ABSTRACT
Current vaccines against influenza virus infection rely on the induction of neutralizing antibodies targeting the globular head of the viral hemagglutinin (HA). Protection against seasonal antigenic drift or sporadic pandemic outbreaks requires further vaccine development to induce cross-protective humoral responses, potentially to the more conserved HA stalk region. Here, we present a novel viral vaccine adjuvant comprised of two synthetic ligands for Toll-like receptor 4 (TLR4) and TLR7. 1Z105 is a substituted pyrimido[5,4-b]indole specific for the TLR4-MD2 complex, and 1V270 is a phospholipid-conjugated TLR7 agonist. Separately, 1Z105 induces rapid Th2-associated IgG1 responses, and 1V270 potently generates Th1 cellular immunity. 1Z105 and 1V270 in combination with recombinant HA from the A/Puerto Rico/8/1934 strain (rPR/8 HA) effectively induces rapid and sustained humoral immunity that is protective against lethal challenge with a homologous virus. More importantly, immunization with the combined adjuvant and rPR/8 HA, a commercially available split vaccine, or chimeric rHA antigens significantly improves protection against both heterologous and heterosubtypic challenge viruses. Heterosubtypic protection is associated with broadly reactive antibodies to HA stalk epitopes. Histological examination and cytokine profiling reveal that intramuscular (i.m.) administration of 1Z105 and 1V270 is less reactogenic than a squalene-based adjuvant, AddaVax. In summary, the combination of 1Z105 and 1V270 with a recombinant HA induces rapid, long-lasting, and balanced Th1- and Th2-type immunity; demonstrates efficacy in a variety of murine influenza virus vaccine models assaying homologous, heterologous, and heterosubtypic challenge viruses; and has an excellent safety profile.
IMPORTANCE Novel adjuvants are needed to enhance immunogenicity and increase the protective breadth of influenza virus vaccines to reduce the seasonal disease burden and ensure pandemic preparedness. We show here that the combination of synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands is a potent adjuvant for recombinant influenza virus hemagglutinin, inducing rapid and sustained immunity that is protective against influenza viruses in homologous, heterologous, and heterosubtypic challenge models. Combining TLR4 and TLR7 ligands balances Th1- and Th2-type immune responses for long-lived cellular and neutralizing humoral immunity against the viral hemagglutinin. The combined adjuvant has an attractive safety profile and the potential to augment seasonal-vaccine breadth, contribute to a broadly neutralizing universal vaccine formulation, and improve response time in an emerging pandemic.
INTRODUCTION
Influenza A and B viruses remain a substantial public health burden, with seasonal epidemics resulting in significant morbidity, mortality, and economic loss (1–3). Pandemic outbreaks occur when antigenically novel influenza A viruses emerge in a population with little preexisting immunity (4). Pandemic viruses spread more rapidly and cause more severe disease than epidemic strains, as observed for the 1918 Spanish influenza, the 1957 Asian influenza, the 1968 Hong Kong influenza, and the 2009 swine origin influenza (4) viruses. Vaccination is the most effective means of limiting the spread of influenza viruses; however, the vaccine strain must be closely matched to the circulating strain, and efficacy varies from year to year (1, 5, 6). Current vaccines rely on the induction of neutralizing antibodies targeting the globular head of the viral hemagglutinin (HA) (7). Mismatch resulting from antigenic drift in HA is common with vaccines designed to manage seasonal epidemics (8), and prediction of the next pandemic virus is currently all but impossible. New vaccine formulations that enhance the breadth of protection afforded by immunization to influenza A and B viruses are needed. It is thus a high priority to develop novel antigens targeting conserved viral epitopes, as opposed to the highly variable antigenic regions of the viral HA, as well as adjuvants that enhance vaccine antigenicity and induce a protective immune response (9–12).
Seasonal influenza virus vaccines currently administered in the United States do not contain an adjuvant. Adjuvants spare antigen, enhance vaccine immunogenicity, direct the quality of the immune response, and may also increase the protective breadth of vaccines (12, 13). Pattern recognition receptors of the innate immune system are common adjuvant targets (12, 13). Small synthetic molecules targeting innate immune receptors are ideal adjuvant candidates, as they act via well-defined signaling pathways, may be chemically optimized for efficacy and safety, and may be produced on a large scale with high purity at minimal cost. Accordingly, two low-molecular-weight synthetic Toll-like receptor (TLR) ligands, 1Z105 and 1V270, a TLR4 ligand and a TLR7 ligand, respectively, are being developed as novel vaccine adjuvants. 1Z105 is a substituted pyrimido[5,4-b]indole that was derived from a hit identified in a small-molecule screen for NF-κB activators (14, 15). At this time 1Z105, as well as its related compounds, is among the few small, synthetic, nonlipid-like TLR4 ligands described in the literature and perhaps the only one with demonstrated adjuvant properties (14). The AS04 adjuvant licensed in GlaxoSmithKline's Cervarix vaccine provides a precedent for the safety and efficacy of a TLR4 ligand, namely, monophosphoryl lipid A (MPLA), as an adjuvant for a recombinant viral vaccine (16). 1V270 contains a known TLR7 agonist (1V136) conjugated to a phospholipid that has previously been reported to possess immunological activity (17–19). Here, 1Z105 was further characterized in vitro for its ability to activate antigen presentation in murine and human cells. Subsequently, 1Z105 and 1V270 were assayed for preclinical efficacy and safety as single agents or as a combined adjuvant with different influenza virus HA antigens in several murine models of influenza virus immunization and challenge.
Initial work in vivo assessed the abilities of 1Z105 and 1V270 to induce humoral and cellular immunity when administered with ovalbumin (OVA) as a model antigen. They compared favorably to AddaVax, a squalene-based oil-in-water emulsion similar to MF59, which is included in some European influenza virus vaccine formulations (13, 20). The adjuvants were subsequently tested with recombinant HA protein and a commercially available trivalent subvirion vaccine for their abilities to induce protective immunity to challenges with homologous and heterologous influenza viruses. Furthermore, the combined adjuvant was added to recombinantly produced chimeric hemagglutinin antigens containing the highly conserved HA stalk domain and assayed for protective efficacy against a heterosubtypic challenge virus after a sequential-vaccination regimen (21–24). The combined adjuvant was highly effective in all cases. While various adjuvants under development exhibit efficacy in preclinical models, U.S. regulators have considerable safety concerns regarding adjuvants and vaccine reactogenicity that must be addressed (12, 13, 25–27). 1Z105 and 1V270 demonstrate little in vitro toxicity (14, 19). Moreover, intramuscular (i.m.) injection did not induce substantial local or systemic inflammation.
MATERIALS AND METHODS
Animals.
Animal experiments performed at the University of California San Diego, La Jolla, CA, USA (UCSD) were approved by the UCSD Institutional Animal Care and Use Committee (IACUC) (S09331), and animal experiments performed at the Icahn School of Medicine were approved by the Mount Sinai IACUC (LA13-00084). Seven- to 9-week-old C57BL/6 (wild-type [WT]) and OT-1 transgenic mice in which the CD8+ T cells express a T cell receptor specific for the ovalbumin (OVA257–264) peptide (C57BL/6 background) were purchased from the Jackson Laboratories (Bar Harbor, MA). TrifLps2/Lps2 mice were kindly provided by Bruce Beutler (University of Texas Southwestern Medical Center, Dallas, TX, USA) (28). Tlr4−/− and Myd88−/− mice were a gift from Shizuo Akira (Osaka University, Osaka, Japan). These strains were backcrossed for 10 generations onto the C57BL/6 background at the University of California, San Diego. For immunization with influenza virus antigens, 6- to 8-week-old WT female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, MA). The animals were anesthetized with ketamine/xylazine before intranasal viral infection.
Reagents.
Compounds 1Z105 and 1V270 (Fig. 1) were synthesized in our laboratory as previously described (14, 19), dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO) as 20 to 100 mM stock solutions, and stored at −20°C. The endotoxin levels of these drugs were determined by Endosafe (Charles River Laboratory, Wilmington, MA, USA) and were less than 10 endotoxin units (EU)/μmol. MPLA and AddaVax were purchased from Invivogen (San Diego, CA). OVA (endotoxin levels, 12 to 18 EU/mg protein) was purchased from Worthington Biochemical Co. (Lakewood, NJ).
FIG 1.
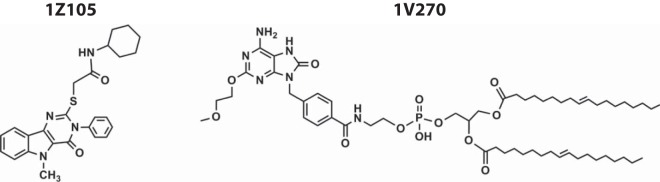
Structures of 1Z105 and 1V270.
The recombinant hemagglutinin (rHA) antigens derived from influenza viruses A/Puerto Rico/8/1934 (H1N1) (PR/8) and A/California/04/2009 (H1N1) (Cal/09) and the chimeric HAs (cHAs) (cH2/1PR/8 and cH6/1PR/8) were expressed from baculovirus in High Five cells in the laboratory as soluble trimers utilizing the T4 phage fibritin natural trimerization domain and a C-terminal 6× His tag for purification as previously described (29). Both chimeric HAs utilize the PR/8 stalk, as previously described, and their globular heads are derived from A/Singapore/1-MA12E/1957 (H2N2) and A/mallard/Sweden/81/2002 (H6N1) (23). These proteins were purified with Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Hilden, Germany). A second version of recombinant PR/8 and Cal/09 HAs utilizing a streptomycin (Strep) tag purification domain and the GCN4 leucine zipper as a trimerization domain was expressed in SF9 cells and purified with a StrepTactin Sepharose column (GE Healthcare Life Sciences, Pittsburgh, PA). Recombinant HA from A/Vietenam/1203/2004 (H5N1) was purchased from Sino Biologicals (Beijing, China). The cH5/3 construct incorporates the HA globular head of A/Vietenam/1203/2004 and the HA stalk domain of A/Perth/16/2009. The virus was rescued as a “6-plus-2” reassortant utilizing an N3 subtype neuraminidase derived from A/swine/Missouri/4296424/2006 in the PR/8 background. The 2009–2010 formulation of Fluzone was manufactured by Sanofi-Pasteur. The influenza viruses were all grown in 8- to 10-day-old embryonated chicken eggs. Viruses used as enzyme-linked immunosorbent assay (ELISA) substrates were subsequently purified and concentrated by centrifugation through a 30% sucrose gradient.
