaccines or antivirals are currently available for the prevention or treatment of CHIKV infection. Treatment is directed primarily toward relieving symptoms, especially joint pain (4, 5).
CHIKV has an approximately 12-kb positive-sense RNA genome that encodes four nonstructural proteins (nsP1 to -4); in addition, five structural proteins are expressed from subgenomic (SG) RNA synthesized in infected cells. The nonstructural proteins are the essential components of the viral replicase and transcriptase complex (6). They are initially synthesized in the form of a large nonstructural polyprotein (P1234) or two nonstructural polyproteins (P1234 and P123). These precursor molecules are subsequently cleaved in a highly regulated manner by the protease activity of nsP2 (7–10). nsP1 possesses membrane-binding properties and methyl- and guanylyltransferase activities, which are required for capping genomic and SG RNA (11, 12). nsP3 contains a macrodomain that binds to poly(ADP-ribose) and RNA molecules (13), a zinc-binding domain (14), and an unstructured region that interacts with host factors (15). nsP4 has an RNA-dependent RNA polymerase activity that is required for synthesizing progeny RNA (16). Alphavirus nonstructural proteins interact with each other (17) and with multiple host proteins (18, 19).
CHIKV nsP2 is a multifunctional protein. The nucleoside triphosphatase (NTPase) and RNA-dependent 5′-triphosphatase activities map to the N-terminal half of the protein, and the basic proteolytic activity is contained in its C-terminal region (20–22). Only complete nsP2 possesses RNA helicase activity (23). In addition to its role in viral RNA replication, nsP2 is also responsible for shutting off host cell transcription, by causing degradation of Rpb1 (a catalytic subunit of cellular RNA polymerase II [24]), and for inhibiting antiviral signaling (25). The expression of individual nsP2 from Old World alphaviruses causes the shutdown of cellular transcription and translation (26, 27). nsP2 is also involved in the shutdown of host cell translation. The mechanisms by which translation is shut down are unclear, although it has been shown that nsP2 interacts with several ribosomal proteins that may affect protein synthesis (28).
Mutations in the region of the viral genome encoding nsP2 are associated with the establishment of persistent infection and the prolonged survival of infected vertebrate cells. Such cytotoxicity-reducing mutations have been discovered for the nsP2s of Sindbis virus (SINV) (29, 30), Semliki Forest virus (SFV) (29, 31–33), and CHIKV (34, 35). Mutations in nsP2 may result in the reduced stability of late replication complexes and decreased positive-strand RNA synthesis (36). Such mutations have been put to use in the context of alphavirus replicon vectors (37). These vectors are self-replicating RNAs that encode nonstructural proteins but not structural proteins. Alphavirus replicons are characterized by their high biosafety, ease of manipulation, and ability to be introduced into a broad range of host cells (38). The incorporation of cytotoxicity-reducing mutations allows the creation of stable cell lines containing persistently replicating alphavirus replicons (34, 39).
In this study, we sought to develop CHIKV replicons carrying reporter genes that would be capable of persistent replication in vertebrate cells, preferably of human origin. To achieve this goal, we first rationally substituted the Pro718 residue of CHIKV nsP2 in the context of a replicon based on a virus isolated during the Indian Ocean outbreak (ECSA genotype, LR2006 OPY1 strain) and selected noncytotoxic replicons in BHK-21 cells. Different sets of adaptive mutations in the nsP2 region were identified in selected replicons. The most promising mutant replicon was subsequently adapted to persist in the human hepatocellular carcinoma cell line Huh7; this ability was found to be due to a combination of two novel mutations outside the nsP2 region. We investigated properties of the noncytotoxic replicons by analyzing RNA replication, nonstructural polyprotein production, and processing profiles; subcellular localization of nsP2 proteins for corresponding viruses was also analyzed. Enzymatic activities of mutant forms of recombinant nsP2 were analyzed to investigate the possible link between impaired protein properties and noncytopathic phenotypes of replicons or viruses. This study provides evidence that mutations associated with the noncytotoxic phenotype of CHIKV compromise the enzymatic activities of nsP2, whereas their effects on viruses and replicons strongly depend on the context and appear to vary not only between different alphaviruses but also across different genotypes.
MATERIALS AND METHODS
Cells and media.
BHK-21 cells (ATCC) were grown in Glasgow's minimal essential medium (GMEM) (Gibco) containing 10% fetal bovine serum (FBS), 2% tryptose phosphate broth (TPB), 200 mM HEPES, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Huh7 cells were grown in Dulbecco's modified Eagle's medium (DMEM) (PAA) containing 10% FBS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. The CHIKV replicon-containing cell lines were maintained in corresponding growth medium supplemented with puromycin (final concentration, 5 μg/ml). Cells were grown in a humidified incubator at 37°C in a 5% CO2 atmosphere.
Selection of CHIKV replicons in BHK-21 and Huh7 cells.
CHIKV replicons with mutations allowing stable, noncytotoxic growth in BHK-21 cells were obtained as described previously (34). Briefly, after selection, total RNA was isolated from cells using TRIzol reagent (Life Technologies). RNA was reverse transcribed using the first-strand cDNA synthesis kit from Thermo Scientific. Sequences corresponding to CHIKV replicons were PCR amplified using a set of primers designed to generate overlapping PCR fragments covering the full genome of the CHIKV replicon. The obtained fragments were sequenced, and mutations acquired during selection were identified.
Huh7 cells were transfected with CHIKV replicon transcripts harboring three genomic alterations: (i) a Renilla luciferase (Rluc)-encoding sequence inserted in the nsP3 region, (ii) a Gly-Glu-Glu-Gly-Ser insertion (here designated the 5A mutation) between amino acid residues 647 and 648 of nsP2, and (iii) a Pro718-to-Gly substitution (here designated the PG mutation) in the methyltransferase-like (MTL) domain of nsP2 (34). Cells were subjected to puromycin selection; colonies were picked and allowed to expand to cell lines. Replicon RNA was isolated and analyzed as described above.
Construction of mutant CHIKV replicons, genomes, and expression constructs.
CHIKV replicon vectors were constructed as described previously (34) except that the sequence encoding enhanced green fluorescent protein (EGFP) was replaced with a sequence encoding ZsGreen. CHIKV replicons are referred to here as ChikvRep; the designation ChikvRepRluc is used for replicons that also contain an Rluc insertion in the nsP3 region. Identified mutations were introduced singly or in combination into cDNA clones of ChikvRep, ChikvRepRluc, or an infectious cDNA (icDNA) clone of CHIKV-LR2006 OPY1 (34) using site-specific mutagenesis and subcloning. All rescued viruses are referred to using the designation CHIKV followed by the designations of the mutations that had been introduced.
The presence of introduced mutations was verified by sequencing cDNA clones that were obtained. Because reversions were possible, the presence of mutations was also verified by analysis of all obtained cell lines and viral stocks. RNA was extracted from each obtained virus stock or cell line using an RNeasy minikit (Qiagen) or TRIzol reagent (Life Technologies), respectively. Reverse transcription was carried out using a first-strand cDNA synthesis kit (Thermo Scientific). CHIKV-specific cDNA fragments were PCR amplified using pairs of primers flanking regions containing the introduced mutations and sequenced.
Plasmid constructs for the expression of wild-type (wt) nsP2 and of nsP2 with the 5A and PG mutations (nsP2-5A-PG; constructs with other mutations are designated similarly here) were constructed as follows. A sequence encoding CHIKV nsP2 was fused to the sequence for ubiquitin. Ubiquitin is cleaved off by cellular enzymes, allowing the production of nsP2 with its native N-terminal Gly residue. Then, sequences encoding the Ubi-nsP2 and Ubi-nsP2-5A-PG proteins were cloned under the control of the human EF-1α promoter in the context of modified pEF5/FRT/V5-DEST vector (Life Technologies).
RNA transcription and transfection of BHK-21 and Huh7 cells.
Plasmids containing cDNA of CHIKV replicons or viruses were linearized by NotI digestion. RNA was synthesized from these cDNAs in vitro using the mMESSAGE mMACHINE SP6 transcription kit (Ambion). BHK-21 cells (8 × 106) or Huh7 cells (3 × 106) were transfected with RNA by electroporation with a Bio-Rad Gene Pulser II unit, as follows. BHK-21 cells were resuspended in phosphate-buffered saline (PBS) and electroporated with two pulses at 850 V and 25 μF; Huh7 cells were resuspended in a mixture of cytomix (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4/KH2PO4, 25 mM HEPES [pH 7.6], 2 mM EGTA [pH 7.6], and 5 mM MgC12), 2 mM ATP, and 1.25% dimethyl sulfoxide (DMSO) and electroporated at 270 V and 950 μF with one pulse. All electroporations were performed in 4-mm cuvettes (Thermo Fisher Scientific). Expression plasmids encoding Ubi-nsP2 and Ubi-nsP2-5A-PG were used to transfect BHK-21 cells using Lipofectamine 2000 (Life Technologies) according to the manufacturer's protocol.
Virological methods.
The titers of collected virus stocks were determined using standard plaque assays. For infectious-center assays (ICAs), 8 × 106 BHK-21 cells were electroporated with 1 μg of in vitro-synthesized RNA. Tenfold dilutions of transfected cells were seeded onto 6-well tissue culture plates containing 1.5 × 106 BHK-21 cells per well. After 2 h of incubation at 37°C, the cell culture medium was aspirated, and the cells were overlaid with 2 ml of GMEM supplemented with 2% fetal calf serum (FCS) containing 0.8% carboxymethyl cellulose (Sigma Life Science). Plaques were stained with crystal violet after 2 to 3 days of incubation at 37°C.
Cell survival assay.
The survival of transfected cells containing different CHIKV replicons was measured using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) method (40). Briefly, 50 μg of in vitro-synthesized CHIKV replicon RNAs were electroporated into 8 × 106 BHK-21 cells or 3 × 106 Huh7 cells, which were then seeded into 96-well plates at a density of 5 × 104 or 1.4 × 104 cells/well, respectively. Puromycin at a final concentration of 5 μg/ml was added to the cells 24 h after electroporation. At the appropriate time points, MTT reagent at a final concentration of 0.5 mg/ml was added to the wells, and plates were incubated at 37°C in a 5% CO2 incubator for 2 h. Subsequently, the supernatant was removed, 100 μl of DMSO was added per well, and the plates were gently shaken for 15 min. The optical densities were measured at 540 nm using a microplate reader (Sunrise Tecan).
Immunoblot analysis.
BHK-21 cells (8 × 106) were transfected with 50 μg of in vitro-synthesized CHIKV replicon RNAs. At 16 h posttransfection (p.t.), cells were lysed using SDS gel-loading buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 200 mM dithiothreitol [DTT], and 0.2% bromophenol blue). Lysates corresponding to 50,000 cells were loaded into each well; proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes, and detected using rabbit polyclonal antiserum against CHIKV nsP2 (generated in-house). The membranes were then incubated with secondary goat anti-rabbit antibodies conjugated to horseradish peroxidase (LabAs Ltd.). Western blots were visualized using an ECL immunoblot detection kit (GE Healthcare).
Northern blot analysis.
BHK-21 cells (8 × 106) or Huh7 cells (3 × 106) were transfected with 50 μg of in vitro-synthesized CHIKV replicon RNAs. At 16 h p.t., total RNA was extracted using TRIzol reagent (Life Technologies) according to the manufacturer's instructions. Equal amounts of total RNA (5 μg) were denatured for 10 min at 70°C in 2-fold RNA loading dye (Thermo Scientific), cooled on ice, and separated by electrophoresis in 1.4% agarose gels supplemented with 0.2 M formaldehyde. RNA was transferred to a Hybond-N+ filter (GE Healthcare) and fixed using a UV Stratalinker 1800 (Stratagene). A digoxigenin (DIG)-labeled RNA detection probe complementary to the 3′ untranslated region (UTR) of the CHIKV genome was generated using a DIG hybridization kit (Roche). Filters were hybridized overnight; blots were washed and developed according to the manufacturer's protocols (Northern blot with DIG, Roche).
