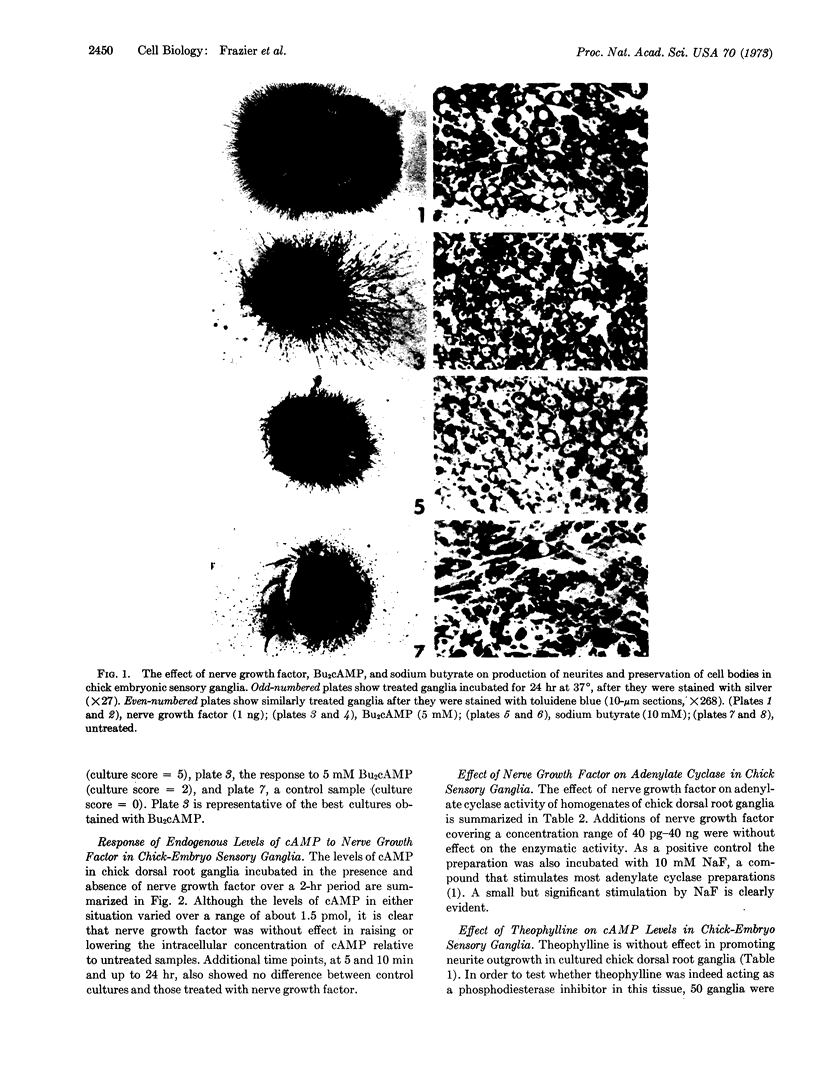
Abstract
The suggested role of adenosine 3′:5′-cyclic monophosphate as the “second messenger” in the neurite outgrowth from chick embryonic sensory ganglia mediated by nerve growth factor was examined. Although N6,O2-dibutyryl adenosine 3′:5′-cyclic monophosphate induces fiber outgrowth at concentrations of 1-5 mM, this response is morphologically distinct from that produced by nerve growth factor, is pH dependent, is mimicked by sodium butyrate, and does not occur in sympathetic ganglia. In addition, nerve growth factor does not alter the amounts of intracellular cyclic AMP during incubations up to 24 hr and does not stimulate adenylate cyclase in broken-cell preparations. Addition of theophylline, an inhibitor of phosphodiesterase, causes increases in intracellular levels of cyclic AMP but does not affect fiber outgrowth. These observations indicate that the nerve growth factor response is not mediated through cyclic AMp and that stimulation of sensory ganglia by exogenous cyclic AMP derivatives is probably of limited physiological significance. These findings are also compatible with the developing hypothesis, based on structural similarities, that nerve growth factor and insulin exert their effects on their respective responsive tissues by related mechanisms.
Keywords: adenylate cyclase, theophylline, phosphodiesterase, insulin, sodium butyrate
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti R. H., Bradshaw R. A. Nerve growth factor from mouse submaxillary gland: amino acid sequence. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2417–2420. doi: 10.1073/pnas.68.10.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W. A., Angeletti R. H., Bradshaw R. A. Nerve growth factor and insulin. Science. 1972 May 5;176(4034):482–488. doi: 10.1126/science.176.4034.482. [DOI] [PubMed] [Google Scholar]
- Haas D. C., Hier D. B., Arnason G. W., Young M. On a possible relationship of cyclic AMP to the mechanism of action of nerve growth factor. Proc Soc Exp Biol Med. 1972 May;140(1):45–47. doi: 10.3181/00379727-140-36392. [DOI] [PubMed] [Google Scholar]
- Hier D. B., Arnason B. G., Young M. Studies on the mechanism of action of nerve growth factor. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2268–2272. doi: 10.1073/pnas.69.8.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Cyclic AMP in metabolism. Nat New Biol. 1971 Jan 6;229(1):5–7. doi: 10.1038/newbio229005a0. [DOI] [PubMed] [Google Scholar]
- Roisen F. J., Murphy R. A., Braden W. G. Neurite development in vitro. I. The effects of adenosine 3'5'-cyclic monophosphate (cyclic AMP). J Neurobiol. 1972;3(4):347–368. doi: 10.1002/neu.480030408. [DOI] [PubMed] [Google Scholar]
- Roisen F. J., Murphy R. A., Pichichero M. E., Braden W. G. Cyclic adenosine monophosphate stimulation of axonal elongation. Science. 1972 Jan 7;175(4017):73–74. doi: 10.1126/science.175.4017.73. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Ferrendelli J. A., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. 3. Effect of ischemia, changes during development and regional distribution of adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mouse brain. J Biol Chem. 1972 Feb 25;247(4):1121–1124. [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]