In vivo immunization studies using model antigen and influenza virus antigens.
C57BL/6 WT, Myd88−/−, or TrifLps2/Lps2 mice were i.m. immunized with 20 μg of OVA with 1Z105 (89.4 μg/dose, equivalent to 200 nmol/animal), 1V270 (10.8 μg/dose, equivalent to 10 nmol/animal), or a combination of 1Z105 (89.4 μg/dose) and 1V270 (10.8 μg/dose) in a total volume of 50 μl on days 0 and 14. Vehicle (10% DMSO in saline) and AddaVax (1:1 ratio with antigen in saline) were used as controls. WT BALB/c mice were i.m. immunized with rHA (5 μg per mouse for rPR/8, rCal/09, cH2/1, and cH6/1 HAs and 2 μg per mouse for rVN/04 HA) plus 1Z105 (89.4 μg/dose), 1V270 (10.8 μg/dose), or a combination of 1Z105 (89.4 μg/dose) and 1V270 (10.8 μg/dose) in 10% DMSO–phosphate-buffered saline (PBS). AddaVax was used at a 1:1 ratio with antigen in PBS. The control groups received antigen in 10% DMSO-PBS (no adjuvant), a combination of 1Z105 (89.4 μg/dose) and 1V270 (10.8 μg/dose) in 10% DMSO-PBS without antigen (adjuvant only), or the vehicle. Fluzone was used at 50 ng/HA per mouse, and all immunizations were delivered into the gastrocnemius muscle in a total volume of 50 μl.
Alignment of influenza virus HA protein sequences utilized in this study.
HA protein sequences in vaccination and challenge strains relevant to this work were aligned to generate a phylogenetic tree with MEGA5. The Caption Expert tool in Mega 5.1 was used as follows. The evolutionary history was inferred using the neighbor-joining method (30). The optimal tree with the sum of branch lengths equaling 2.82138273 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches (31). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method (32) and are in units of the number of amino acid substitutions per site. The analysis involved 11 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 545 positions in the final data set. Evolutionary analyses were conducted in MEGA5 (33).
In vitro assay for antigen uptake and costimulatory-molecule expression in mouse bone marrow-derived dendritic cells.
Mouse bone marrow-derived dendritic cells (mBMDCs) were prepared from wild-type or Tlr4−/− C57BL/6 mice as described previously (34). mBMDCs (105 cells per well) were plated in 96-well plates in 200 μl of complete RPMI 1640. The cells were incubated with 10 μM 1Z105 or vehicle for 18 h at 37°C, 5% CO2; 10 μg/ml OVA conjugated with Alexa Fluor 488 (Life Technologies) was added to the culture during the last 30 min of incubation. Cells incubated at 4°C served as negative controls. MPLA (1 μg/ml) was used as a positive control. In studies for expression of costimulatory molecules, mBMDCs were incubated with 1Z105 (2 or 10 μM) or MPLA (0.04 µg/ml) overnight and stained for surface expression of CD40 and CD86. The cells were stained for CD11c, and OVA uptake and expression of CD40 and CD86 in the CD11chi-gated population was determined by flow cytometry.
Cytokine induction of human monocyte-derived dendritic cells.
Human primary dendritic cells (DCs) were generated as previously described (35, 36). Briefly, CD14+ cells were isolated from buffy coats of healthy human donors (New York Blood Center) with anti-human CD14 antibody-labeled magnetic beads and iron-based MiniMACS liquid separation columns (Miltenyi Biotec, San Diego, CA). For the generation of immature DCs, CD14+ cells were incubated at 37°C for 5 days in complete RPMI 1640 supplemented with 500 U/ml human granulocyte-macrophage colony-stimulating factor (hGM-CSF) and 1,000 U/ml human interleukin 4 (hIL-4) (Peprotech, Rocky Hill, NJ). DCs were incubated with 10 μM 1Z105, 50 ng/ml of lipopolysaccharide (LPS) (L2654, Escherichia coli 026:B6; Sigma-Aldrich), or vehicle (0.5% DMSO in medium) for 18 h. The supernatants were stored at −20°C for later quantification of released cytokines. Quantification of IL-1β, IL-6, IL-8, IL-12p70, and tumor necrosis factor alpha (TNF-α) released in supernatants was performed using the Milliplex Multiplex Assays (Luminex; Millipore, Billerica, MA) according to the manufacturer's instructions. Data were analyzed using the Milliplex Analyst software (Millipore).
OT-1 CD8 T cell proliferation assay.
Naive CD8+ T cells from OT-1 C57BL/6 mice were isolated using an EasySep Mouse CD8+ T Cell Isolation Kit and stained with carboxyfluorescein succinimidyl ester (CFSE) (10 μM). WT BMDCs were incubated with 1Z105 (2 μM) and MPLA (1 μg/ml) overnight, and OVA (10 μg/ml) was added to the culture for the last 4 h of incubation. The cells were washed and cultured with CFSE-labeled CD8+ OT-1 T cells for 3 days. OT-1 T cell proliferation was monitored by CFSE dilution using a flow cytometer.
In vivo immunization study using OVA and influenza virus rHA antigens.
C57BL/6 WT, Myd88−/−, or TrifLps2/Lps2 mice were i.m. immunized with 20 μg OVA with 1Z105 (200 nmol/animal, equivalent to 89.4 μg/dose), 1V270 (10.8 μg/dose, equivalent to 10 nmol/animal), or a combination of 1Z105 (89.4 μg/dose) and 1V270 (10.8 μg/dose) in a total volume of 50 μl on days 0 and 7. Vehicle (10% DMSO in saline) and AddaVax (1:1 ratio with antigen in saline) were used as controls. Sera were collected on days 0, 7, 14, 21, 28, and 35. Mice were sacrificed on day 35, and the spleens were harvested. Approximately 2.5 × 106/ml spleen cells was dispersed into round-bottom microtiter plates in triplicate in a total volume of 200 μl complete RPMI 1640 and restimulated with either 100 μg/ml OVA or medium alone. The cultures were then incubated at 37°C, 5% CO2, and the supernatants were harvested after 72 h. The levels of gamma interferon (IFN-γ) in the culture supernatants were measured by ELISA (BD Bioscience, San Jose, CA) according to the manufacturer's instructions. In parallel, splenocytes were stimulated with 10 μg/ml OVA class I (OVA257–264) or class II (OVA323–339) peptide on anti-IFN-γ antibody-coated enzyme-linked immunospot (ELISpot) plates for 18 h. The results are reported as numbers of IFN-γ spot-forming cells (SFC) per million cells as described below.
BALB/c mice were i.m. immunized with rHA (5 μg per mouse for rPR/8, rCal/09, cH2/1, and cH6/1 HAs and 2 μg per mouse for rVN/04 HA) plus 1Z105 (89.4 μg/dose), 1V270 (10.8 μg/dose), or a combination of 1Z105 (89.4 μg/dose) and 1V270 (10.8 μg/dose) in 10% DMSO-PBS. AddaVax was used at a 1:1 ratio with antigen in PBS. Control groups received antigen in 10% DMSO-PBS or a combination of 1Z105 (89.4 μg/dose) and 1V270 (10.8 μg/dose) in 10% DMSO-PBS without antigen. Fluzone was used at 50 ng/HA per mouse, and all immunizations were delivered i.m. into the gastrocnemius muscle in a total volume of 50 μl.
Splenocytes were stimulated for 20 h on anti-IFN-γ antibody-coated ELISpot plates with a pool of PR/8 peptides acquired from BEI Resources (Manassas, VA) at a final concentration of 2 μg/ml in DMEM (NR-18973; the first 25 peptides were pooled for a stock concentration of 20 μg/μl per peptide in DMSO). IFN-γ spot-forming cells were detected with the IFN-γ ELISpot ALP kit (3321-2A; Mabtech, Cincinnati, OH). Antigen-specific B cells from splenocytes were quantified 5 days after reexposure to antigen (5 μg of rPR/8 HA in PBS only administered i.m.) by plating them on ELISpot plates coated with rPR/8 protein (Strep tag purified) for 20 h, and detection of secreted antibody was performed with an anti-mouse IgG secondary antibody conjugated to horseradish peroxidase (HRP).
Measurement of antigen-specific antibodies.
Anti-OVA antibodies of the IgG subclasses IgGl and IgG2c were measured by ELISA as previously described (19). Each ELISA plate contained a titration of a previously quantitated serum to generate a standard curve. The titer of this standard was calculated as the reciprocal of the highest dilution of serum that gave an absorbance reading that was double the background. Serum samples were tested at a 1:100 dilution and reported as U/ml based on comparison with the standard curve. Anti-influenza virus antibodies were measured by ELISA using purified virus or rCal/09 (Strep tag purified) as the substrate by standard methods. Endpoint titers were defined as the highest dilution of serum that resulted in a signal three times above the background level. Total IgG, IgG1, and IgG2a were detected as previously described (22). Hemagglutination-inhibiting (HAI) titers to the PR/8 strain were assayed using trypsin-heat-periodate-inactivated sera according to established WHO methods (37).
Murine influenza virus challenge.
All challenge viruses were grown in 8- to 10-day-old embryonated eggs. Murine 50% lethal doses (mLD50) were determined in 6- to 10-week-old female BALB/c mice. The challenge viruses included A/Puerto Rico/8/1934(H1N1) (10 mLD50; 300 PFU), mouse-adapted (5 passages through mouse lungs before amplification in embryonated eggs) A/Netherlands/602/2009(H1N1) (5 mLD50; 100 PFU), mouse-adapted B/Florida/04/2006 (25 mLD50; 80,000 PFU), and a 6-plus-2 reassortant of the HA (a low-pathogenic form with the polybasic cleavage site removed) and neuraminidase (NA) from A/Vietnam/1203/2004(H5N1) in the PR/8 background (5 mLD50; 110 PFU). Mice were anesthetized with ketamine/xylazine and infected intranasally in a volume of 50 μl of PBS.
Histological examination for reactogenicity.
BALB/c mice were injected with 1Z105 (89.4 μg/dose), 1V270 (10.8 μg/dose), or a combination of 1Z105 (89.4 μg/dose) and 1V270 (10.8 μg/dose) in the gastrocnemius muscles in a total volume of 50 μl in the absence of antigen; 10% DMSO and 50% AddaVax in saline were used as controls. Twenty-four hours after injection, muscle tissues at the injection sites and sera were harvested. The muscles were fixed in 10% buffered formalin and embedded in paraffin. Sections 5 μm thick were stained with hematoxylin and eosin (H&E) and examined under a microscope.