Extraction of nuclear and cytoplasmic fractions.
BHK-21 cells were infected with CHIKV and its mutant variants at a multiplicity of infection (MOI) of 5 PFU/cell. Cells were washed and collected at 4, 8, and 12 h postinfection (p.i.). Nuclear and cytoplasmic fractions were prepared from the cells using NE-PER nuclear and cytoplasmic extraction reagent according to the manufacturer's (Thermo Scientific) protocols. A total of 5% of each fraction was loaded per well, and proteins were separated by SDS-PAGE and analyzed by Western blotting as described above. α-Tubulin was detected using an anti-α-tubulin mouse monoclonal antibody (Santa Cruz Biotechnology).
Immunofluorescence microscopy.
BHK-21 cells were grown on glass coverslips and infected with wt CHIKV or CHIKV-5A-PG at an MOI of 5 PFU/cell in serum-free medium; mock-infected cells were used as controls. At 12 h p.i., cells were washed with PBS, fixed with 4% paraformaldehyde, quenched with 50 mM NH4Cl, and permeabilized with 0.1% Triton X-100 for 2 min at room temperature. Cells were washed with PBS, blocked with 5% FBS in PBS, and stained for 1 h with rabbit anti-nsP2 antibodies. Anti-rabbit Alexa-568-conjugated goat antibodies (Life Technologies) were used as secondary antibodies. SlowFade Gold reagent with 4′,6′-diamidino-2-phenylindole (DAPI) (Life Technologies) was used to counterstain nuclei. Immunofluorescence images were obtained and analyzed using an LSM710 confocal microscope (Zeiss).
In vitro transcription/translation and immunoprecipitation.
In vitro transcription and translation were carried out using the TNT-coupled SP6 rabbit reticulocyte lysate system (Promega) according to the manufacturer's protocol. Reaction mixtures (25 μl) containing 10 μCi of [35S]methionine (Perkin-Elmer) and 1 μg of a plasmid containing cDNA of wt ChikvRep, ChikvRep-5A, ChikvRep-PG, ChikvRep-5A-PG, or ChikvRep-FL-5A-PG-IL were incubated for 1 h at 30°C; translation was stopped by adding cycloheximide to a final concentration of 1 mM. Samples were immediately collected (pulse) or incubated for 15 or 60 min under the same conditions (chase). RNase A was added to collected samples at a final concentration of 10 ng/μl, and the mixtures were incubated for another 5 min. Products of in vitro translation and processing reactions were denatured by boiling in LDS-NET150 buffer (50 mM Tris-HCl [pH 8.0], 2% lithium dodecyl sulfate, 150 mM NaCl, 5 mM EDTA, and 1% NP-40), diluted 100-fold with NET150 buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM EDTA, and 1% NP-40), and incubated for 1 h at 4°C with rabbit polyclonal antiserum against CHIKV nsP2. Immunocomplexes were precipitated with protein A-Sepharose CL-4B (Sigma-Aldrich) overnight at 4°C and then washed four times with NET buffer containing 400 mM NaCl. The precipitated proteins were denatured by heating in SDS gel-loading buffer, separated by SDS-PAGE, and visualized using a Typhoon imager (GE Healthcare).
Metabolic labeling.
Pulse-chase labeling of infected BHK-21 cells was carried out as described previously (8). Briefly, BHK-21 cells were infected with wt CHIKV, CHIKV-5A, CHIKV-PG, CHIKV-5A-PG, or CHIKV-FL-5A-PG-IL at an MOI of 20 PFU/cell. At 3 h p.i. the cells were starved in methionine-cysteine-free DMEM (Life Technologies) for 30 min and then labeled with 50 μCi of [35S]methionine-cysteine mixture (Perkin-Elmer) for 15 min (pulse). In the chase samples, the pulse was followed by a chase for 15 or 60 min in medium containing an excess of unlabeled methionine and cysteine. The cells were then lysed by boiling in 1% SDS solution, and proteins in the cell lysates were immunoprecipitated and analyzed by SDS-PAGE as described above.
Expression and purification of recombinant nsP2 and its mutant forms.
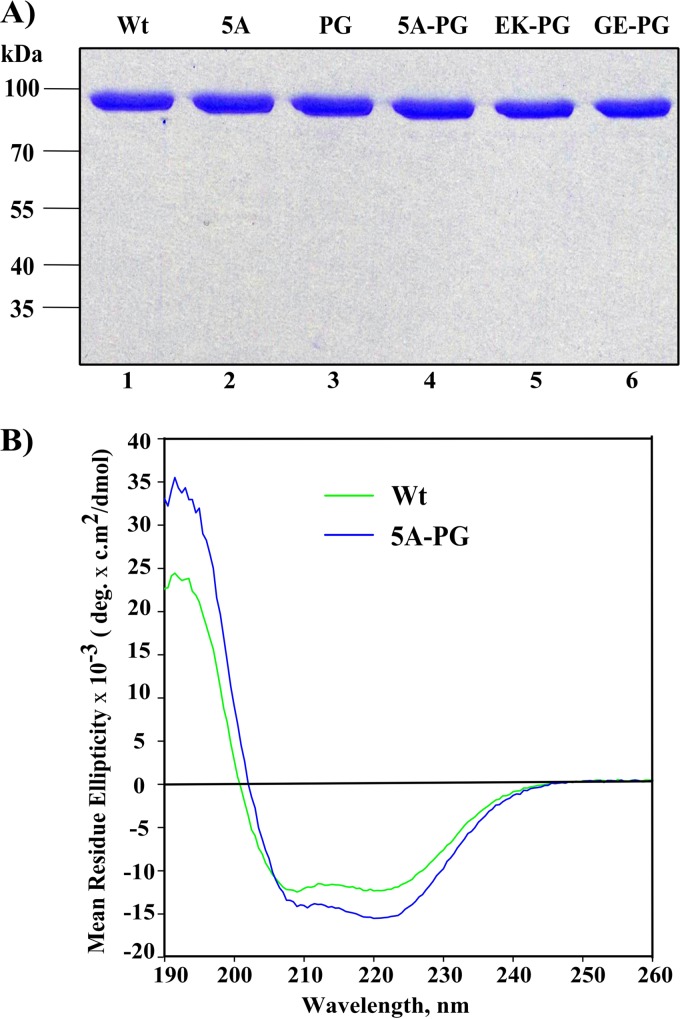
Recombinant nsP2 and its mutant forms were obtained using a bacterial expression system as described before, with minor modifications (41). Briefly, the primary structure of the expressed protein is M-(thioredoxin)-GSSSG-LDRAGG↓(nsP2); the arrow indicates the peptide bond that is cleaved by nsP2 protease activity. After autoproteolysis, we obtained an nsP2 protein without extra N-terminal amino acid residues, which have been shown to have considerable adverse effects on its functionality (9, 23). The mutations associated with the noncytotoxic phenotype of CHIKV replicons were introduced into this expression construct using PCR-based site-directed mutagenesis. All recombinant proteins, with the exception of nsP2-PG, were purified using Ni affinity, ion exchange, and size exclusion chromatographies as described previously (23). For nsP2-PG, the gel filtration step was excluded, due to the low yield of this protein. Concentrations of the purified proteins were measured using the NanoDrop 1000 instrument (Thermo Scientific), taking into account the extinction coefficients and molecular masses of the proteins, which were calculated using the ProtParam software tool (http://web.expasy.org/protparam/). The purities of all the recombinant proteins were verified using 10% SDS-PAGE and Coomassie brilliant blue staining (see Fig. 6A). All protein preparations were flash-frozen in liquid nitrogen and stored at −80°C.
FIG 6.
Expression, purification, and quality analysis of recombinant enzymes. (A) SDS-PAGE analysis of purified recombinant nsP2 proteins. Approximately 1 μg of each purified protein was loaded per lane and analyzed by 10% SDS-PAGE. The gel was stained with Coomassie brilliant blue. (B) Comparison of the CD spectra of wt nsP2 and nsP2-5A-PG recombinant proteins.
CD spectroscopy.
Circular dichroism (CD) spectrum measurements for recombinant wt nsP2 and nsP2-5A-PG were carried out at 20°C in the far-UV region (190 to 260 nm) in buffer containing 10 mM KH2PO4 (pH 7.2) and 100 mM NaF with a protein concentration of 1.12 μM in a 1-mm quartz cuvette cell (Hellma Analytics) using a Chirascan plus CD spectrometer (Applied Photophysics). Before analysis, samples were centrifuged for 10 min at 13,000 × g at 4°C. The concentrations of soluble proteins were measured immediately before CD spectrum measurements were made. CD spectra were acquired three times. The mean spectrum of the negative control (buffer) was subtracted from the data obtained for proteins using the Prodata viewer software (Applied Photophysics). The average values of three independent acquisitions were expressed as mean residue ellipticities.
Expression and purification of substrates for nsP2 protease.
EGFP-10:5-Trx substrates containing short versions of cleavage sites between nsP1/nsP2 (1/2 site), nsP2/nsP3 (2/3 site), and nsP3/nsP4 (3/4 site) were prepared as follows. Sequences encoding 10 amino acid residues from the P side and 5 amino acid residues from the P′ side of each cleavage site were placed between sequences encoding Escherichia coli codon usage-adapted superfolder GFP (42) and thioredoxin in a modified pET28a vector. For the 2/3 site, the longer version of the substrate (EGFP-10:170) was also prepared; in this case, the sequence encoding 10 amino acid residues from the P side of the site followed by the first 170 amino acid residues of nsP3 was fused to the sequence encoding EGFP (see Fig. 7A). Recombinant proteins representing all four substrates were expressed in a bacterial expression system. Induced cell cultures were collected by centrifugation and lysed using an EmulsiFlex-C3 high-pressure homogenizer (Avestin, Germany) in buffer A (50 mM sodium phosphate [pH 7.6], 300 mM NaCl, 1 mM beta-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride [PMSF], 4 μg/ml DNase, and 5% glycerol). The lysates were clarified by centrifugation at 48,000 × g; the resulting supernatants were passed through a DE52 anion-exchange resin (Whatman) precharged with 2 M NaCl and equilibrated with buffer A. These filtered supernatants were then loaded onto columns with Talon resin (Clontech Laboratories) preequilibrated with buffer B (50 mM sodium phosphate [pH 7.6], 300 mM NaCl, 1 mM beta-mercaptoethanol, and 5% glycerol), and the columns were washed with 30 column volumes of buffer B containing 5 mM imidazole. Proteins were eluted with buffer B supplemented with 150 mM imidazole in several 0.5-ml volumes. The fractions containing the largest amounts of recombinant protein were combined and desalted using a PD10 column (GE Healthcare) in buffer C (20 mM Tris-HCl [pH 7.5] and 50 mM NaCl). Finally, the proteins were loaded onto a 1-ml Resource Q anion-exchange column (GE Healthcare) with a flow rate of 1 ml/min (AKTA Purifier; GE Healthcare) and eluted with a gradient of buffer C containing 1 M NaCl. The selected fractions were concentrated and loaded onto a Superdex 75 10/300 GL high-resolution gel filtration column (GE Healthcare) at a flow rate of 0.5 ml/min. Obtained proteins were analyzed by 10% SDS-PAGE and Coomassie brilliant blue staining. Protein concentrations were measured as described above; proteins were flash-frozen and stored at −80°C.
FIG 7.