Quantitative RT-PCR.
Harvested tissues were immediately frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted from tissues or cells using an RNeasy minikit (Qiagen). cDNA synthesis was performed using iScript (Bio-Rad), and real-time (RT) PCRs were performed on the Bio-Rad iCycler IQ. The comparative ΔΔCt method was used to assess fold changes in expression of RNA transcripts between control and drug-treated mice. ΔCT values were determined by subtracting the average glyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA gene threshold cycle (CT) values from each test CT value. ΔΔCT values were normalized by the control calibrator ΔCT value. TaqMan Gene Expression Assays (murine IL-6, keratinocyte chemoattractant [KC], monocyte chemotactic protein 1 [MCP-1], and MIP-1α) were purchased from Life Technologies.
Statistical analysis.
For continuous outcomes, the data are represented as means and standard errors of the mean (SEM). A two-tailed Student t test or Mann-Whitney U test was used to compare two groups, and one-way analysis of variance (ANOVA) with Dunnett's post hoc test was used to compare multiple groups with the control. The ANOVA model assumption of equal variances across groups was checked using Bartlett's test of homogeneity of variances (38). For influenza virus serum HAI and endpoint titer data, the Kruskal-Wallis test with Dunn's correction with multiple comparisons was used to assess significance compared to the no-adjuvant control. When there was clear evidence of variance heterogeneity, nonparametric multiple-contrast tests were used to compare an adjuvant group to the controls with Dunnett's adjustment for multiple comparisons using the R-nparcomp package (39). For weight loss curves, t tests correcting for multiple comparisons by the Holm-Sidak method were used to compare each day's weight to that of the no-adjuvant control, and no adjustment was made for performing comparisons on multiple days for the same endpoints. For survival curves, Kaplan-Meier curves were plotted, and the log rank test was performed to assess significance. Prism 6 (GraphPad Software) statistical software and R (version 3.1.0; http://www.r-project.org) were used to obtain P values for comparisons between groups (a P value of <0.05 was considered significant).
RESULTS
1Z105 enhances dendritic cell maturation and antigen presentation.
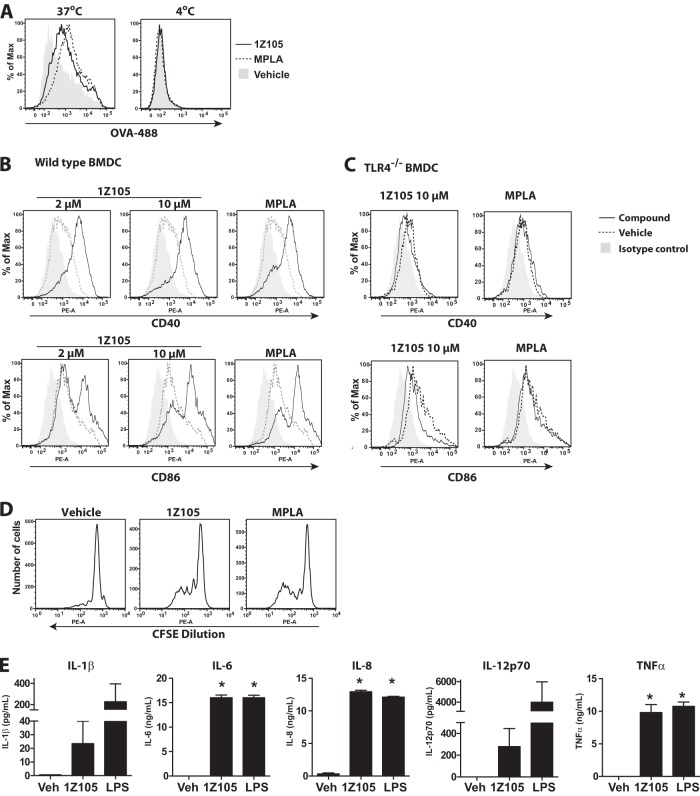
Adjuvants are added to vaccine antigens to potentiate antigen-specific immune responses and induce desired protective responses. Hence, key functions of adjuvants are directed at local immature DCs, inducing them to capture antigen, mature, and migrate to draining lymph nodes, where they prime naive T cells. We previously described the in vitro and in vivo biological activities of the TLR7 agonist 1V270 (17–19). Here, we evaluated the adjuvant activity of a TLR4 agonist, 1Z105 (Fig. 1), with three in vitro assays to evaluate (i) antigen uptake, (ii) DC maturation, and (iii) cross-presentation to prime CD8+ T cells. BMDCs from C57BL/6 mice were compared after overnight exposure to 1Z105; MPLA, which is a semisynthetic TLR4 ligand; or vehicle for antigen uptake as assessed by incubation with fluorescently labeled OVA (Fig. 2A). After incubation with 1Z105 or MPLA at 37°C, an increased number of OVA-associated CD11chi BMDCs were observed, but no effect was observed with BMDCs incubated at 4°C (Fig. 2A). Next, to evaluate whether 1Z105 enhanced DC maturation, the induction of costimulatory molecules (CD40 and CD86) on BMDCs incubated with 1Z105, MPLA, or vehicle was determined by flow cytometry. 1Z105 increased the surface expression of CD40 and CD86 at levels similar to that of MPLA (Fig. 2B). This was not observed in TLR4-deficient BMDCs (Fig. 2C). Lastly, antigen cross-presentation was tested by assaying the proliferation of OVA-specific CD8+ T cells (OT-1) incubated with BMDCs and OVA protein in the presence of 1Z105, MPLA, or vehicle. BMDCs incubated with 1Z105 or MPLA induced proliferation of OT-1 cells, indicating that they efficiently processed the extracellular OVA protein via the major histocompatibility complex (MHC) class I pathway (Fig. 2D). Furthermore, 1Z105 activated human monocyte-derived DCs, resulting in proinflammatory cytokine release (Fig. 2E). These in vitro evaluations strongly suggested that 1Z105 enhanced the antigen-presenting functions of DCs.
FIG 2.
1Z105 enhances the antigen presentation function of murine dendritic cells and is active in human dendritic cells. (A) Upregulation of antigen uptake by 1Z105. BMDCs prepared from C57BL/6 mice were incubated with 10 μM 1Z105 or MPLA overnight. Antigen, Alexa Fluor 488-conjugated ovalbumin (OVA-488), was added to the culture for the last 30 min of incubation. The cells were washed and stained for CD11c. OVA-associated dendritic cells in the CD11chi population were evaluated by flow cytometry. Cells incubated at 4°C served as a negative control. (B and C) Enhancement of expression of costimulatory molecules by 1Z105. WT (B) or Tlr4−/− (C) BMDCs were incubated with 1Z105 (10 or 2 μM) or MPLA (0.04 μg/ml) overnight, and expression of CD40 and CD86 was assessed by flow cytometric assay. (D) 1Z105 promotes antigen cross-presentation. Wild-type BMDCs were incubated with 1Z105 (2 μM) overnight, and OVA (10 μg/ml) was added to the culture for the last 4 h of incubation. The cells were washed and cultured with CFSE-labeled CD8+ OT1 T cells for 3 days. OT-1 T cell proliferation was monitored by flow-cytometric assay. The data shown are representative of two independent experiments showing similar results. The flow cytometry data are representative of at least 3 independent experiments. (E) Human myeloid dendritic cells were incubated with 1Z105 (10 μM) for 18 h. Cytokine release of IL-1β, IL-6, IL-8, IL-12p70, and TNF-α was determined by Luminex bead assay. The data are the average and SEM of measurements obtained from hDCs from three independent donors. *, P < 0.05, indicating a significant difference from the vehicle (Veh; 0.5% DMSO) by one-way ANOVA with Dunnett's post hoc testing.
Combination of 1Z105 with a TLR7 ligand, 1V270, induces both Th1- and Th2-associated humoral responses and antigen-specific cellular immune responses.
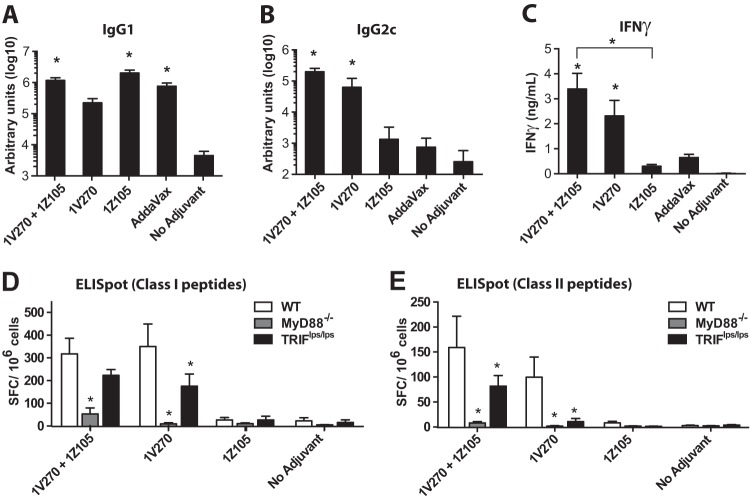
Recent reports indicate that the combination of a TLR4 ligand and a TLR7 ligand enhances the magnitude of antigen-specific cellular and antibody responses as vaccine adjuvants (40, 41). To develop an adjuvant that can be easily prepared and stably replicated in composition, synthetic molecules are preferable to natural products. Hence, we selected a synthetic TLR7 ligand conjugated to a phospholipid, 1V270 (Fig. 1), previously shown to have protective effects in a murine infectious model (17). C57BL/6 mice were immunized i.m. with a model antigen, OVA (20 μg/animal), plus 1Z105 (89.4 μg/dose), 1V270 (10.8 μg/dose), or a combination of 1Z105 and 1V270. AddaVax and unadjuvanted OVA in vehicle (no adjuvant) were used as controls. Immunoglobulin levels on day 35 in the sera indicated that 1Z105 induced significantly higher IgG1 (Fig. 3A), but not IgG2c (Fig. 3B), antibody titers than OVA alone, so that the antibody profile was similar to that of AddaVax. In contrast, 1V270 induced significantly higher IgG2c titers, but it did not induce IgG1 comparably to 1Z105. When the two ligands were combined, significant induction of both IgG1 and IgG2c was observed.
FIG 3.