Protease activities of purified recombinant nsP2 and its mutant variants. (A) A schematic representation of the CHIKV nonstructural polyprotein and of substrates containing short (EGFP-10:5-Trx) or long (EGFP-10:170) versions of the nsP2 protease cleavage sites. (B) Cleavage of substrates containing short versions of cleavage sites by recombinant enzymes. The indicated substrates at a total concentration of 6 μM were treated with 0.7 μM enzymes for 60 min; the enzyme names are indicated above the panel. Reactions were stopped by the addition of SDS gel-loading buffer, and reaction products were analyzed by 12% SDS-PAGE and Coomassie brilliant blue staining. The names and positions of the detected proteins are shown at the left of the panel. Note that the second cleavage product, 5-Trx, was too short to be detected under these conditions and is not shown. (C) Cleavage of a substrate containing the long version of the 2/3 site (EGFP-10:170) by recombinant enzymes for 6 and 60 min (indicated above the panel). Reactions and an analysis of the reaction products were carried out in the same way as described for panel B. Dotted lines indicate where separate images were merged. In panels B and C, the results from one of four reproducible experiments are shown. “% of product” indicates the relative amount of detected cleavage product; the amount of product obtained from wt enzyme was set at 100%.
In vitro nsP2 protease activity assay.
A protease assay with substrates containing short versions of nsP2 cleavage sites was carried out using 0.7 μM recombinant enzyme and 6 μM each substrate in assay buffer D (10 mM HEPES [pH 7.2] and 1 mM beta-mercaptoethanol) in a final reaction volume of 8 μl. After incubation at 30°C for 60 min, the reactions were terminated by adding equal volumes of 2-fold SDS gel-loading buffer. After boiling, samples were resolved using 12% SDS-PAGE. In the case of the EGFP-10:170 substrate, the reaction volume was increased to 12 μl. Aliquots (5.5 μl) of the reaction mixtures were taken at 6 and 60 min; reactions were stopped and reaction products analyzed as described above.
GTPase activity assay.
The GTP hydrolysis rate was analyzed using the EnzCheck phosphate assay kit (Life Technologies) according to the manufacturer's protocol. Briefly, the reactions were carried out using 10 μl of 20-fold reaction buffer (1 M Tris-HCl [pH 7.5], 20 mM MgCl2, and 2 mM NaN3), 40 μl of 2-amino-6-mercapto-7-methyl purine riboside substrate, 1 unit of purine nucleoside phosphorylase, and 56 nM recombinant enzyme. The volume was adjusted to 170 μl, and mixtures were preincubated at 22°C for 10 min; the reactions were then initiated by the addition of 30 μl of 1 mM GTP. Continuous spectrophotometric measurements were carried out using an Ultrospec 7000 spectrophotometer (GE Healthcare). For the GTPase stimulation assay, 1.75 μM ribo(U)18 oligonucleotide was included in the reaction mixture.
RNA helicase assay.
The RNA helicase assay was performed as described previously (23). Briefly, 50 pM of helicase substrate [RNA with a 16-bp duplex region and a 12-base single-stranded 5′ poly(A) overhang] and 12.5 nM enzyme were incubated with reaction buffer E (20 mM HEPES [pH 7.2], 10 mM NaCl, and 2 mM DTT) in a final volume of 15 μl at 22°C for 15 min. The reaction mixture was then divided into 3 tubes. To the first tube, 3 mM ATP:Mg2+ was added, while equal volumes of water were added to other two. Reactions were terminated after incubation at 30°C for 60 min; one of the tubes that received water was heated at 95°C. RNA products were resolved by native PAGE and analyzed as described previously (23).
RESULTS
Development of stable BHK-21 CHIKV replicon cell lines.
To adapt CHIKV replicons for noncytotoxic growth in BHK-21 cells, a Pro718-to-Gly substitution in the MTL domain of nsP2 (PG mutation) was introduced into the ChikvRep construct, which carries sequences encoding EGFP followed by foot-and-mouth disease virus 2A autoprotease and puromycin acetyltransferase (Pac) under the control of the SG promoter. An analogous substitution (at Pro726 of nsP2) has been shown to result in a noncytotoxic phenotype in SINV and is a major contributor to the noncytotoxic phenotypes of SFV and WA CHIKV replicons (30–32, 35). In contrast, it was found that ChikvRep-PG, based on a virus with an ECSA genotype, was highly cytotoxic (34). In the MTT-based cell survival assay, a large majority of BHK-21 cells transfected with ChikvRep-PG RNA died within 72 h p.t.; no clear difference was observed with respect to cells transfected with wt ChikvRep RNA (Fig. 1A). Nevertheless, under prolonged puromycin selection, ChikvRep-PG allowed the formation of puromycin-resistant colonies (34); in contrast, if wt replicon RNA was used, such colonies were not obtained in this study or in a previous study (34). Sequencing of replicon RNA extracted from stable cell lines originating from independent transfection/selection experiments identified two combinations of potential cytotoxicity-reducing mutations in the nsP2 region (Fig. 1A). The first combination (here referred to as 5A-PG) consisted of the original PG mutation together with an insertion of five amino acid residues (Gly-Glu-Glu-Gly-Ser; 5A mutation) between amino acid residues 647 and 648 of nsP2 (34). The MTT-based cell survival assay revealed that the 5A mutation alone was not sufficient to confer the noncytotoxic phenotype of ChikvRep. In contrast, ChikvRep-5A-PG clearly lacked cytotoxic properties (Fig. 1A). The second combination consisted of the PG mutation together with two new point mutations in nsP2. The first of these point mutations, Glu116 to Lys (EK mutation), was identified in the N-terminal domain of nsP2, while the second change, Gly620 to Glu (GE mutation), was located in the beginning of the MTL domain (Fig. 1A). ChikvRep-EK-GE and ChikvRep-EK, as well as ChikvRep-GE-PG and ChikvRep-PG, were cytotoxic. In contrast, combination of EK and PG mutations or the EK, GE, and PG mutations resulted in similar reductions in cytotoxicity (Fig. 1A; Table 1), and cells transfected with ChikvRep-EK-PG or ChikvRep-EK-GE-PG were dividing at 72 h p.t. Thus, the major contribution to the noncytotoxic phenotype of these replicons clearly originated from the combination of the EK and PG mutations. This finding was unexpected, because up to this point, all mutations associated with the noncytotoxic phenotype of CHIKV replicons have been mapped to the MTL domain (34, 35). However, as was observed with the cell lines obtained as result of puromycin selection, cells containing ChikvRep-EK-GE-PG (or ChikvRep-EK-PG) exhibited much slower growth than cells containing ChikvRep-5A-PG. Consistent with these results, fluorescence of marker protein was also much stronger in cells containing ChikvRep-5A-PG (data not shown).
FIG 1.
Schematic presentation of the CHIKV replicons and the survival of cell cultures transfected with the original and mutant replicons. Upper panels, design of ChikvRep and ChikvRepRluc, used to transfect BHK-21 (A) and Huh7 cells (B), respectively. The positions of the original introduced mutations (PG and 5A) and mutations acquired during selection (FL, EK, GE, IL, and 5A) are shown. NTD, N-terminal domain; MTL, methyltransferase-like domain of nsP2. Lower panels, results of the cell survival assay. BHK-21 (A) or Huh7 (B) cells were transfected by electroporation with replicons carrying the mutations indicated below the graph. Control, mock-transfected cells. Puromycin-containing medium (puro) was added to the transfected cells at 24 h p.t. Viability of the cells (measured as the optical density at 540 nm (OD540) was analyzed using MTT reagent at 24 h p.t. (white bars) and subsequently at 24 h (gray bars) and 48 h (black bars) after the addition of puromycin. The viability of control cells before the addition of puromycin is set to 1; all other results were normalized to this value. The experiment was performed in triplicate and repeated twice; the results of one experiment are shown. Error bars indicate standard deviations.
TABLE 1.
Biological effects of the analyzed mutations and their combinations
| Mutation | Reduction of cytotoxicity | Effect on positive-strand RNA synthesis in: |
Effect on levels of nsP2 in: |
Infectivity of in vitro-transcribed RNA in BHK-21 cells (PFU/μg) | Effect on nuclear localization | Processing in defined enzymatic assaya |
Processing of P1234 in cell-free assayb | GTPase activityc | Effect on oligonucleotide stimulation of GTPase activity | RNA helicase activityc | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BHK-21 cells | Huh7 cells | BHK-21 cells | Huh7 cells | 1/2 substrate | Long 2/3 substrate | 3/4 substrate | ||||||||
| PG | No | No effect | Decrease | Increase | Increase | 1.3 × 105 | Increase | 18 | >90 | 33 | 18 | 57 ± 2.1 | Abolishes | 84 ± 14 |
| 5A | No | Decrease | Severe decrease | Decrease | Decrease | 2 × 104 | No effect | 87 | >90 | 79 | 30 | 89 ± 4.6 | No effect | 96 ± 20 |
| 5A-PG | Yes | Severe decrease | Severe decrease | Decrease | Severe decrease | 2.4 × 104 | Increase | 11 | >90 | 29 | 2.6 | 70 ± 1.1 | Abolishes | 72 ± 10 |
| EK | No | Severe decrease | NAd | Severe decrease | NA | 56 | NA | NA | NA | NA | NA | NA | NA | NA |
| GE | No | Increase | NA | Increase | NA | 1.1 × 105 | NA | NA | NA | NA | NA | NA | NA | NA |
| EK-GE | No | Severe decrease | NA | Severe decrease | NA | 24 | NA | NA | NA | NA | NA | NA | NA | NA |
| EK-PG | Yes | Severe decrease | NA | Severe decrease | NA | 24 | NA | 34 | >90 | 39 | NA | 49 ± 4.8 | No effect | 62 ± 17 |
| GE-PG | No | Decrease | NA | Increase | NA | 1 × 105 | NA | 27 | >90 | 38 | NA | 78 ± 5.1 | Abolishes | 83 ± 11 |
| EK-GE-PG | Yes | Severe decrease | NA | Severe decrease | NA | 24 | NA | NA | NA | NA | NA | NA | NA | NA |
| FL | No | NA | Increase | NA | Increase | 1.8 × 105 | NA | NA | NA | NA | NA | NA | NA | NA |
| IL | No | NA | Increase | NA | Increase | 1.4 × 105 | NA | NA | NA | NA | NA | NA | NA | NA |
| FL-IL | No | NA | Increase | NA | Increase | 1.4 × 105 | NA | NA | NA | NA | NA | NA | NA | NA |
| FL-PG-IL | No | NA | Increase | NA | Increase | 2 × 104 | NA | NA | NA | NA | NA | NA | NA | NA |
| FL-5A-IL | No | NA | Decrease | NA | Increase | 1,920 | NA | NA | NA | NA | NA | NA | NA | NA |
| 5A-PG-IL | Yes | NA | Severe decrease | NA | Decrease | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| FL-5A-PG | Yes | NA | Severe decrease | NA | Decrease | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| FL-5A-PG-IL | Yes | NA | Severe decrease | NA | Decrease | 96 | NA | NA | NA | NA | 3.1 | NA | NA | NA |
The amount of reaction product produced by nsP2 at 60 min was taken as 100.
The activity of wt nsP2 at 0 min (pulse), estimated by the ratio of label incorporated into nsP2 to the label incorporated into P1234, was taken as 100.
The activity of wt nsP2 was taken as 100. Results are means ± standard deviations.
NA, not analyzed.
Development of stable human-origin cell lines carrying the CHIKV replicon.
The BHK-21 cell line containing the ChikvRepRluc-5A-PG replicon (termed CHIKV-NCT in a previous study) proved to be a useful tool for screening inhibitors of CHIKV replication (34). However, BHK-21 cells are not suitable for screening host factors using human small interfering RNA (siRNA) libraries or for detecting antiviral effects of compounds targeting such host factors. In contrast, Huh7 cells have been successfully used for the development of hepatitis C virus replicon cell lines that have been used to screen antiviral compounds and identify host factors essential for viral replication (43). Therefore, we investigated whether selected combinations of mutations exerted the same effects in human cell lines as in BHK-21 cells. For this, HeLa (ATCC) and Huh7 cells, which, similarly to BHK-21 cells, are unable to produce type-I interferon, were used. Cells transfected with ChikvRep-EK-GE-PG RNA died after puromycin addition at a rate similar to that observed for mock-transfected cells (data not shown). Under the same conditions, HeLa and Huh7 cells transfected with ChikvRep-5A-PG survived for several days; however, starting in the second week p.t., a gradual reduction in the number of viable cells occurred, and antibiotic-resistant colonies were not formed.
Interestingly, puromycin-resistant colonies were obtained only for ChikvRepRluc-5A-PG RNA-transfected Huh7 cells and not for Huh7 or HeLa cells transfected with any other replicon (including ChikvRep-5A-PG) RNA. Sequence analysis revealed two additional point mutations in the adapted ChikvRepRluc-5A-PG RNA. The first change was located in the C-terminal half of nsP1 (Phe391 to Leu, designated FL). The second mutation was located in nsP3 (Ile175 to Leu, IL mutation). Cell survival assays revealed that in the absence of the PG and 5A mutations, neither the FL mutation alone, the IL mutation alone, nor the combination of these mutations was able to reduce the cytotoxicity of ChikvRepRluc RNA (Fig. 1B; Table 1). Only cells transfected with ChikvRepRluc-FL-5A-PG, ChikvRepRluc-5A-PG-IL, or ChikvRepRluc-FL-5A-PG-IL RNAs survived for 72 h (Fig. 1B; Table 1). Although no difference between the survival of cells containing any of these three replicons and the survival of cells transfected with the parental ChikvRepRluc-5A-PG were observed during the first 72 h p.t., only Huh7 cells transfected with ChikvRepRluc-FL-5A-PG, ChikvRepRluc-5A-PG-IL, or ChikvRepRluc-FL-5A-PG-IL formed stable replicon cell lines. Furthermore, cell lines containing ChikvRepRluc-FL-5A-PG or ChikvRepRluc-5A-PG-IL exhibited slower growth than cells containing ChikvRepRluc-FL-5A-PG-IL (data not shown).
The Huh7 cell line containing ChikvRepRluc-FL-5A-PG-IL exhibited rapid growth and high levels of expression of ZsGreen and Rluc. Head-to-head comparison with BHK-21 cells containing the ChikvRepRluc-5A-PG replicon revealed that under puromycin selection, both cell lines maintained similarly high levels of marker expression (data not shown). The same result was observed for Huh7 cells with the ChikvRepRluc-FL-5A-PG-IL replicon in the absence of puromycin selection: even after 10 passages, all cells expressed ZsGreen, with no decrease in Rluc activity. In contrast, in the absence of puromycin, cells lacking ZsGreen fluorescence rapidly appeared in the culture of BHK-21 cells harboring ChikvRepRluc-5A-PG; after 10 passages, ZsGreen expression was maintained in fewer than 20% of BHK-21 cells, and Rluc activity was markedly (nearly 100-fold) decreased (data not shown). Thus, the Huh7 cell line harboring the ChikvRepRluc-FL-5A-PG-IL replicon has the potential to be a convenient and safe tool for studies investigating host factors or inhibitors of CHIKV replication.
Analysis of viral RNA and nsP2 synthesis in cells transfected with CHIKV replicon RNAs.
nsP2 mutations associated with a noncytotoxic phenotype are known to severely reduce the synthesis of alphavirus positive-strand RNAs (30–32, 35) and, to a lesser extent, also synthesis of nonstructural proteins (31, 44). To determine to what extent mutations identified in this study alter positive-strand RNA and nonstructural protein levels, BHK-21 and Huh7 cells were transfected with in vitro-synthesized RNAs based on ChikvRep and ChikvRepRluc, respectively, and the levels of positive-strand RNAs and nsP2 proteins in transfected cells were analyzed at 16 h p.t.
Consistent with its high cytotoxicity, transfection with ChikvRep-PG RNA resulted in the production of almost wt levels of positive-strand RNA in BHK-21 cells (Fig. 2A). Relatively minor reductions in the levels of these RNAs in ChikvRepRluc-PG-transfected Huh7 cells (Fig. 2B) are consistent with increased survival of such cells in the previous experiment (Fig. 1B). These findings are in clear contrast to findings made using SINV (30, 44) and SFV (31) replicons. Even more strikingly, a mutation at the same position (Pro718 to Ser) in the replicon based on the WA genotype (37997 strain) of CHIKV also caused a severe reduction in positive-strand RNA synthesis (35). Consistent with findings from the cell survival assay (Fig. 1A), the GE mutation did not have a negative effect on the synthesis of positive-strand RNA in cells transfected with the ChikvRep-GE replicon, and this mutation had only a relatively minor effect in combination with the PG mutation as well (compare ChikvRep-PG with ChikvRep-GE-PG in Fig. 2A). In contrast to ChikvRep-PG (and ChikvRepRluc-PG), the ChikvRep-5A and ChikvRepRluc-5A replicons displayed considerably reduced levels of RNA synthesis (Fig. 2A and B; Table 1). The effect of the 5A mutation on nsP2 protein levels was less prominent (Fig. 2C and D). As expected, the combination of the 5A and PG mutations resulted in a severe reduction in positive-strand RNA levels (Fig. 2A and B) and a clear reduction in nsP2 levels (Fig. 2C and D; Table 1). The effect of the EK mutation was even more prominent, as neither the corresponding positive-strand RNA nor nsP2 could be detected (Fig. 2A and C). These products became detectable only when the membranes were strongly overexposed (data not shown). This finding correlates with the observation that BHK-21 cells containing ChikvRep-5A-PG expressed ZsGreen at a level similar to that in cells transfected with the wt replicon, whereas ZsGreen expression in cells containing the ChikvRep-EK-PG replicon was much lower (data not shown). Thus, the extremely low levels of replicon RNA in Huh7 cells transfected with ChikvRep-EK-PG were most likely not sufficient to provide Pac expression at levels necessary for protection against puromycin. The same may also be true for ChikvRepRluc-5A-PG, as the defects in RNA and viral protein synthesis caused by the 5A-PG mutations were clearly more prominent in Huh7 cells (compare Fig. 2A with B and Fig. 2C with D).
FIG 2.
Effects of the identified mutations on the synthesis of positive-strand RNAs and the nsP2 protein in cells transfected with CHIKV replicon RNAs. (A and B) Northern blot analysis was performed using total RNAs extracted from BHK-21 (A) or Huh7 (B) cell transfected with the ChikvRep (BHK-21) or ChikvRepRluc (Huh7) RNAs indicated above the panels. Replicon structures are shown in Fig. 1A and B, respectively. Total RNA was isolated at 16 h p.t.; 5 μg of each RNA sample was separated by agarose gel electrophoresis. CHIKV RNAs were detected by hybridization with a probe complementary to the 3′ UTR of the CHIKV genome. (C and D) For Western blot analysis, BHK-21 (C) or Huh7 (D) cells transfected with the same replicon RNAs as cells used for panels A and B were lysed at 16 h p.t. in SDS gel-loading buffer. Material corresponding to 50,000 transfected cells was loaded into each well of the gel. Proteins were separated by 10% SDS-PAGE, and nsP2 was detected using rabbit anti-nsP2 antiserum. Dotted lines on panels A, B, and C indicate where separate images have been merged. The experiment was repeated twice with similar results.
The introduction of the FL, IL, or FL-IL mutation into ChikvRepRluc resulted in slightly increased levels of nsP2 and SG RNAs (Fig. 2B and D; Table 1). Introduction of these mutations into ChikvRepRluc-5A, ChikvRepRluc-PG, and, importantly, ChikvRepRluc-5A-PG also resulted in a clear increase in nsP2 levels; the effect was largest for the FL-IL combination (Fig. 2D). Consistent with these findings, levels of SG RNAs produced by these replicons (Fig. 2B) were similarly increased. Due to their very low level of expression, it was impossible to confirm whether this was also the case for genomic RNAs. Thus, the contributions of the FL and IL mutations to the noncytotoxic phenotype of the CHIKV replicon are different from those of the PG, 5A, and EK mutations. The FL and IL mutations act in an additive manner and boost SG RNA (and possibly also genomic RNA) synthesis in Huh7 cells, which in turn allows synthesis of Pac at levels sufficient to provide resistance to puromycin.
Effects of the identified mutations on the infectivity of viral genomes.
The effects of FL and IL mutations on the replication of CHIKV replicon RNA in Huh7 cells did not depend on the presence of Rluc in the nsP3 region (data not shown). Therefore, to analyze the effects of the identified mutations in the context of a complete CHIKV genome, all changes were introduced into an icDNA clone of CHIKV LR2006 OPY1 which lacks the Rluc reporter (34). As no established ICA protocol was available for Huh7 cells, the infectivity of in vitro-synthesized RNA transcripts was analyzed in BHK-21 cells. This assay revealed that all RNAs that contained the EK mutation had very low infectivity (Fig. 3; Table 1). Furthermore, the EK mutation always reverted in the genomes of rescued viruses. Consistently, rescued (revertant) viruses were cytotoxic, but due to the very small numbers of initially infected cells, cytopathic effect (CPE) was observed only at 48 h p.t. These findings are highly consistent with other properties associated with the EK mutation (Table 1), confirming that the EK mutation causes severe defects in RNA replication. The low level of RNA replication was sufficient for noncytotoxic growth of the corresponding replicon in BHK-21 (but not in HeLa or Huh7) cells; in contrast, it was not acceptable in the context of infectious virus. On their own, the GE, FL, IL, and FL-IL mutations had virtually no effect on the infectivity of in vitro-synthesized RNAs. The corresponding viruses were highly cytotoxic (CPE detected at 24 h p.t.) and generated plaques similar to those observed with wt CHIKV (Fig. 3; Table 1), confirming that these mutations are not directly associated with the noncytotoxic phenotype. Importantly, ICA revealed that the RNA of CHIKV-PG was also highly infectious; moreover, the rescued virus was cytotoxic and formed large plaques (Fig. 3). Due to the wt-like properties of the corresponding viruses, the FL, GE, PG, and IL mutations were stably maintained in viral progeny (Fig. 3).
FIG 3.
Effects of mutations on the efficiency of viral rescue and on the properties of rescued viruses. A total of 1 μg of in vitro-transcribed RNAs from the CHIKV icDNA clone (wt) or from icDNA clones harboring the identified mutations or combinations of these mutations was used to transfect BHK-21 cells. The infectivity of the transcripts was analyzed by ICA and is presented as PFU per μg of RNA. The plaque size, whether cytopathic effects (CPE) were present in cell culture at 24 and 48 h p.t., and the results of sequence analysis of the mutated regions are shown. N/A, not applicable. The results of one of two reproducible experiments are shown.
Compared to wt CHIKV, the infectivity of CHIKV-5A genomes was reduced approximately 10-fold; addition of the 5A mutation into the genome of CHIKV-PG caused a similar reduction. CHIKV-5A and CHIKV-5A-PG both generated small plaques, and no CPE was detectable at 24 h p.t.; however, at 48 h p.t., CPE was detected for CHIKV-5A-infected cells but not for those infected with CHIKV-5A-PG (Fig. 3). This finding is consistent with the noncytotoxic growth of the ChikvRep-5A-PG in BHK-21 cells (Fig. 1A). Sequencing of the mutant genomes revealed that both rescued viruses preserved the introduced mutations.