Assessment of the adjuvant properties of 1Z105 using a model antigen, OVA. (A to C) C57BL/6 mice (n = 8 to 16/group) were i.m. immunized with OVA (20 μg/mouse) plus 1Z105, 1V270, or a combination of 1Z105 and 1V270 in a 50-μl volume on days 0 and 14. AddaVax and vehicle were used as controls. The sera were collected on day 35, and the serum IgG1 (A) and IgG2c (B) levels were tested. (C) Splenocytes were incubated with OVA (100 μg/ml) for 3 days, and IFN-γ levels in the culture supernatants were determined by ELISA. Data were pooled from three experiments showing similar trends. (D and E) Myd88−/−, Triflps/lps, or wild-type mice (n = 8/group) were i.m. immunized with ovalbumin mixed with the indicated adjuvant or vehicle in a 50-μl volume on days 0 and 14. On day 35, splenocytes were incubated with OVA MHC class I peptides (OVA257–264) (D) or class II peptides (OVA323–339) (E) overnight, and IFN-γ-producing cells were detected by ELISpot assay. The data shown are pooled from 2 or 3 independent experiments showing similar trends. *, P < 0.05 compared to no adjuvant (A to C) or the WT (D and E) by one-way ANOVA with Dunnett's post hoc testing.
Furthermore, 1V270 and the combination of 1V270 and 1Z105 promoted OVA-specific IFN-γ release by T cells restimulated ex vivo (Fig. 3C). OVA-specific ELISpot assays demonstrated that splenocytes from mice immunized with antigen plus 1V270, or a combination of 1Z105 and 1V270, contained increased frequencies of IFN-γ-releasing T cells specific for OVA class I (Fig. 3D) and class II (Fig. 3E) peptides. These increased antigen-specific T cell frequencies were partially dependent upon the MyD88 and TRIF pathways (Fig. 3D and E) (42). These data indicate that MyD88 signaling is required for the adjuvant activity of 1V270 alone and in combination with 1Z105. TLR7 signaling utilizes MyD88, and the partial requirement for TRIF suggests that it may affect MyD88 signaling in antigen-presenting cells (APCs) (43). TRIF is required for the TLR4-mediated expansion of T cells and may contribute to T cell development when 1V270 and 1Z105 are used in combination (44). Collectively, these findings indicated that the combination of 1Z105 and 1V270 could induce robust Th1- and Th2-associated humoral responses, in addition to MHC class I- and II-restricted T cell responses.
1Z105 and 1V270 with a recombinant hemagglutinin antigen induce rapid protective immunity to a homologous strain of influenza A virus after a single immunization.
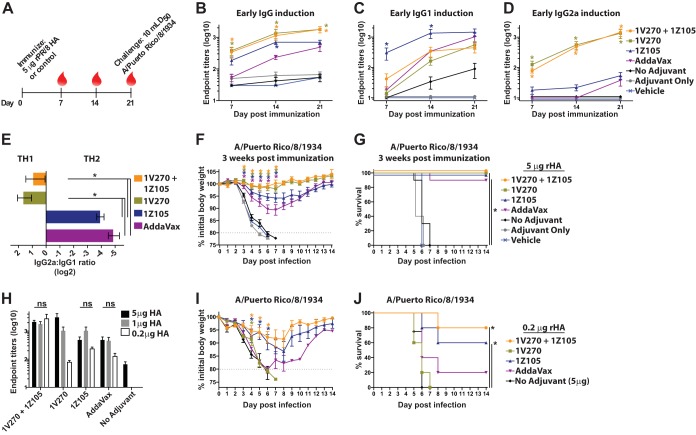
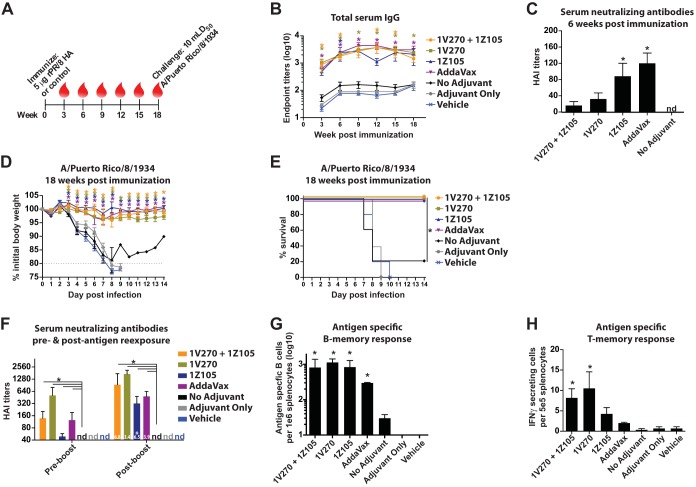
In order to assess the efficacy of 1Z105 and 1V270 as bona fide vaccine adjuvants, the mouse model for influenza virus vaccination and challenge was employed. Several HA antigens and challenge viruses were utilized to test various models of HA-directed immunity. The phylogenetic relationships of the HAs relevant to this work are depicted in Fig. 4. Current vaccines rely upon the induction of strain-specific neutralizing antibodies directed toward the antigenic regions of the influenza virus HA (7). Therefore, rHA derived from A/Puerto Rico/8/1934 (PR/8) was chosen as the vaccine antigen in order to concentrate upon HA-specific immunity that is protective against a homologous challenge. BALB/c mice were immunized with 5 μg of rPR/8 HA on day 0 and bled on days 7, 14, and 21, as schematized in Fig. 5A. The early seroresponse was assessed for the induction of antigen-specific total IgG (Fig. 5B), IgG1 (Fig. 5C), and IgG2a (Fig. 5D) as early as 7 days after a single immunization. Both 1Z105 and 1V270, in addition to AddaVax, which was included as a reference, induced rapid seroconversion to the rPR/8 HA. 1Z105 and AddaVax produced predominately IgG1 in a Th2-type response, as demonstrated by the IgG2a/IgG1 ratio (Fig. 5E). 1V270, alone and in combination with 1Z105, induced both IgG1 and IgG2a in a more balanced Th1-Th2-type response (Fig. 5E). The control groups included mice receiving rPR/8 HA without adjuvant (no adjuvant) or 1V270 and 1Z105 without antigen (adjuvant only) and animals receiving the vehicle.
FIG 4.
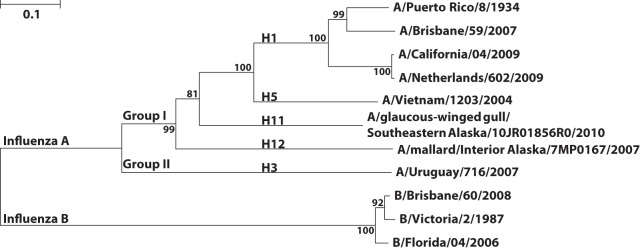
Phylogenetic relationship of influenza virus hemagglutinins in the study. The phylogenetic tree illustrates the relationship among influenza virus HAs that are pertinent to this study. B/Victoria/2/1987 was included as a reference for the influenza B virus HAs. Amino acid sequences were aligned by ClustalW, and the tree was constructed using the neighbor-joining method with Mega 5.10. Bootstrap values for 1,000 replicates are listed at the branches, and the units are the number of amino acid substitutions per site.
FIG 5.
1Z105 and 1V270 induce rapid protective immunity to influenza A virus after a single immunization with rHA. (A) BALB/c mice (n = 5/group) were immunized i.m. with rPR/8 HA (5 μg/mouse) with the indicated adjuvant or vehicle on day 0. Sera were collected at 7, 14, and 21 days after immunization, as indicated by the red drops. (B to D) At 7, 14, and 21 days postimmunization, HA-specific total IgG (B), IgG1 (C), and IgG2a (D) serum antibody titers were assayed by ELISA with PR/8 virus as a substrate. (E) The Th1-Th2 immune balance shown by the IgG2a/IgG1 ratio expressed on a log 2 scale. (F and G) Three weeks after immunization with rPR/8 HA, the immunized mice (n = 10) were administered 10 mLD50 of PR/8 virus and monitored for morbidity, as measured by body weight loss (F), and mortality (G) induced by the viral challenge. (H to J) BALB/c mice (n = 5/group) were immunized with rPR/8 HA with the indicated adjuvant or vehicle on day 0. (H) The rHA was administered at 5, 1, or 0.2 μg/animal, and serum IgG antibody titers were assessed by ELISA 3 weeks after the immunization. The bars indicate that there was no significant (ns) difference in endpoint titers between mice receiving different antigen doses with the same adjuvant. (I and J) Three weeks after immunization with 0.2 μg/animal of rHA, the immunized mice (n = 5) were administered 10 mLD50 of PR/8 virus and monitored for morbidity, as measured by body weight loss (I), and mortality (J) induced by the viral challenge. “No adjuvant” indicates animals that received the antigen in the absence of adjuvant, adjuvant-only animals received both 1Z105 and 1V270 in the absence of antigen, and mice in the vehicle group received an injection of 10% DMSO in PBS without antigen or adjuvant. The data shown are means and SEM; *, P < 0.05 compared to no adjuvant by the Kruskal-Wallis test for serum antibody titers. Weight loss data were compared to no adjuvant with multiple t tests, and survival data were compared using a Mantel-Cox test; a P value of <0.05 was considered significant.
Three weeks after immunization with 5 μg of rPR/8 HA, all groups were challenged with 10 mLD50 of PR/8 virus. Vaccination with HA adjuvanted by 1Z105 and 1V270, alone or in combination, provided protective efficacy against morbidity, as measured by weight loss (Fig. 5F), and mortality (Fig. 5G) in response to the viral challenge. 1V270, alone or in combination with 1Z105, also restricted weight loss compared to AddaVax. The adjuvant-only control group demonstrated morbidity and mortality similar to those vaccinated with vehicle, confirming that the protection afforded by 1V270 and 1Z105 is mediated by an enhanced response to the antigen rather than the nonspecific induction of antiviral immunity.