Compared to that of CHIKV-PG, the infectivity of CHIKV-FL-PG-IL RNAs was reduced 6.5-fold. The rescued virus was highly cytotoxic and formed large plaques (Fig. 3). Similarly, the infectivity of CHIKV-FL-5A-IL RNAs was reduced approximately 10-fold compared with that of CHIKV-5A. As CHIKV-FL-5A-IL RNAs were nearly 100-fold less infectious than those of wt CHIKV, the appearance of CPE was also delayed. The biggest reduction in infectivity caused by the FL and IL mutations was observed for CHIKV-FL-5A-PG-IL RNAs, which were nearly 250-fold less infectious than CHIKV-5A-PG RNAs (or over 2,000-fold less infectious than wt CHIKV RNAs) (Fig. 3; Table 1). Thus, as in Huh7 cells the FL and IL mutations increased the replication of ChikvRepRluc-5A-PG (Fig. 2B and D), they may represent cell type-specific adaptations. Hence, in BHK-21 cells (and/or in the context of the full-length CHIKV genome), these mutations reduced the infectivity of CHIKV containing the 5A, PG, and 5A-PG mutations. Interestingly, CHIKV-FL-5A-PG-IL was able to produce CPE, while the more infectious CHIKV-5A-PG was not (Fig. 3). No reversion of these mutations was observed, even in CHIKV-FL-5A-PG-IL, which exhibited a level of infectivity similar to that of CHIKV-EK. However, unlike CHIKV-EK, the mutant CHIKV-FL-5A-PG-IL cannot easily regain fitness by reversion: changes of more than one nucleotide would be required for the 5A and PG mutations to revert to wt sequences, and reversion of either the FL or IL mutation alone does not result in a sufficient gain of fitness.
The identified mutations do not block nuclear localization of nsP2.
Nuclear localization of nsP2 is a prerequisite for the cytotoxic properties of Old World alphavirus replicons (25, 45). Interestingly, the 5A mutation in CHIKV nsP2 is located in the region corresponding to the hypothetical nuclear localization signal (NLS) of SFV nsP2 (46). Previous analyses carried out using BHK-21 cells transfected with ChikvRep, ChikvRep-PG, and ChikvRep-5A-PG RNAs failed to determine clearly whether the 5A and/or PG mutations affect the nuclear localization of nsP2. Compared to wt nsP2, more prominent nuclear localization was detected for nsP2-PG, while nsP2-5A-PG was found to be largely, but not completely, excluded from nuclei (34). The uncertainty around the effects of these mutations on the nuclear localization of nsP2 originates from the fact that the growth kinetics and nsP2 levels observed with ChikvRep differ markedly from those observed with ChikvRep-5A-PG (Fig. 2A and C), thereby making comparison difficult.
Due to these limitations, we performed immunofluorescence analyses of cells transiently expressing nsP2 or nsP2-5A-PG. In this experiment, cells transfected with the wt nsP2 expression vector exhibited cytotoxic effects and died rapidly (data not shown); hence, no comparable images were obtained. Thus, the experiment was repeated using cells infected with wt CHIKV or CHIKV-5A-PG. This analysis did not reveal consistent differences between these two viruses; in both cases, nsP2 was detected in both cellular compartments (Fig. 4A). It should also be noted that for both viruses, cells with nsP2 predominantly located in nucleus and cells with the majority of nsP2 located in the cytoplasm were both detected (Fig. 4A). Thus, the situation with CHIKV-5A-PG was clearly different from that with the extensively analyzed SFV-RDR virus (46); instead, localization of CHIKV-5A-PG nsP2 resembled that of nsP2 encoded by SFV replicons adapted to form stable cell lines (32).
FIG 4.
Analysis of the subcellular localization of nsP2 in BHK-21 cells infected with wt or mutant versions of CHIKV. (A) The localization of nsP2 in infected cells was analyzed by immunofluorescence microscopy. BHK-21 cells were infected with wt CHIKV or CHIKV-5A-PG at an MOI of 5 PFU/cell. Cells were fixed at 12 h p.i.; nsP2 was then stained with rabbit anti-nsP2 polyclonal antibody. Anti-rabbit Alexa-568-conjugated goat antibody was used as the secondary antibody. Nuclei were stained with DAPI. (B) Detection of nsP2 in nuclear and cytoplasmic fractions. BHK-21 cells were infected with wt CHIKV, CHIKV-PG, CHIKV-5A, or CHIKV-5A-PG. At 4, 8, or 12 h p.i., cells were collected, and nuclear and cytoplasmic fractions were separated. A total of 5% of each fraction was analyzed. Proteins were resolved using SDS-PAGE and detected using a rabbit polyclonal antibody against CHIKV nsP2. A mouse monoclonal antibody against α-tubulin was used as an internal control. Dotted lines indicate where separate images were merged. The results of one of two reproducible experiments are shown.
To confirm these results, nuclear and cytoplasmic fractions of infected cells were obtained and analyzed. BHK-21 cells were infected with wt CHIKV, CHIKV-PG, CHIKV-5A, or CHIKV-5A-PG at a high MOI; cells were harvested at 4, 8, and 12 h p.i. and fractionated. Western blot analysis of the nuclear and cytoplasmic fractions revealed that CHIKV nsP2 was localized almost equally in the nucleus and in the cytoplasm, similar to the nsP2s of other Old World alphaviruses (47, 48). Localization was slightly dependent on the time of infection, as the nsP2 nuclear fraction was more abundant at earlier time points (4 h and 8 h p.i.). CHIKV-PG nsP2 behaved similarly, except that nuclear localization was more extensive and remained dominant even at 12 h p.i. These data are consistent with observations made using CHIKV and SFV replicons harboring the same mutation (31, 34). CHIKV-5A nsP2 was found to be localized in a pattern very similar to that of wt CHIKV nsP2, while CHIKV-5A-PG nsP2 was shown to be localized in a pattern similar to that of CHIKV-PG nsP2 (Fig. 4B; Table 1). Taken together, these data unequivocally demonstrate that mutations associated with the noncytotoxic phenotype of the CHIKV replicon of ECSA genotype do not prevent the nuclear localization of nsP2. Thus, the lack of cytotoxic effect must originate from another functional defect(s) in mutant nsP2 proteins.
Proteolytic processing of CHIKV nonstructural polyprotein.
Alphavirus RNA replication is performed by replicase complexes, whose formation is regulated by nonstructural polyprotein processing. Thus, mutations that affect nsP2 protease activity also indirectly affect all viral functions for which RNA replication is essential. To our knowledge, the processing of CHIKV nonstructural polyprotein (P1234) has not previously been analyzed in detail; the protease activity of recombinant CHIKV nsP2 has been analyzed using only truncated enzymes and very short peptides corresponding to the protease recognition sites (21). Thus, to analyze the possible effects of the identified mutations on P1234 processing, it was essential to identify the processing requirements of CHIKV P1234.
Radioactive pulse-chase analysis of CHIKV-infected BHK-21 cells revealed that wt P1234 processing was similar to that of P1234 of SFV. In pulsed samples, the P1234, P123, and P12 polyproteins and mature nsP2 were detected; after a 60-min chase, polyproteins become nearly undetectable. A polyprotein that likely represents P23 was detected in minute quantities. Polyprotein processing was similar in cells infected by mutant viruses (Fig. 5A). However, it could be expected that even minor defects in processing may have an impact on alphavirus RNA replication. Thus, P1234 processing was also analyzed using an in vitro transcription/translation system. This analysis revealed that wt P1234 processing was similar to, albeit less efficient than, that observed in infected cells, as evidenced by increased stability of the P1234, P12, and P23 polyproteins (Fig. 5B). Introduction of the 5A mutation clearly reduced processing efficiency, as the ratio of P1234 to mature nsP2 was approximately 3-fold higher than the same ratio for wt P1234; such a difference was observed at all three time points (Fig. 5B). The PG mutation reduced processing efficiency approximately 5-fold. Importantly, in this case, the processing defect was also more specific; as the amount of released nsP2 was reduced, the amount of P1234 (compared to nsP2) was elevated, and the P23 polyprotein became nearly undetectable (Fig. 5B). Interestingly, the effect of the 5A and PG mutations was cumulative; when both of these mutations were present, the ratio of P1234 to nsP2 increased (relative to the ratio of wt P1234 to wt nsP2) 30- to 40-fold at all three time points (Table 1). P23 was nearly undetectable, while P12 levels remained high; this relationship reflected the marked stability of the latter intermediate (Fig. 5B). Addition of the FL and IL mutations had no effect on this pattern. Thus, the PG mutation delays the cleavage of 3/4 (as evidenced by the stability of P1234) and 1/2 (as evidenced by the stability of P12 and the small quantities of mature nsP2 that were generated) sites. In this system, such effects were increased by the presence of the 5A mutation. In contrast, the processing of the 2/3 site was most likely not affected (as evidenced by the presence of P12, which can be formed only via the processing at the 2/3 site in P1234 or P123) (Fig. 5B).
FIG 5.
Analysis of the proteolytic processing of wt and mutant nonstructural polyproteins. The names of viruses and replicon cDNA-containing plasmids used in the assays are indicated at the tops of the panels. The positions of the nonstructural polyproteins and nsP2 are indicated on the right side of the gels. (A) Processing of nonstructural polyproteins in virus-infected BHK-21 cells. BHK-21 cells were infected with wt CHIKV, CHIKV-PG, CHIKV-5A, CHIKV-5A-PG, or CHIKV-FL-5A-PG-IL at an MOI of 20 PFU/cell. At 3 h p.i., cells were starved in methionine-free and cysteine-free medium, labeled for 15 min with [35S]methionine-cysteine (pulse, 0′), and chased for 15 or 60 min in medium containing excess unlabeled methionine and cysteine. The cells were lysed in 1% SDS, and proteins were denatured by boiling and analyzed via immunoprecipitation with anti-nsP2 followed by SDS-PAGE and visualization with a Typhoon imager. (B) Processing of in vitro-translated wt and mutant nonstructural polyproteins. The reaction was carried out using the TNT SP6 rabbit reticulocyte system and plasmids containing the indicated ChikvRep cDNAs as templates. The produced nonstructural polyproteins and the products of their processing were denatured by boiling in 1% SDS. The samples were analyzed as described for panel A. The ratio is the amount of label that was incorporated into unprocessed P1234 compared with the amount incorporated into individual nsP2 protein. Results from one of two reproducible experiments are shown.
Purification of recombinant nsP2 and its mutant variants.
The inconsistencies of the effects observed in different processing assays (Fig. 5A and B) suggested that it would be important to validate the obtained data using a well-defined enzymatic reaction. The process of expression and purification of CHIKV nsP2 developed in an earlier study (23) was used to obtain homogeneous preparations of wt nsP2, together with nsP2-5A, nsP2-PG, nsP2-5A-PG, nsP2-EK-PG, and nsP2-GE-PG (Fig. 6A). The PG mutation clearly reduced the yield of recombinant nsP2; this effect was alleviated to some extent by the addition of the EK, GE, or 5A mutation. As reduced yields often reflect problems with the folding and/or stability of recombinant proteins, the folding of proteins encoded by cytotoxic (wt nsP2) and noncytotoxic (nsP2-5A-PG) replicons was compared using CD spectroscopy. This analysis revealed that folding of these proteins was comparable: both proteins displayed well-folded, largely alpha-helical patterns (49). No conformational deformity that could indicate aggregation or denaturation was detected for the mutant protein (Fig. 6B).
Proteolytic activities of purified recombinant nsP2 proteins.
The design of substrates used in the protease assay (Fig. 7A) was based on previous studies of SFV nsP2 protease activity (8, 9). wt CHIKV nsP2 efficiently cleaved EGFP-10:5-Trx substrates containing 1/2 and 3/4 sites but was virtually unable to process a similar substrate containing the 2/3 site (Fig. 7B). In contrast, the EGFP-10:170 substrate was efficiently cleaved (Fig. 7C). Thus, the processing requirements of CHIKV nsP2 are similar to these previously described for SFV nsP2 (8, 9).
As expected, none of the mutant enzymes was able to cleave the EGFP-10:5-Trx substrate corresponding to the 2/3 site (Fig. 7B, middle panel). Changes observed in the cleavage of substrates representing the 1/2 and 3/4 sites were similar (Table 1). First, compared to the wt enzyme, nsP2-5A cleaved these substrates slightly less efficiently. Second, all recombinant enzymes containing PG mutations had a severe defect in the processing of these substrates; this effect was somewhat more prominent for the substrate representing the 1/2 site (Fig. 7B). Third, most likely due to the strong effect of the PG mutation, no clear additive effect between the 5A- and PG mutations was observed (Fig. 7B). Strikingly, all mutant forms of nsP2 cleaved the EGFP-10:170 substrate nearly as efficiently as wt nsP2 (Fig. 7C; Table 1). Thus, this assay also confirmed that all mutant nsP2s are enzymatically functional proteins that harbor only specific defects originating from the introduced mutations. Small differences in folding, revealed by CD spectroscopy (Fig. 6B), may therefore reflect specific conformational changes caused by the 5A and PG mutations. Accordingly, functional differences in the wt and mutant proteins observed in subsequent assays also represent direct consequences of the introduced mutations.
GTPase and RNA helicase activities of the recombinant nsP2 proteins.
The ability of SINV nsP2 to cause degradation of Rpb1 can be abolished by a point mutation in the NTP-binding site that is critical for the NTPase/RNA helicase/RNA triphosphatase activities of the protein and also by a Pro726-to-Gly or Pro726-to-Leu substitution (24). However, the exact roles of the NTPase/RNA helicase activities of nsP2 in Rpb1 degradation remain unknown. In addition, the intracellular NTP concentration is among the key determinants of CPE (50–52). Thus, we hypothesized that the noncytotoxic properties of nsP2-5A-PG and nsP2-EK-PG might stem from compromised NTPase activity. To examine this hypothesis, GTPase activities of the recombinant nsP2 proteins were measured (Table 1). nsP2-5A had a GTPase activity level very similar to that of wt nsP2 (Fig. 8A). In contrast, the GTPase activity of nsP2-PG was reduced by approximately 40% (P = 0.0015). The GTPase activity of nsP2-EK-PG was similar to that of nsP2-PG. Unexpectedly, introduction of the 5A or GE mutation into nsP2-PG resulted in a 10 to 20% increase in GTPase activity (P = 0.015 for nsP2-5A-PG and P = 0.031 for nsP2-GE-PG).
FIG 8.
GTPase and RNA helicase activities of wt and mutant nsP2 proteins. (A) The GTPase activities of recombinant enzymes were analyzed using 150 μM GTP and a 56 nM concentration of the indicated enzyme. The results are normalized to the activity of the wt enzyme, which was set at 100%. (B) Stimulatory effect of 1.75 μM poly(U)18 oligonucleotides on the GTPase activity of recombinant enzymes. Enzyme activities in the absence of the poly(U)18 oligonucleotide were set at 1. (C) RNA helicase activities of purified recombinant nsP2 proteins analyzed with 50 pM double-stranded RNA (dsRNA) substrates and 12.5 nM concentrations of the indicated enzymes. Data from one reproducible experiment are shown. (D) Quantitative representation of helicase activity. Results are normalized to the activity of wt enzyme, which was set at 100%. The quantitative data represented in panels A, B and D represent the average from at least three independent experiments; the error bars represent standard deviations. Statistically significant differences are shown (Student's t test; *, P < 0.05; **, P < 0.01).
The activities of many viral NTPases, including that of CHIKV nsP2 (23), can be stimulated by the addition of nucleic acids. Consistent with previous results (23), the GTPase activity of wt nsP2 was slightly stimulated by the addition of a poly(U)18 RNA oligonucleotide (Fig. 8B). The same was true for nsP2-5A but not for nsP2-PG, nsP2-5A-PG, or nsP2-GE-PG, all of which failed to demonstrate any increase in GTPase activity (Fig. 8B; Table 1). Thus, the PG mutation likely affected the structure of the MTL domain of nsP2, which is proposed to be responsible for RNA binding, and may have hampered RNA binding and/or the interaction between different domains of nsP2. This hypothesis is indirectly supported by the observation that the introduction of the EK mutation into nsP2-PG restored stimulation of GTPase activity by oligonucleotide (Fig. 8B). The EK mutation is located in the area of nsP2 that is hypothesized to interact with the MTL domain (23). A charge reversion from negative to positive can increase nsP2 RNA binding and/or facilitate interaction of the N-terminal domain with RNA bound to the MTL domain. Thus, the PG mutation reduced the basal NTPase activity of nsP2 and completely eliminated its ability to be stimulated by nucleic acids. Nevertheless, these effects are not necessarily directly responsible for the noncytotoxic properties of the corresponding replicons. Thus, the GTPase activities of both nsP2-5A-PG and nsP2-PG cannot be stimulated with RNA oligonucleotide (Fig. 8A), but the GTPase activity of nsP2-5A-PG (from a noncytotoxic replicon) is significantly greater than the GTPase activity of nsP2-PG (from a cytotoxic replicon) (Fig. 8B; Table 1).
The finding that the GTPase activities of several nsP2 mutants were not stimulated by RNA oligonucleotides (Fig. 8B) suggests that the RNA helicase activities of the corresponding recombinant enzymes may also be compromised. Thus, the RNA helicase activities of recombinant enzymes were analyzed using an RNA helicase substrate containing a 16-bp duplex region (23). wt nsP2 and nsP2-5A unwound the RNA substrate with similar efficiency, while recombinant proteins containing the PG mutation demonstrated reduced unwinding activity (Fig. 8C and D; Table 1). Though the observed differences did not reach statistical significance, this finding suggests that the PG mutation may be associated with a negative effect on RNA unwinding activity. Importantly, the addition of the 5A or EK mutation to nsP2-PG resulted in an additional decrease in RNA unwinding activity (Fig. 8C and D). Although this effect was small, it was nonetheless in the opposite direction of the effects of these mutations in the GTPase assays, in which either the 5A or EK mutation rescued some of the functional defects of nsP2 caused by the PG mutation (Fig. 8A and B; Table 1). Because nsP2-5A-PG and nsP2-EK-PG are the proteins that carry the combinations of mutations that are associated with the noncytotoxic growth of CHIKV replicons (Fig. 1A), it is tempting to speculate that there may be a direct connection between the RNA helicase activity of nsP2 and the cytotoxicity of replicon vectors.
DISCUSSION
Alphavirus infection in vertebrate cells leads to the rapid development of CPE. CPE due to infection with the Old World alphaviruses can be caused by several factors, including degradation of the Rpb1 protein (24), translation block (53), and endoplasmic reticulum (ER) stress (54). For these viruses, nsP2 is the critical factor involved in both the shutdown of transcription and translation and the disruption of antiviral signaling (25, 26, 35). Because nsP2 is an obligatory part of the virus replication complex, replicon vectors based on Old World alphaviruses also display cytotoxic properties that hamper the construction of stable cell lines for the production of recombinant proteins (39) or for the screening of antiviral compounds (34).
The construction and use of BHK-21 cell-based CHIKV replicon cell lines with the cytotoxic properties of CHIKV nsP2 eliminated by the combination of the 5A and PG mutations have been described previously (34). This combination of mutations in the corresponding regions of nsP2 (around amino acid residue 648 and at residue Pro718) appears to represent a requirement for the noncytotoxic phenotype of replicons for viruses of the SFV serogroup (34, 35). Moreover, such combinations of mutations also result in significant reductions in RNA replication (Fig. 2). In contrast, the effects of individual mutations in SFV and WA CHIKV replicons differed from those observed with CHIKV of the ECSA genotype. Thus, substitution of the Pro718 residue of nsP2 is sufficient to reduce cytotoxicity and RNA replication in both SFV and CHIKV of the WA genotype (31, 35), but in the ECSA genotype virus, such effects were minimal or undetectable (Fig. 1, 2, and 3; Table 1). Similarly, mutations introduced into the putative NLS region of SFV nsP2 or into the corresponding region of CHIKV (WA genotype, KR649AA mutation) blocked the nuclear transport of nsP2; this did not occur with nsP2 of the CHIKV ECSA genotype (Fig. 4B). Thus, the effects of individual mutations associated with the noncytotoxic phenotype of alphavirus replicons strongly depend on their overall context. Consequently, conclusions based on the analysis of one viral genotype do not necessarily apply to other genotypes of the same virus. Although the Pro718 substitution in the CHIKV WA genotype resulted in reduced cytotoxicity, additional mutations (KR649AA or D711G) were still needed to obtain stable cell lines (35). Like the 5A and PG mutations, these changes were located in the MTL-like domain of nsP2. In the current study, an additional combination of mutations (EK and PG) resulting in a noncytotoxic replicon was also identified. Interestingly, the EK substitution resides in the N-terminal domain of nsP2, which has not previously been associated with the noncytotoxic phenotype of CHIKV replicons. However, mutations associated with noncytotoxic phenotype of replicon vectors have been found in the N-terminal halves of nsP2 proteins of SINV and SFV (29, 33, 55). Thus, the combination of EK and PG mutations served as a valuable comparison for previously identified sets of mutations.
The nuclear fraction of nsP2 is responsible for counteracting the interferon response (56–58) by blocking the Jak-Stat signaling pathway (25, 59) and/or causing the shutdown of transcription of interferon-stimulated genes (26, 31). However, on its own, localization of nsP2 into the nucleus is not sufficient for these effects (45). It is also not clear how nsP2 enters the nucleus; none of the predicted NLS sequences in the SINV nsP2 were involved in nuclear localization of the protein (45). For SFV, disruption of the predicted NLS blocked the nuclear transport of nsP2 only at higher (37°C) and not at lower (28°C) temperatures (31). Our data showed that the 5A mutation (located in a region corresponding to the SFV NLS region) did not affect the nuclear transport of CHIKV nsP2 (Fig. 4). Because cells containing ChikvRep-5A-PG were viable, it can be concluded that the nuclear fraction of nsP2-5A-PG was unable to induce the degradation of Rpb1. Whether these mutations affect the ability of the virus to block Jak-Stat signaling and induce or antagonize the interferon response represents topics for future studies.
Other biological effects of mutations associated with the reduced cytotoxicity of alphavirus replicons have been studied using infected or transfected cells. Severely reduced RNA replication and reduced nsP2 levels have been identified as common properties of noncytotoxic replicons of Old World alphaviruses (30–32, 35). In transient-expression systems, mutations blocking the NTPase/RNA triphosphatase/RNA helicase activities of nsP2 abolished its ability to induce Rpb1 degradation (24). However, these findings cannot be verified using replicons (or viruses) because this mutation is lethal for CHIKV (our unpublished data). Strong evidence exists that protease activity is not directly required for nsP2 cytotoxicity (24, 27). However, mutations that render the replicon noncytotoxic also often affect nonstructural polyprotein processing (30, 32), which, in turn, likely contributes to observed reductions in replication. Furthermore, the release of nsP2, mediated by its protease activity, is essential for the suppression of antiviral effects (60). nsP2 has multiple functions, and functional cross talk appears to exist between its different domains. Thus, different functional defects generated by the mutations (or by combinations of mutations) analyzed here could directly and/or indirectly contribute to the noncytotoxic phenotype.
In contrast with previous findings made for SINV, in which PG-type mutations led to hyperprocessivity of P123 (30), both the 5A and PG mutations slowed processing of the CHIKV nonstructural polyprotein (Fig. 5B). The ability of nsP2-PG to process the nsP1/nsP2 and nsP3/nsP4 junctions in trans in substrates containing 15 amino acid residues from recognition/cleavage sites was severely compromised (Fig. 7B). This defect was less prominent in in vitro-translated P1234 (compare Fig. 5B and 7B), which may indicate that the ability of nsP2 to cleave these sites in cis was affected to a lesser extent (Fig. 5B). However, we favor an alternative explanation. Pro is a rigid amino acid residue that is known to be associated with the restriction of rotation of the peptide bond (61). It could be hypothesized that the replacement of Pro718 with Gly causes unfavorable flexibility in nsP2, thereby preventing nsP2-PG from optimally interacting with the 15-amino-acid substrate region. This is one possible explanation for why the defect caused by the PG mutation was most apparent under conditions when processing was based on the recognition of short sequences representing cleavage sites. For SFV, it has been demonstrated that in the context of the full nonstructural polyprotein, the presentation of cleavage sites can override the specificity of the enzyme (10); most likely this is also true for CHIKV. Indeed, processing of the 2/3 site, recognition of which depends almost exclusively on macromolecular assembly-derived mechanisms (9, 10), was not affected by the PG mutation (Fig. 7C; Table 1). It could be hypothesized that multiple interactions between the nsP2-PG and nsP3 macrodomain in the EGFP-10:170 substrate compensated for the increased flexibility in nsP2-PG, allowing proper formation of the protease-substrate complex. The effect of the PG mutation may be further diminished in the cellular environment due to the compartmentalization of nonstructural proteins to virus replication sites, due to interactions between nonstructural polyproteins and replicating viral RNA and/or due to interactions with host components. These hypotheses may be able to explain why processing defects were too small to be observed in infected cells (Fig. 5A). However, this does not mean that the processing defect caused by the PG mutation has no effect on infectious virus; data from our group (S. Saul et al., unpublished data) and others (62) clearly reveal that small differences in the rate of release of mature nsP2 have major effects on the biological properties of alphaviruses.