In addition to enhancing the immune response and inducing a bias in the T helper response, adjuvants are known to reduce the amount of antigen needed to induce a protective response. 1Z105 and 1V270 were assayed for their antigen-sparing properties by immunizing mice with 5, 1, or 0.2 μg of rPR/8 HA. In Fig. 5H, the total serum IgG endpoint titers were assayed by ELISA 3 weeks after immunization. For the combination of 1V270 and 1Z105, 1Z105 alone, and AddaVax, no significant difference in serum IgG titers was detected among the 3 rHA doses, indicating robust antigen-sparing properties. Mice receiving 0.2 μg of rHA were challenged 3 weeks postimmunization with 10 mLD50 of PR/8 virus and followed for morbidity (Fig. 5I) and mortality (Fig. 5J). 1Z105, alone or combined with 1V270, significantly minimized morbidity and mortality compared to the no-adjuvant control group receiving 5 μg of rHA. In terms of antigen sparing, the combination of 1V270 and 1Z105 conferred an advantage over using either adjuvant alone when endpoint titers (Fig. 5H), morbidity (Fig. 5I), and mortality (Fig. 5J) were compared. In summary, the data presented in Fig. 5 suggested that 1Z105 and 1V270 had efficacies comparable to or better than that of AddaVax as influenza virus vaccine adjuvants and warranted characterization with more contemporary antigens.
1Z105 and 1V270 induce rapid protective immunity to the pandemic H1N1 virus and an avian H5N1 subtype virus and enhance the immunogenicity of Fluzone.
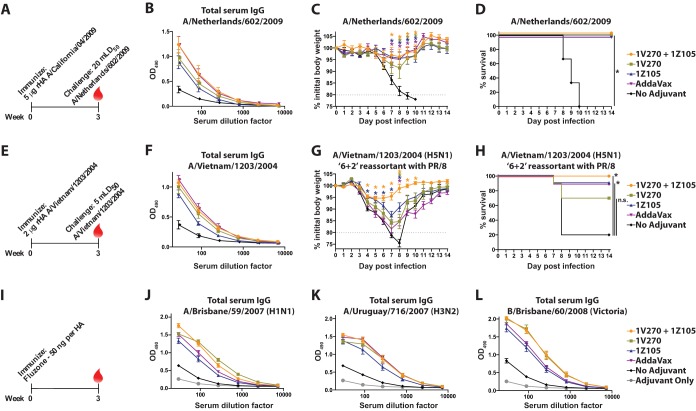
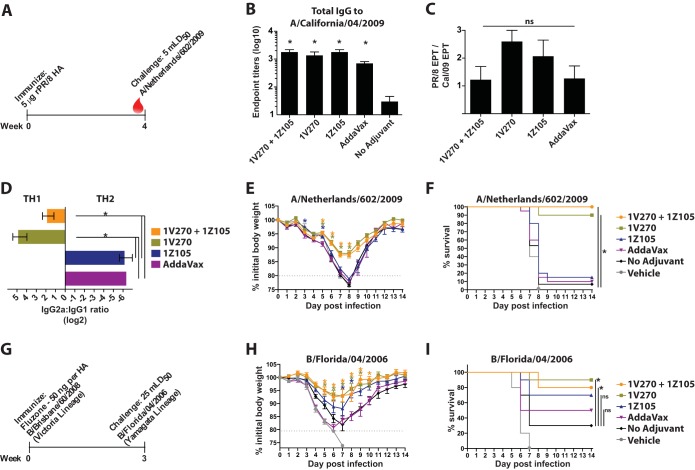
After assessing the efficacy of 1Z105 and 1V270 with rPR/8 HA, antigens more relevant to the development of present-day influenza virus vaccines were tested with the TLR ligands. rHA derived from Cal/09 was used to determine the efficacy of 1Z105 and 1V270 for the induction of protective immunity to the 2009 pandemic H1N1 (pH1N1) virus (Fig. 6A). 1Z105 and 1V270, alone and in combination, and AddaVax enhanced the IgG seroresponse (Fig. 6B) and served to restrict morbidity, as measured by weight loss (Fig. 6C), and mortality (Fig. 6D) after a lethal challenge with a mouse-adapted pH1N1 virus, A/Netherlands/602/2009, which is essentially homologous to Cal/09 (Fig. 4).
FIG 6.
1Z105 and 1V270 induce rapid protective immunity to the pH1N1 virus and an avian H5N1 subtype virus and enhance the immunogenicity of Fluzone. (A) BALB/c mice (n = 3/group) were immunized with rHA (5 μg/animal) derived from the pH1N1 virus (A/California/04/2009) on day 0 and bled 3 weeks later (red drop). (B) Total serum IgG titers were assayed by ELISA 3 weeks after immunization. (C and D) Three weeks after immunization, the mice were administered 5 mLD50 of a mouse-adapted pH1N1 virus (A/Netherlands/602/2009), which is essentially homologous to Cal/09, and monitored for morbidity (C) and mortality (D) induced by the challenge. (E) BALB/c mice (n = 10/group) were immunized with rHA derived from a highly pathogenic avian H5N1 virus (A/Vietnam/1203/2004) (2 μg/animal) on day 0. (F) Total serum IgG titers were assayed by ELISA 3 weeks after immunization. (G and H) Three weeks after a single immunization, the mice were challenged with 5 mLD50 of a 6-plus-2 reassortant of the VN/04 HA and NA (H5N1) in the PR/8 background and monitored for morbidity (G) and mortality (H) induced by the challenge. The data for the H5N1 challenge were combined from two experiments for a total of 10 mice/group. (I) BALB/c mice (n = 10/group) were immunized with 50 ng/HA of 2009–2010 Fluzone. (J to L) Serum IgG levels 3 weeks after immunization to each of the homologous viral components of the vaccine, including A/Brisbane/59/2007 (H1N1) (J), A/Uruguay/716/2007 (H3N2) (K), and B/Brisbane/60/2008 (Victoria lineage) (L), were assayed by ELISA. “No adjuvant” indicates animals that received the antigen in the absence of adjuvant, and mice in the vehicle group received an injection of 10% DMSO in PBS without antigen or adjuvant. The data shown are means and SEM. Weight loss data were compared to no adjuvant with multiple t tests, and survival data were compared using a Mantel-Cox test; *, P < 0.05, which was considered significant.
Avian-origin influenza A viruses of the H5N1 subtype are of concern for their potential to cause a human pandemic with a high mortality rate, and efforts to develop vaccines against H5 subtype avian influenza viruses have demonstrated that H5 subtype HAs are poorly immunogenic and may require the use of an adjuvant (45). Mice were immunized with 2 μg of rHA derived from A/Vietnam/1203/2004 (VN/04), a highly pathogenic avian H5N1 subtype virus, plus adjuvant or in vehicle alone (Fig. 6E). 1Z105, 1V270, and AddaVax enhanced the IgG seroresponse (Fig. 6F) when administered with rHA. 1Z105, alone or combined with 1V270, significantly minimized morbidity (Fig. 6G) and mortality (Fig. 6H) induced by 5 mLD50 of a 6-plus-2 reassortant virus consisting of the VN/04 HA and NA in the PR/8 background. Notably, the combination of 1Z105 and 1V270 minimized morbidity, as assayed by weight loss, more effectively than either adjuvant alone and provided 100% survival after challenge.
The vast majority of influenza virus vaccines in present-day use consist of split virions grown in embryonated chicken eggs. These vaccines are time-consuming to produce and depend upon the availability of eggs. The use of adjuvants with split vaccines can enhance their immunogenicity and reduce the amount of antigen required to generate a seroresponse (20, 46). 1Z105 and 1V270 were assayed for the ability to enhance the seroresponse to the 2009–2010 seasonal Fluzone, a trivalent vaccine consisting of three viral components: A/Brisbane/59/2007 (H1N1), A/Uruguay/716/2007 (H3N2), and B/Brisbane/60/2008 (Victoria lineage). Mice were immunized with Fluzone and bled 3 weeks later (Fig. 6I). 1Z105 and 1V270, alone and in combination, and AddaVax enhanced the seroresponse to all three viral components, as assayed by ELISA (Fig. 6J to L). These data confirmed that 1V270 and 1Z105 rapidly induce a protective humoral response comparable to or better than AddaVax when administered with relevant influenza virus antigens, and this indicated a need to characterize the long-term effects of the adjuvant.
1Z105 and 1V270 induce sustained protective immunity to influenza A virus after a single immunization with rHA.
After characterizing the rapid response to rPR/8 HA induced by 1Z105 and 1V270, the long-term response to antigen was assessed. Mice were immunized with 5 μg of rPR/8 HA and bled every 3 weeks up to 18 weeks postimmunization, at which point they were challenged to determine whether protective immunity was sustained (Fig. 7A). The total serum IgG reactive to antigen was measured by ELISA (Fig. 7B). 1Z105 and 1V270, alone and in combination, and AddaVax induced a robust and sustained IgG response. Sera were assayed for neutralizing capacity by their ability to inhibit PR/8 virus from hemagglutinating chicken red blood cells. HAI titers were detectable by 6 weeks postimmunization, as the serum endpoint titers continued to rise (Fig. 7B and C). After 18 weeks, mice were challenged with 10 mLD50 of PR/8 virus and followed for morbidity (Fig. 7D) and mortality (Fig. 7E). 1V270 and 1Z105 significantly reduced weight loss and provided 100% survival after the lethal challenge, indicating that antigen-specific antiviral immunity induced by the adjuvants is long lived.
FIG 7.
1Z105 and 1V270 induce sustained protective immunity to influenza A virus after a single immunization with rHA antigen. (A) BALB/c mice were i.m. immunized with rPR/8 HA (5 μg/animal) on day 0 with the indicated adjuvant or vehicle, and sera were collected every 3 weeks (red drops). (B) Levels of antigen-specific total IgG were measured by ELISA. (C) The serum HAI titer to PR/8 virus was assayed at 6 weeks after immunization, with a detection limit of 1:40 serum dilution; nd, not detectable. (D and E) Eighteen weeks after immunization, mice (n = 5/group) were administered 10 mLD50 of PR/8 virus and monitored for morbidity, as measured by body weight loss (D), and mortality (E) induced by the viral challenge. (F) (Left) Six months after immunization, all groups of mice were bled (n = 10/group), and HAI titers to PR/8 virus were assayed. (F and G) Subsequently, all groups of mice (including adjuvant-only and vehicle groups; n = 3/group) were i.m. administered 5 μg/animal (in PBS only) of rPR/8 HA to stimulate a memory response. Five days after exposure to the unadjuvanted protein, the mice were bled to assess HAI to PR/8 virus, with the fold increase over preboost HAI titers indicated in the bars (F, right), and splenocytes were isolated and assayed for ex vivo PR/8 HA-specific antibody production by ELISpot with rPR/8 HA with a different trimerization domain and purification tag from the immunogen as a substrate (G). (H) Six months after immunization in a separate cohort of mice not receiving a protein boost, splenocytes were isolated, stimulated ex vivo overnight with a peptide pool derived from PR/8 HA, and assayed by ELISpot for IFN-γ production. The data shown are means and SEM; *, P < 0.05 compared to no adjuvant by Kruskal-Wallis test or nonparametric multiple-contrast test for serum antibody titers and ELISpot assays. Weight loss data were compared to no adjuvant with multiple t tests, and survival data were compared using a Mantel-Cox test; P values of <0.05 were considered significant. *, P < 0.05 by t test for fold increase in postboost over preboost HAI titers.