The effect of the 5A mutation on the GTPase and RNA helicase activities of nsP2 was minor, whereas the PG mutation caused an approximately 40% reduction in both GTPase and RNA helicase activities (Fig. 8; Table 1). In addition, the PG mutation completely abolished the ability of RNA oligonucleotides to stimulate GTPase activity (Fig. 8B), thus indicating that properties of the RNA-binding site have been compromised. In case of noncytotoxic SINV, SFV, or WA CHIKV mutants, such data could have been interpreted as clear experimental support for a connection between the noncytotoxic phenotype of replicons and the reduced NTPase/RNA helicase activities of nsP2. In the CHIKV ECSA genotype used in this study, the situation is more intriguing: the PG mutation causes multiple, clear defects in the enzymatic activities of nsP2, but its effects in the contexts of replicons and viruses are minor (Table 1). There are several possible explanations for this inconsistency. First, the defect caused by the PG mutation may be compensated for in infected cells, and the EK or 5A mutation may act by suppressing such compensations. Second, defects caused by the PG mutation alone may not be severe enough, and the role of the second mutation may be to increase the functional defect in nsP2. In this case, the PG and 5A (or EK) mutations should have additive (or cumulative) effects on the crucial activity (or activities) of nsP2. Finally, these possibilities may be combined, and if so, additive effects of mutations on nsP2 activities may be small and difficult to reveal using in vitro reactions.
The cumulative effect of the 5A and PG mutations on nsP2 protease activity was clearly detected using an in vitro transcription/translation system (Fig. 5B). This may represent one reason for the observed reductions in RNA replication, which was affected by the PG and 5A mutations in a similar manner (Fig. 2B; Table 1); at the same time, decreased protease activity is unlikely to be the main reason for the noncytotoxic phenotype. No negative cooperative effect of the PG and 5A mutations was observed in the GTPase stimulation assay. Furthermore, introduction of the EK mutation into nsP2-PG rescued the defect caused by the PG mutation. The same effect was revealed for basal GTPase activity, except that in this case, the defect caused by the PG mutation was partly rescued by the 5A mutation (Table 1). Thus, additional changes obtained in antibiotic selection experiments not only led to reduced cytotoxicity of the replicon but also compensated for some of the defects caused by the PG mutation. Thus, a set of multiple and complex requirements may govern whether replicons with such additional changes survive; this may markedly reduce the number of acceptable additional mutations. Indeed, in antibiotic selection experiments, very similar combinations of mutations are obtained not only in parallel experiments but also for different strains of alphaviruses and different alphaviruses.
Only the RNA helicase activity of nsP2-PG was further reduced by the addition of either the EK or 5A mutation (Fig. 8C and D). Though the observed additional decrease was small, it is tempting to speculate that the reduction in the RNA helicase activity of nsP2 could be the primary explanation for the noncytotoxic phenotype of CHIKV replicons. This assumption is indirectly supported by the results obtained for nsP2 of SINV expressed using the Venezuelan equine encephalitis virus replicon system. It was observed that to lose cytotoxic properties, the nsP2-coding sequence accumulated mutations that prevented the translation of the C-terminal third of the protein (27). This region is dispensable for NTPase activity (22, 63) but is absolutely required for the RNA helicase activity of nsP2 (23). For SFV, the Ser259-to-Pro mutation in nsP2 is shown to contribute to reduced cytotoxicity of replicon vectors (33). As this mutation is located close to the NTP-binding site in the RecA-like domain, it may also reduce RNA helicase activity of the protein. Detailed analysis of the effects of such previously identified mutations on enzymatic activities of nsP2 proteins of different alphaviruses would be required to verify these hypotheses.
If the noncytotoxic properties of CHIKV replicons originate from defects in basic enzymatic activities of nsP2, then it is logical to assume that the corresponding replicons should have similar (noncytotoxic) phenotypes in different vertebrate cell lines. However, under puromycin selection, neither ChikvRep-5A-PG nor ChikvRep-EK-PG was able to support stable growth in human cell lines. Additional mutations required for continuous growth of replicon-harboring Huh7 cells were mapped to nsP1 and nsP3. BHK-21 cells are known to be extremely permissive for alphaviruses, with active replication and viral gene expression. In most other cell lines, alphavirus replication/gene expression occurs at considerably (and often much) lower levels. Thus, it is plausible that Pac expression by ChikvRep-5A-PG was sufficient to protect BHK-21 cells from the effects of puromycin but was too inefficient to do the same for Huh7 cells (Fig. 2B and D) or other cells of human origin. The mechanisms behind the increased replication of ChikvRepRluc-FL-5A-PG-IL containing the FL and IL mutations in Huh7 cells (Fig. 2B and D) remain unclear. Leu at amino acid residue 391 of nsP1 has been found previously in several natural CHIKV isolates from the Indian Ocean outbreak (e.g., GenBank accession numbers FJ959103, FJ000067, HM045812, and HM045815) but has not been associated with increased viral fitness; the Ile175-to-Leu mutation in CHIKV nsP3 has not been previously reported. However, it could be hypothesized that these mutations may act by stabilizing viral replication complexes. Both of these changes are located in the presumably unstructured regions between the predicted domains of nsP1 and nsP3, and these regions may play a role in stabilizing the replication complexes. Because the FL and IL mutations increased the level of replication and gene expression in Huh7 cells but dampened the infectivity of the corresponding RNAs in BHK-21 cells (Table 1), we propose that these changes may have altered interactions of the viral replicase with host components.
In conclusion, this study presents the first evidence that mutations resulting in the noncytotoxic phenotype of the CHIKV replicon also affect multiple enzymatic activities of nsP2. These data increase our understanding of mechanisms that are essential for noncytotoxic replication. Our results may also be clinically relevant, as CHIKV is known to cause chronic infection and to persist in infected macrophages (64–66). Human cell lines harboring persistently replicating CHIKV replicons represent promising tools for the search for cellular factors involved in CHIKV replication, as well as for inhibitors acting via such host factors.
ACKNOWLEDGMENTS
We thank Arvi Freiberg and Margus Rätsep for their help with the measurement and interpretation of CD spectra.
This work was supported by institutional research funding (IUT20-27) from the Estonian Ministry of Education and Research, the European Union 7th Framework Integrated Chikungunya Research project (grant agreement number 261202), the Estonian Science Foundation (grants 9400 and 9421), and the European Union through the European Regional Development Fund via the Center of Excellence in Chemical Biology. P.K.D. is the recipient of a scholarship from the European Union Social Fund through Activity 4 of the Doctoral Studies and Internationalization Program.
REFERENCES
- 1.Powers AM, Brault AC, Tesh RB, Weaver SC. 2000. Re-emergence of Chikungunya and O'nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol 81:471–479. [DOI] [PubMed] [Google Scholar]
- 2.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney M-C, Lavenir R, Pardigon N, Reynes J-M, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel M-P, Bréhin A-C, Cubito N, Desprès P, Kunst F, Rey FA, Zeller H, Brisse S. 2006. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med 3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, de Lamballerie X. 2014. Chikungunya in the Americas. Lancet 383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 4.Powers AM, Logue CH. 2007. Changing patterns of Chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 5.Pialoux G, Gaüzère B-A, Jauréguiberry S, Strobel M. 2007. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 6.Strauss JH, Strauss EG. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev 58:491–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasiljeva L, Merits A, Golubtsov A, Sizemskaja V, Kääriäinen L, Ahola T. 2003. Regulation of the sequential processing of Semliki Forest virus replicase polyprotein. J Biol Chem 278:41636–41645. doi: 10.1074/jbc.M307481200. [DOI] [PubMed] [Google Scholar]
- 8.Lulla A, Lulla V, Tints K, Ahola T, Merits A. 2006. Molecular determinants of substrate specificity for Semliki Forest virus nonstructural protease. J Virol 80:5413–5422. doi: 10.1128/JVI.00229-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lulla A, Lulla V, Merits A. 2012. Macromolecular assembly-driven processing of the 2/3 cleavage site in the alphavirus replicase polyprotein. J Virol 86:553–565. doi: 10.1128/JVI.05195-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lulla V, Karo-Astover L, Rausalu K, Merits A, Lulla A. 2013. Presentation overrides specificity: probing the plasticity of alphaviral proteolytic activity through mutational analysis. J Virol 87:10207–10220. doi: 10.1128/JVI.01485-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahola T, Kääriäinen L. 1995. Reaction in alphavirus mRNA capping: formation of a covalent complex of nonstructural protein nsP1 with 7-methyl-GMP. Proc Natl Acad Sci U S A 92:507–511. doi: 10.1073/pnas.92.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahola T, Lampio A, Auvinen P, Kääriäinen L. 1999. Semliki Forest virus mRNA capping enzyme requires association with anionic membrane phospholipids for activity. EMBO J 18:3164–3172. doi: 10.1093/emboj/18.11.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malet H, Coutard B, Jamal S, Dutartre H, Papageorgiou N, Neuvonen M, Ahola T, Forrester N, Gould EA, Lafitte D, Ferron F, Lescar J, Gorbalenya AE, de Lamballerie X, Canard B. 2009. The crystal structures of Chikungunya and Venezuelan equine encephalitis virus nsP3 macro domains define a conserved adenosine binding pocket. J Virol 83:6534–6545. doi: 10.1128/JVI.00189-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin G, Yost SA, Miller MT, Elrod EJ, Grakoui A, Marcotrigiano J. 2012. Structural and functional insights into alphavirus polyprotein processing and pathogenesis. Proc Natl Acad Sci U S A 109:16534–16539. doi: 10.1073/pnas.1210418109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panas MD, Ahola T, McInerney GM. 2014. The C-terminal repeat domains of nsP3 from the Old World alphaviruses bind directly to G3BP. J Virol 88:5888–5893. doi: 10.1128/JVI.00439-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubach JK, Wasik BR, Rupp JC, Kuhn RJ, Hardy RW, Smith JL. 2009. Characterization of purified Sindbis virus nsP4 RNA-dependent RNA polymerase activity in vitro. Virology 384:201–208. doi: 10.1016/j.virol.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rana J, Rajasekharan S, Gulati S, Dudha N, Gupta A, Chaudhary VK, Gupta S. 2014. Network mapping among the functional domains of Chikungunya virus nonstructural proteins. Proteins 82:2403–2411. doi: 10.1002/prot.24602. [DOI] [PubMed] [Google Scholar]
- 18.Atasheva S, Gorchakov R, English R, Frolov I, Frolova E. 2007. Development of Sindbis viruses encoding nsP2/GFP chimeric proteins and their application for studying nsP2 functioning. J Virol 81:5046–5057. doi: 10.1128/JVI.02746-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristea IM, Carroll J-WN, Rout MP, Rice CM, Chait BT, MacDonald MR. 2006. Tracking and elucidating alphavirus-host protein interactions. J Biol Chem 281:30269–30278. doi: 10.1074/jbc.M603980200. [DOI] [PubMed] [Google Scholar]