To gain a better appreciation of the quality of the long-lived immune response, mice were immunized one time with 5 μg of rPR/8 HA with or without adjuvant and assayed for memory response upon reexposure to antigen. In one cohort (Fig. 7F and G), all groups of mice (including those originally receiving adjuvant only or vehicle) were i.m. administered 5 μg of rPR/8 HA in PBS only more than 6 months after the primary immunization. The mice were bled prior to and 5 days after antigen reexposure and assayed for serum HAI titers to PR/8 virus (Fig. 7F). All of the adjuvants induced sustained HAI titers after the primary immunization that were significantly greater than those of the no-adjuvant control group (Fig. 7F, left). Five days after reexposure to antigen, serum HAI titers in the adjuvanted groups were significantly elevated compared to the unadjuvanted control (Fig. 7F, right). The fold increase in HAI titer induced by the boost (indicated on the bars in Fig. 7F, right) was significant only in the 1Z105 group at 5 days after immunization; however, significant expansion of antigen-specific B cells from splenocyte preparations was readily detectable by ELISpot in all of the adjuvanted groups (Fig. 7G). These antigen-specific B cells do not represent a primary response to the antigen exposure, as they were undetectable in the groups that received adjuvant only and vehicle at the time of the primary immunization.
In a second cohort, mice were immunized with 5 μg of rPR/8 HA with or without adjuvant, and splenocytes were isolated 6 months after the immunization, stimulated overnight with a peptide pool derived from PR/8 HA, and assayed for IFN-γ-producing T cells by ELISpot (Fig. 7H). Similar to the prior observations in studies using OVA, the Th1-type adjuvant 1V270, alone or combined with 1Z105, induced antigen-specific IFN-γ T cells that were long lived. These data confirmed that 1V270 and 1Z105 are comparable to or better than AddaVax by several measures, and this prompted work to evaluate the efficacy of 1V270 and 1Z105 as adjuvants in heterologous vaccination and challenge models.
1V270, alone or in combination with 1Z105, induces cross-protective immunity to heterologous influenza viruses.
Some influenza virus vaccine adjuvants have been demonstrated to induce cross-protective responses (13, 22, 45), and cross-protection has been associated with the induction of Th1-type immune responses (47). Therefore, we sought to assay whether 1V270 and 1Z105 could induce heterologous protection from influenza viruses in two different assays. First, mice were immunized with 5 μg of rPR/8 HA with or without adjuvant and assayed for cross-reactive antibodies and cross-protective immunity to the pandemic 2009 H1N1 virus (Fig. 4 and 8A). 1V270 and 1Z105, alone and in combination, as well as AddaVax, induced significantly higher heteroreactive serum IgG titers than unadjuvanted protein alone (Fig. 8B). We did not observe a significant difference in the ability of any adjuvant to induce more cross-reactive total IgG to the pandemic HA relative to the total IgG induced to the PR/8 immunogen (Fig. 8C). The subtypes of these cross-reactive antibodies were similar to those discussed above, with adjuvants containing 1V270 inducing more IgG2a while 1Z105 and AddaVax produced predominately IgG1 (Fig. 8D). Animals were subsequently challenged with 5 mLD50 of a mouse-adapted pandemic H1N1 virus (A/Netherlands/602/2009) and followed for morbidity, as measured by weight loss (Fig. 8E), and mortality (Fig. 8F) induced by the heterologous viral challenge. 1V270, alone and combined with 1Z105, significantly reduced weight loss and mortality resulting from the viral challenge. As single agents, the Th2-type adjuvants 1Z105 and AddaVax did not induce protective immunity in this heterologous-challenge model. The cross-protective efficacy of the adjuvants correlates with the induction of a Th1-type response (Fig. 8D) rather than total cross-reactive serum IgG levels (Fig. 8C).
FIG 8.
1V270, alone or in combination with 1Z105, induces cross-protective immunity to heterologous influenza viruses. (A) BALB/c mice received a single immunization of rPR/8 HA (5 μg/animal) on day 0. (B) Three weeks after immunization, the cross-reactive serum IgG was assayed by ELISA using as a substrate rCal/09 HA, which has a trimerization domain and purification tag different than those utilized for immunization. (C) Total serum IgG titers reactive to pandemic H1 and PR/8 HAs, both purified rHAs with a trimerization domain (GCN4 leucine zipper) and a purification tag (streptavidin purification domain) different than those of the PR/8 immunogen (T4 phage fibritin natural trimerization domain and C-terminal 6× His tag), were determined by ELISA and presented as the PR/8-to-Cal/09 endpoint titer (EPT) ratio. (D) IgG2a and IgG1 serum antibodies cross-reactive to the Cal/09 HA were assayed by ELISA and presented as the IgG2a/gG1 ratio. (E and F) Four weeks after immunization, mice (n = 20/group) were administered 5 mLD50 of a heterologous H1N1 virus, a mouse-adapted pandemic H1N1 strain (A/Netherlands/602/2009), and monitored for morbidity, as measured by body weight loss (E), and mortality (F) induced by the viral challenge. (G) BALB/c mice (10 mice/group) were immunized with 2009–2010 Fluzone, containing B/Brisbane/60/2008 (Victoria lineage), with the indicated adjuvant or vehicle. (H and I) Mice were challenged 3 to 4 weeks after immunization with 25 mLD50 of a heterologous mouse-adapted virus, B/Florida/04/2006 (Yamagata lineage), and monitored for morbidity, as measured by body weight loss (H), and mortality (I) induced by the viral challenge. The data were combined from two independent challenges. The data shown are means and SEM; *, P < 0.05 compared to no adjuvant by a nonparametric multiple-contrast test for serum antibody titers. PR/8-to-Cal/09 endpoint titer ratios were not significantly (ns) different by one-way ANOVA. Weight loss data were compared to no adjuvant with multiple t tests, and survival data were compared using a Mantel-Cox test; a P value of <0.05 was considered significant.
Influenza B viruses are unique among human influenza viruses in that two closely related strains from two distinct lineages, Victoria and Yamagata, cocirculate. Despite a high degree of homology between B virus HAs from different lineages (Fig. 4), the vaccines are not necessarily cross-protective (48). Mice were immunized once with the 2009–2010 seasonal Fluzone, which contains B/Brisbane/60/2008 (Victoria lineage) (Fig. 8G). Three to 4 weeks after the immunization, mice were challenged with 25 mLD50 of a mouse-adapted heterologous influenza B virus (B/Florida/04/2006; Yamagata lineage) (Fig. 8G), and they were followed for morbidity, as measured by weight loss (Fig. 8H), and mortality (Fig. 8I). 1V270, alone and in combination with 1Z105, significantly reduced morbidity and mortality compared to the no-adjuvant group. 1Z105 also reduced morbidity by restricting weight loss on days 7 and 8 postinfection. These data indicated that 1V270 and the combined 1Z105 and 1V270 adjuvant induce cross-protective immunity. Therefore, we sought to further evaluate their adjuvant activities using candidate universal vaccine constructs.
1V270, alone or combined with 1Z105, induces protective heterosubtypic immunity to the conserved HA stalk domain when administered with chimeric hemagglutinins.
In addition to the challenge of addressing antigenic drift within an HA subtype, influenza A viruses sporadically reassort to produce antigenically novel viruses capable of causing serious pandemic outbreaks in a human population with little preexisting immunity (4). Therefore, the development of universal influenza virus vaccines based upon conserved epitopes is being fervently pursued. Among the most promising vaccine targets is the conserved HA stalk domain (21, 24, 49–54), and adjuvants have been demonstrated to play an essential role in the development of HA stalk-directed immunity in a mouse model (22).
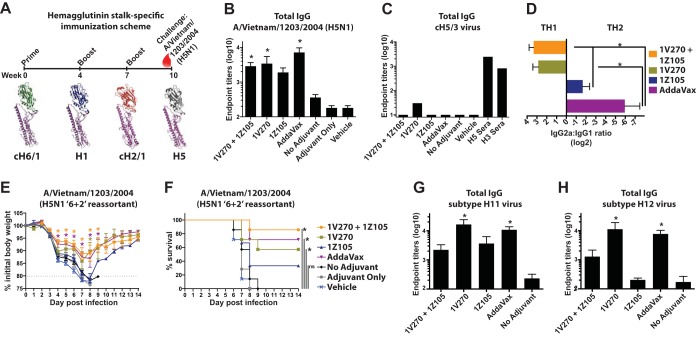
1Z105 and 1V270 were assayed for their abilities to induce HA stalk-directed immunity and to protect against a heterosubtypic viral challenge via sequential immunization with candidate universal vaccine constructs, cHAs (Fig. 9A) (21, 22, 24). cHAs allow the antigenic separation of the globular head domain and the stalk domain by making novel combinations between the globular heads and stalks of different HA subtypes (23). In this immunization strategy, mice were repeatedly exposed to the rH1 subtype stalk (PR/8 strain), while the globular head domain was varied for each immunization by sequentially administering recombinant cH6/1, H1, and cH2/1 (Fig. 9A). Mice remained naive to the H5, H11, and H12 subtype globular heads.
FIG 9.