- 20.Vasiljeva L, Merits A, Auvinen P, Kääriäinen L. 2000. Identification of a novel function of the alphavirus capping apparatus. RNA 5′-triphosphatase activity of Nsp2. J Biol Chem 275:17281–17287. doi: 10.1074/jbc.M910340199. [DOI] [PubMed] [Google Scholar]
- 21.Pastorino BAM, Peyrefitte CN, Almeras L, Grandadam M, Rolland D, Tolou HJ, Bessaud M. 2008. Expression and biochemical characterization of nsP2 cysteine protease of Chikungunya virus. Virus Res 131:293–298. doi: 10.1016/j.virusres.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karpe YA, Aher PP, Lole KS. 2011. NTPase and 5′-RNA triphosphatase activities of Chikungunya virus nsP2 protein. PLoS One 6:e22336. doi: 10.1371/journal.pone.0022336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das PK, Merits A, Lulla A. 2014. Functional cross-talk between distant domains of Chikungunya virus non-structural protein 2 is decisive for its RNA-modulating activity. J Biol Chem 289:5635–5653. doi: 10.1074/jbc.M113.503433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akhrymuk I, Kulemzin SV, Frolova EI. 2012. Evasion of the innate immune response: the Old World alphavirus nsP2 protein induces rapid degradation of Rpb1, a catalytic subunit of RNA polymerase II. J Virol 86:7180–7191. doi: 10.1128/JVI.00541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fros JJ, Liu WJ, Prow NA, Geertsema C, Ligtenberg M, Vanlandingham DL, Schnettler E, Vlak JM, Suhrbier A, Khromykh AA, Pijlman GP. 2010. Chikungunya virus nonstructural protein 2 inhibits type I/II interferon-stimulated JAK-STAT signaling. J Virol 84:10877–10887. doi: 10.1128/JVI.00949-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorchakov R, Frolova E, Frolov I. 2005. Inhibition of transcription and translation in Sindbis virus-infected cells. J Virol 79:9397–9409. doi: 10.1128/JVI.79.15.9397-9409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garmashova N, Gorchakov R, Frolova E, Frolov I. 2006. Sindbis virus nonstructural protein nsP2 is cytotoxic and inhibits cellular transcription. J Virol 80:5686–5696. doi: 10.1128/JVI.02739-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery SA, Berglund P, Beard CW, Johnston RE. 2006. Ribosomal protein S6 associates with alphavirus nonstructural protein 2 and mediates expression from alphavirus messages. J Virol 80:7729–7739. doi: 10.1128/JVI.00425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perri S, Driver DA, Gardner JP, Sherrill S, Belli BA, Dubensky TW, Polo JM. 2000. Replicon vectors derived from Sindbis virus and Semliki Forest virus that establish persistent replication in host cells. J Virol 74:9802–9807. doi: 10.1128/JVI.74.20.9802-9807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frolov I, Agapov E, Hoffman TA, Prágai BM, Lippa M, Schlesinger S, Rice CM. 1999. Selection of RNA replicons capable of persistent noncytopathic replication in mammalian cells. J Virol 73:3854–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamm K, Merits A, Sarand I. 2008. Mutations in the nuclear localization signal of nsP2 influencing RNA synthesis, protein expression and cytotoxicity of Semliki Forest virus. J Gen Virol 89:676–686. doi: 10.1099/vir.0.83320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casales E, Rodriguez-Madoz JR, Ruiz-Guillen M, Razquin N, Cuevas Y, Prieto J, Smerdou C. 2008. Development of a new noncytopathic Semliki Forest virus vector providing high expression levels and stability. Virology 376:242–251. doi: 10.1016/j.virol.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Lundstrom K, Rotmann D, Hermann D, Schneider EM, Ehrengruber MU. 2001. Novel mutant Semliki Forest virus vectors: gene expression and localization studies in neuronal cells. Histochem Cell Biol 115:83–91. doi: 10.1007/s004180000223. [DOI] [PubMed] [Google Scholar]
- 34.Pohjala L, Utt A, Varjak M, Lulla A, Merits A, Ahola T, Tammela P. 2011. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS One 6:e28923. doi: 10.1371/journal.pone.0028923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fros JJ, van der Maten E, Vlak JM, Pijlman GP. 2013. The C-terminal domain of chikungunya virus nsP2 independently governs viral RNA replication, cytopathicity, and inhibition of interferon signaling. J Virol 87:10394–10400. doi: 10.1128/JVI.00884-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawicki DL, Perri S, Polo JM, Sawicki SG. 2006. Role for nsP2 proteins in the cessation of alphavirus minus-strand synthesis by host cells. J Virol 80:360–371. doi: 10.1128/JVI.80.1.360-371.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhême C, Ehrengruber MU, Grandgirard D. 2005. Alphaviral cytotoxicity and its implication in vector development. Exp Physiol 90:45–52. doi: 10.1113/expphysiol.2004.028142. [DOI] [PubMed] [Google Scholar]
- 38.Atkins GJ, Fleeton MN, Sheahan BJ. 2008. Therapeutic and prophylactic applications of alphavirus vectors. Expert Rev Mol Med 10:e33. doi: 10.1017/S1462399408000859. [DOI] [PubMed] [Google Scholar]
- 39.Casales E, Aranda A, Quetglas JI, Ruiz-Guillen M, Rodriguez-Madoz JR, Prieto J, Smerdou C. 2010. A novel system for the production of high levels of functional human therapeutic proteins in stable cells with a Semliki Forest virus noncytopathic vector. New Biotechnol 27:138–148. doi: 10.1016/j.nbt.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 41.Russo AT, Watowich SJ. 2006. Purification, crystallization and X-ray diffraction analysis of the C-terminal protease domain of Venezuelan equine encephalitis virus nsP2. Acta Crystallograph Sect F Struct Biol Cryst Commun 62:514–517. doi: 10.1107/S1744309106014667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pédelacq J-D, Cabantous S, Tran T, Terwilliger TC, Waldo GS. 2006. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol 24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- 43.Horscroft N, Lai VCH, Cheney W, Yao N, Wu JZ, Hong Z, Zhong W. 2005. Replicon cell culture system as a valuable tool in antiviral drug discovery against hepatitis C virus. Antivir Chem Chemother 16:1–12. [DOI] [PubMed] [Google Scholar]
- 44.Sanz MA, García-Moreno M, Carrasco L. 20October2014. Inhibition of host protein synthesis by Sindbis virus: correlation with viral RNA replication and release of nuclear proteins to the cytoplasm. Cell Microbiol doi: 10.1111/cmi.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frolov I, Garmashova N, Atasheva S, Frolova EI. 2009. Random insertion mutagenesis of Sindbis virus nonstructural protein 2 and selection of variants incapable of downregulating cellular transcription. J Virol 83:9031–9044. doi: 10.1128/JVI.00850-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rikkonen M, Peränen J, Kääriäinen L. 1994. Nuclear targeting of Semliki Forest virus nsP2. Arch Virol Suppl 9:369–377. [DOI] [PubMed] [Google Scholar]
- 47.Peränen J, Rikkonen M, Liljeström P, Kääriäinen L. 1990. Nuclear localization of Semliki Forest virus-specific nonstructural protein nsP2. J Virol 64:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rikkonen M. 1996. Functional significance of the nuclear-targeting and NTP-binding motifs of Semliki Forest virus nonstructural protein nsP2. Virology 218:352–361. doi: 10.1006/viro.1996.0204. [DOI] [PubMed] [Google Scholar]
- 49.Greenfield NJ. 2006. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc 1:2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dvir A. 2002. Promoter escape by RNA polymerase II. Biochim Biophys Acta 1577:208–223. doi: 10.1016/S0167-4781(02)00453-0. [DOI] [PubMed] [Google Scholar]
- 51.Chandra D, Bratton SB, Person MD, Tian Y, Martin AG, Ayres M, Fearnhead HO, Gandhi V, Tang DG. 2006. Intracellular nucleotides act as critical prosurvival factors by binding to cytochrome C and inhibiting apoptosome. Cell 125:1333–1346. doi: 10.1016/j.cell.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 52.Eguchi Y, Shimizu S, Tsujimoto Y. 1997. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res 57:1835–1840. [PubMed] [Google Scholar]
- 53.Gorchakov R, Frolova E, Williams BRG, Rice CM, Frolov I. 2004. PKR-dependent and -independent mechanisms are involved in translational shutoff during Sindbis virus infection. J Virol 78:8455–8467. doi: 10.1128/JVI.78.16.8455-8467.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barry G, Fragkoudis R, Ferguson MC, Lulla A, Merits A, Kohl A, Fazakerley JK. 2010. Semliki forest virus-induced endoplasmic reticulum stress accelerates apoptotic death of mammalian cells. J Virol 84:7369–7377. doi: 10.1128/JVI.02310-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lundstrom K, Abenavoli A, Malgaroli A, Ehrengruber MU. 2003. Novel Semliki Forest virus vectors with reduced cytotoxicity and temperature sensitivity for long-term enhancement of transgene expression. Mol Ther J Am Soc Gene Ther 7:202–209. doi: 10.1016/S1525-0016(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 56.Breakwell L, Dosenovic P, Karlsson Hedestam GB, D'Amato M, Liljeström P, Fazakerley J, McInerney GM. 2007. Semliki Forest virus nonstructural protein 2 is involved in suppression of the type I interferon response. J Virol 81:8677–8684. doi: 10.1128/JVI.02411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frolov I, Akhrymuk M, Akhrymuk I, Atasheva S, Frolova EI. 2012. Early events in alphavirus replication determine the outcome of infection. J Virol 86:5055–5066. doi: 10.1128/JVI.07223-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frolova EI, Fayzulin RZ, Cook SH, Griffin DE, Rice CM, Frolov I. 2002. Roles of nonstructural protein nsP2 and alpha/beta interferons in determining the outcome of Sindbis virus infection. J Virol 76:11254–11264. doi: 10.1128/JVI.76.22.11254-11264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simmons JD, White LJ, Morrison TE, Montgomery SA, Whitmore AC, Johnston RE, Heise MT. 2009. Venezuelan equine encephalitis virus disrupts STAT1 signaling by distinct mechanisms independent of host shutoff. J Virol 83:10571–10581. doi: 10.1128/JVI.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gorchakov R, Frolova E, Sawicki S, Atasheva S, Sawicki D, Frolov I. 2008. A new role for ns polyprotein cleavage in Sindbis virus replication. J Virol 82:6218–6231. doi: 10.1128/JVI.02624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williamson MP. 1994. The structure and function of proline-rich regions in proteins. Biochem J 297:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruz CC, Suthar MS, Montgomery SA, Shabman R, Simmons J, Johnston RE, Morrison TE, Heise MT. 2010. Modulation of type I IFN induction by a virulence determinant within the alphavirus nsP1 protein. Virology 399:1–10. doi: 10.1016/j.virol.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rikkonen M, Peränen J, Kääriäinen L. 1994. ATPase and GTPase activities associated with Semliki Forest virus nonstructural protein nsP2. J Virol 68:5804–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoarau J-J, Jaffar Bandjee M-C, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzère BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debré P, Autran B, Gasque P. 2010. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol 184:5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 65.Hawman DW, Stoermer KA, Montgomery SA, Pal P, Oko L, Diamond MS, Morrison TE. 2013. Chronic joint disease caused by persistent Chikungunya virus infection is controlled by the adaptive immune response. J Virol 87:13878–13888. doi: 10.1128/JVI.02666-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poo YS, Rudd PA, Gardner J, Wilson JAC, Larcher T, Colle M-A, Le TT, Nakaya HI, Warrilow D, Allcock R, Bielefeldt-Ohmann H, Schroder WA, Khromykh AA, Lopez JA, Suhrbier A. 2014. Multiple immune factors are involved in controlling acute and chronic Chikungunya virus infection. PLoS Negl Trop Dis 8:e3354. doi: 10.1371/journal.pntd.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]