1V270, alone or in combination with 1Z105, induces protective heterosubtypic immunity based upon the conserved HA stalk domain. (A to C) (A) Vaccination scheme for the induction of broadly protective and HA stalk-specific immunity using chimeric hemagglutinins. Three sequential immunizations with rHA (5 μg/animal), with each stalk component derived from A/Puerto Rico/8/1934, included a prime with cH6/1PR/8 followed by boosts with H1 (PR/8 strain) and cH2/1PR/8 proteins administered with the indicated adjuvant or vehicle control. The immunized animals remained naive to the subtype H5 globular head in the heterosubtypic challenge virus, a 6-plus-2 reassortant of A/Vietnam/1203/2004 (H5N1) in the PR/8 background, in order to assay for reactivity and protection on the basis of the conserved group I HA stalk domain. Three weeks after the third immunization, sera were collected (A, red drop), and total IgG serum titers to the H5 subtype HA (individual mouse sera) (B) and the cH5/3 HA (pooled group sera) (C) were assayed by ELISA. (D) IgG1 and IgG2a serum titers (presented as the IgG2a/IgG1 ratio) cross-reactive to the heterosubtypic H5 subtype HA (VN/04) were assayed by ELISA. (E and F) Subsequently, the mice (n = 7/group) were administered 10 mLD50 of the reassortant H5N1 virus and monitored for morbidity, as measured by body weight loss (E), and mortality (F) induced by the viral challenge. (G and H) The sera were further analyzed by ELISA for reactivity to more divergent group I HAs, including subtype H11 (G) and H12 (H) viruses. “No adjuvant” indicates animals that received the antigen in the absence of adjuvant, adjuvant-only animals received both 1Z105 and 1V270 in the absence of antigen, and mice in the vehicle group received an injection of 10% DMSO in PBS without antigen or adjuvant. The data shown are means and SEM; *, P < 0.05 compared to no adjuvant by a Kruskal-Wallis test for serum antibody titers. Weight loss data were compared to no adjuvant with multiple t tests, and survival data were compared using a Mantel-Cox test; P values of <0.05 were considered significant.
Serum IgG levels with heterosubtypic reactivity to the VN/04 (H5) HA, based upon the conservation between group I HA stalks, were assayed by ELISA (Fig. 4 and 9B). Compared to the no-adjuvant control group, 1V270 and 1Z105, 1V270, and AddaVax induced significantly higher reactivity to the H5 subtype HA. To confirm that this reactivity is specific to the group I stalk and not the H5 subtype head, the sera were assayed by ELISA with cH5/3 (the H5 subtype globular head on the H3 subtype group II stalk) virus as the substrate (Fig. 9C). As expected, seroreactivity was ablated by replacing the group I stalk with the group II stalk. The subtype profiles were similar to those seen in other assays with 1V270, alone and in combination with 1Z105, inducing IgG2a while 1Z105 and AddaVax alone induced primarily IgG1 (Fig. 9D). Mice were challenged with 5 mLD50 of a 6-plus-2 reassortant virus consisting of the VN/04 HA and NA (H5N1) in the PR/8 background and followed for morbidity, as measured by weight loss (Fig. 9E), and mortality (Fig. 9F). 1V270, alone or combined with 1Z105, and AddaVax significantly restricted weight loss and mortality induced by the viral challenge. To further assay the breadth of HA stalk-based seroreactivity, we assayed more distant group I HAs, including those of the H11 (Fig. 9G) and H12 (Fig. 4 and 9H) subtypes. 1V270 and AddaVax induced IgG titers that reacted to H11 and H12 subtypes significantly better than the unadjuvanted control.
The accumulated data indicated that 1V270 and 1Z105, and their combination, induced rapid, potent, long-lasting, and cross-protective immunity against influenza virus infection. Therefore, preclinical safety studies were initiated to assess the reactogenicity of 1V270 and 1Z105.
Administration of the combined 1Z105 and 1V270 adjuvant elicits little reactogenicity.
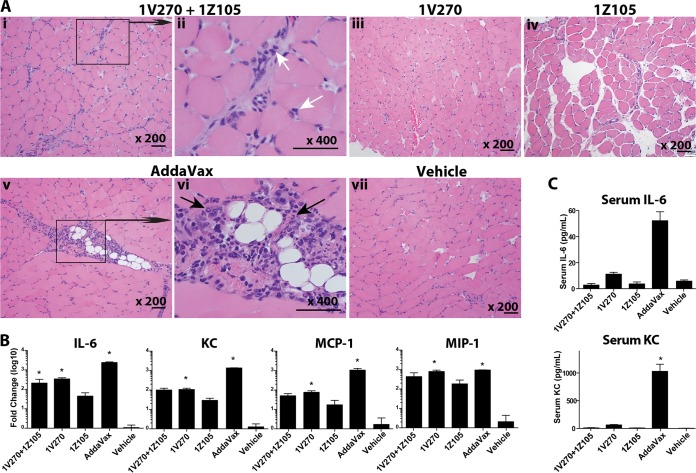
To evaluate the reactogenicity of the adjuvants, gastrocnemius muscles were harvested from BALB/c mice injected with the combination of 1Z105 and 1V270, 1V270, 1Z105, AddaVax, or vehicle alone. Histological examination of H&E-stained muscle sections showed minimal cellular infiltration, with mononuclear cells and a few polynuclear cells in the muscles injected with the combination of 1Z105 and 1V270 (Fig. 10A, i and ii) or 1V270 (iii) or 1Z105 (iv) alone. In contrast, markedly increased cellular infiltration, including both mono- and polymorphonuclear leukocytes, was observed in the AddaVax-injected muscles (Fig. 10A, v and vi, arrows). Ten percent DMSO in PBS was injected into the vehicle control animals (Fig. 10A, vii). Increased cellular infiltration in AddaVax-injected muscle was mirrored by higher local expression of the proinflammatory cytokine IL-6, KC, and MCP-1 (Fig. 10B). Furthermore, increased local expression of IL-6 and KC correlated with increased circulating levels of IL-6 and KC in sera (Fig. 10C). These results indicate that 1Z105 and 1V270, alone or in combination, induce minimal inflammation both at the site of injection and systemically in comparison to AddaVax.
FIG 10.
A combination of 1Z105 and 1V270 induces less local and systemic inflammatory response than AddaVax. BALB/c mice (n = 3 or 4) were injected in the gastrocnemius muscles with 1Z105 (89.4 μg/dose) and 1V270 (10.8 μg/dose), 1V270 (10.8 μg/dose), 1Z105 (89.4 μg/dose), AddaVax (1:1 with saline), or vehicle (10% DMSO in saline) in a 50-μl volume. Twenty-four hours after injection, muscles and sera were harvested. (A) The H&E-stained sections of the injected muscles were examined for cell infiltration. Shown are the muscles injected with 1Z105 plus 1V270 (original magnification, ×200) (i), 1Z105 plus 1V270 (×400) (ii), 1V270 (×200) (iii), 1Z105 (×200) (iv), AddaVax (×200) (v), AddaVax (×400) (vi), and vehicle (×200) (vii). Scale bars, 100 μm. (ii and vi) The white arrows indicate mononuclear cells, and the black arrows indicate polymorphonuclear cells. (B) Gene expression of cytokines and chemokines at the site of injection was determined 24 h after injection. RNA was isolated from muscles, and mRNAs specific for IL-6, KC, MCP-1, and MIP-1α were quantitated by RT-PCR. (C) Systemic cytokine levels (IL-6 and KC) 24 h after injection were examined by Luminex bead assay. *, P < 0.05 compared to the vehicle-injected group by one-way ANOVA. The error bars indicate SEM.
DISCUSSION
The need for novel influenza virus vaccine adjuvants to address the problem of mismatched seasonal vaccines and as components of broadly protective universal vaccine candidates must be balanced with appropriate concerns regarding vaccine safety. The development of small synthetic ligands for innate immune receptors as vaccine adjuvants is an attractive way to simultaneously address the often competing needs of efficacy and safety. Small molecules may be chemically modified to modulate potency, increase storage stability, and minimize cytotoxicity. By specifically targeting receptors in well-understood pathways, the mechanism of action for a small-molecule ligand is far easier to elucidate than for adjuvants without predefined receptor targets, such as oil-in-water emulsions, aluminum salts, or squalene-based emulsions. Accordingly, 1Z105 and 1V270 were optimized from lead compounds targeting TLR4 and TLR7, respectively, to potently activate their receptors while exhibiting little cytotoxicity (14, 17–19).
Current influenza virus vaccines are largely effective only against a matched strain, necessitating accurate prediction of the upcoming epidemic strains during annual vaccine reformulation (1, 5, 6). By enhancing vaccine immunogenicity and improving the quality and persistence of the immune response, adjuvants may provide long-lasting efficacy against drifted strains. Indeed, when 1V270 was administered with rPR/8 HA, it provided a protective immune response against a lethal challenge with the heterologous 2009 pandemic H1N1 virus and showed efficacy with mismatched influenza B virus strains. 1V270 also induced protective immunity to a heterosubtypic virus when administered with chimeric HAs that are in development as universal influenza virus vaccine constructs in an assay focused on the conserved HA stalk domain. 1V270 stimulated robust CD4+ and CD8+ cellular responses, and 1Z105 and 1V270 complemented each other in the antibody profiles that they generated. 1Z105 potently generated IgG1 responses, and 1V270 was a strong inducer of IgG2a and IgG2c. The IgG2a subtype is the most potent activator of the Fc receptor-dependent cellular responses in BALB/c mice, and this activation is critical for the in vivo viral clearance and protective efficacy mediated by broadly neutralizing antibodies against the HA stalk (55). However, for maximal broadly cross-reactive HA immunity, it may be preferable to induce all IgG subclasses as a part of a balanced Th1-Th2 response.
Used in combination, the two synthetic TLR adjuvants, 1Z105 and 1V270, rapidly induce a balanced Th1- and Th2-type response that is protective against homologous and heterologous influenza viruses. Combining 1Z105 with 1V270 in different ratios during the formulation process is a potential means to modulate the Th1/Th2 ratio induced, to refine efficacy and safety, and to reduce the amount of adjuvant administered to induce a protective response. In addition to generating neutralizing humoral immunity, a robust cellular response was also induced by the combined adjuvant, which may play an important role in cross-protective immunity. The protective response was sustained, and evidence of robust B and T cell memory was observed more than 6 months after a single immunization. 1Z105 and 1V270 in combination also demonstrated efficacy when coupled with candidate universal influenza virus vaccine constructs, inducing broadly protective immunity to the HA stalk region. Effective adjuvants will almost certainly be critical for the realization of broadly protective influenza virus vaccines, and the combination of 1Z105 and 1V270 is a promising candidate for this application. Indeed, recent reports describe adjuvanted H5 subtype vaccines that induced broadly neutralizing stalk antibody responses in human trials (56, 57).
In the last decade, experimental adjuvants for viral vaccines have proliferated. While many have demonstrated efficacy in animal models, few have been able to move through regulatory hurdles because of safety and reactogenicity concerns. Notable exceptions for influenza virus vaccines are MF59 and AS03, both squalene-based oil-in-water emulsions that have been approved for broad use in Europe; however, they commonly cause local inflammatory reactions after injection (20, 46). Oil-in-water emulsions have poorly defined mechanisms of action, but tissue damage and the ensuing inflammatory response may play a role (58). 1Z105 and 1V270 have well-defined mechanisms of action, stimulating key pattern recognition receptors and recapitulating signaling of natural ligands (14, 19), and demonstrated improved safety profiles compared to a squalene-based adjuvant, AddaVax, when injection site cell infiltration and systemic cytokine induction were compared. The combination of 1Z105 and 1V270 with a variety of antigens induced rapid and cross-reactive humoral and cellular immunity to elicit broad protection. Vaccine formulation provides a further means to optimize the adjuvant's safety profile and enhance its adjuvancy. Considering its efficacy and favorable safety profile, the combination of 1Z105 and 1V270 warrants further development for novel formulations of broadly protective influenza virus vaccines.
ACKNOWLEDGMENTS
We acknowledge the NIH Adjuvant Discovery Program for funding (HHSN272200900034C to D.A.C. and HHSN272200900032C to P.P.). We also thank the NIH/NIAID CEIRS CRIP program (HHSN272201400008C to P.P. and A.F.-S.) for additional funding.
We are grateful to Jonathan Runstadler for the subtype H11 and H12 viral isolates.
REFERENCES
- 1.Ohmit SE, Petrie JG, Malosh RE, Cowling BJ, Thompson MG, Shay DK, Monto AS. 2013. Influenza vaccine effectiveness in the community and the household. Clin Infect Dis 56:1363–1369. doi: 10.1093/cid/cit060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson WW, Comanor L, Shay DK. 2006. Epidemiology of seasonal influenza: use of surveillance data and statistical models to estimate the burden of disease. J Infect Dis 194:S82–S91. doi: 10.1086/507558. [DOI] [PubMed] [Google Scholar]
- 3.Izurieta HS, Thompson WW, Kramarz P, Shay DK, Davis RL, DeStefano F, Black S, Shinefield H, Fukuda K. 2000. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 4.Smith GJ, Bahl J, Vijaykrishna D, Zhang J, Poon LL, Chen H, Webster RG, Peiris JS, Guan Y. 2009. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci U S A 106:11709–11712. doi: 10.1073/pnas.0904991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, Rotthoff J, Boulton M, Victor JC. 2009. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med 361:1260–1267. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 6.Petrie JG, Ohmit SE, Johnson E, Cross RT, Monto AS. 2011. Efficacy studies of influenza vaccines: effect of end points used and characteristics of vaccine failures. J Infect Dis 203:1309–1315. doi: 10.1093/infdis/jir015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox RJ. 2013. Correlates of protection to influenza virus, where do we go from here? Hum Vaccin Immunother 9:405–408. doi: 10.4161/hv.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannoun C. 2013. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines 12:1085–1094. doi: 10.1586/14760584.2013.824709. [DOI] [PubMed] [Google Scholar]
- 9.Krammer F, Palese P. 2014. Universal influenza virus vaccines: need for clinical trials. Nat Immunol 15:3–5. doi: 10.1038/nri3797. [DOI] [PubMed] [Google Scholar]
- 10.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krammer F, Palese P, Steel J. 2015. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol 386:301–321. doi: 10.1007/82_2014_408. [DOI] [PubMed] [Google Scholar]
- 12.Koff WC, Burton DR, Johnson PR, Walker BD, King CR, Nabel GJ, Ahmed R, Bhan MK, Plotkin SA. 2013. Accelerating next-generation vaccine development for global disease prevention. Science 340:1232910. doi: 10.1126/science.1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed SG, Orr MT, Fox CB. 2013. Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 14.Chan M, Hayashi T, Mathewson RD, Nour A, Hayashi Y, Yao S, Tawatao RI, Crain B, Tsigelny IF, Kouznetsova VL, Messer K, Pu M, Corr M, Carson DA, Cottam HB. 2013. Identification of substituted pyrimido[5,4-b]indoles as selective Toll-like receptor 4 ligands. J Med Chem 56:4206–4223. doi: 10.1021/jm301694x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pu M, Hayashi T, Cottam H, Mulvaney J, Arkin M, Corr M, Carson D, Messer K. 2012. Analysis of high-throughput screening assays using cluster enrichment. Stat Med 31:4175–4189. doi: 10.1002/sim.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angelo MG, Taylor S, Struyf F, Tavares Da Silva F, Arellano F, David MP, Dubin G, Rosillon D, Baril L. 2014. Strategies for continuous evaluation of the benefit-risk profile of HPV-16/18-AS04-adjuvanted vaccine. Expert Rev Vaccines doi: 10.1586/14760584.2014.959931. [DOI] [PubMed] [Google Scholar]
- 17.Wu CC, Crain B, Yao S, Sabet M, Lao FS, Tawatao RI, Chan M, Smee DF, Julander JG, Cottam HB, Guiney DG, Corr M, Carson DA, Hayashi T. 2014. Innate immune protection against infectious diseases by pulmonary administration of a phospholipid-conjugated TLR7 ligand. J Innate Immun 6:315–324. doi: 10.1159/000355217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi T, Chan M, Norton JT, Wu CC, Yao S, Cottam HB, Tawatao RI, Corr M, Carson DA, Daniels GA. 2011. Additive melanoma suppression with intralesional phospholipid-conjugated TLR7 agonists and systemic IL-2. Melanoma Res 21:66–75. doi: 10.1097/CMR.0b013e328340ce6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan M, Hayashi T, Kuy CS, Gray CS, Wu CC, Corr M, Wrasidlo W, Cottam HB, Carson DA. 2009. Synthesis and immunological characterization of Toll-like receptor 7 agonistic conjugates. Bioconjug Chem 20:1194–1200. doi: 10.1021/bc900054q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. 2009. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 21.Krammer F, Pica N, Hai R, Margine I, Palese P. 2013. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol 87:6542–6550. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goff PH, Eggink D, Seibert CW, Hai R, Martinez-Gil L, Krammer F, Palese P. 2013. Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One 8:e79194. doi: 10.1371/journal.pone.0079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, Margine I, Albrecht RA, Palese P. 2012. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol 86:5774–5781. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, Runstadler JA, Wilson PC, Albrecht RA, Garcia-Sastre A, Palese P. 2013. Hemagglutinin stalk-based universal vaccine constructs protect against group 2 influenza A viruses. J Virol 87:10435–10446. doi: 10.1128/JVI.01715-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmed SS, Schur PH, MacDonald NE, Steinman L. 2014. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J Autoimmun 50:1–11. doi: 10.1016/j.jaut.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 26.Fox CB, Haensler J. 2013. An update on safety and immunogenicity of vaccines containing emulsion-based adjuvants. Expert Rev Vaccines 12:747–758. doi: 10.1586/14760584.2013.811188. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed SS, Plotkin SA, Black S, Coffman RL. 2011. Assessing the safety of adjuvanted vaccines. Sci Transl Med 3:93rv92. doi: 10.1126/scitranslmed.3002302. [DOI] [PubMed] [Google Scholar]
- 28.Hoebe K, Du X, Georgel P, Janssen E, Tabeta K, Kim S, Goode J, Lin P, Mann N, Mudd S, Crozat K, Sovath S, Han J, Beutler B. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 29.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. 2012. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One 7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. [DOI] [PubMed] [Google Scholar]
- 31.Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap source. Evolution 39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 32.Zuckerkandl E, Pauling L. 1965. Evolutionary divergence and convergence in proteins. Academic Press, New York, NY. [Google Scholar]
- 33.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu CC, Hayashi T, Takabayashi K, Sabet M, Smee DF, Guiney DD, Cottam HB, Carson DA. 2007. Immunotherapeutic activity of a conjugate of a Toll-like receptor 7 ligand. Proc Natl Acad Sci U S A 104:3990–3995. doi: 10.1073/pnas.0611624104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Sesma A, Marukian S, Ebersole BJ, Kaminski D, Park MS, Yuen T, Sealfon SC, Garcia-Sastre A, Moran TM. 2006. Influenza virus evades innate and adaptive immunity via the NS1 protein. J Virol 80:6295–6304. doi: 10.1128/JVI.02381-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos I, Bernal-Rubio D, Durham N, Belicha-Villanueva A, Lowen AC, Steel J, Fernandez-Sesma A. 2011. Effects of receptor binding specificity of avian influenza virus on the human innate immune response. J Virol 85:4421–4431. doi: 10.1128/JVI.02356-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster R, Cox N, Stöhr K. 2002. WHO manual on animal influenza diagnosis and surveillance. WHO/CDS/CSR/NCS/2002.5. WHO, Geneva, Switzerland. [Google Scholar]
- 38.Bartlett MS. 1937. Properties of sufficiency and statistical tests. Proc R Soc Lond A 160:268–282. doi: 10.1098/rspa.1937.0109. [DOI] [Google Scholar]
- 39.Konietschke F, Hothorn LA, Brunner E. 2012. Rank-based multiple test procedures and simultaneous confidence intervals. Electron J Stat 6:738–759. doi: 10.1214/12-EJS691. [DOI] [Google Scholar]
- 40.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox CB, Sivananthan SJ, Duthie MS, Vergara J, Guderian JA, Moon E, Coblentz D, Reed SG, Carter D. 2014. A nanoliposome delivery system to synergistically trigger TLR4 AND TLR7. J Nanobiotechnology 12:17. doi: 10.1186/1477-3155-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suet Ting Tan R, Lin B, Liu Q, Tucker-Kellogg L, Ho B, Leung BP, Ling Ding J. 2013. The synergy in cytokine production through MyD88-TRIF pathways is co-ordinated with ERK phosphorylation in macrophages. Immunol Cell Biol 91:377–387. doi: 10.1038/icb.2013.13. [DOI] [PubMed] [Google Scholar]
- 43.Hoebe K, Beutler B. 2004. LPS, dsRNA and the interferon bridge to adaptive immune responses: Trif, Tram, and other TIR adaptor proteins. J Endotoxin Res 10:130–136. doi: 10.1177/09680519040100021001. [DOI] [PubMed] [Google Scholar]
- 44.Gandhapudi SK, Chilton PM, Mitchell TC. 2013. TRIF is required for TLR4 mediated adjuvant effects on T cell clonal expansion. PLoS One 8:e56855. doi: 10.1371/journal.pone.0056855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clegg CH, Roque R, Hoeven NV, Perrone L, Baldwin SL, Rininger JA, Bowen RA, Reed SG. 2012. Adjuvant solution for pandemic influenza vaccine production. Proc Natl Acad Sci U S A 109:17585–17590. doi: 10.1073/pnas.1207308109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. 2010. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 28:1740–1745. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Moran TM, Park H, Fernandez-Sesma A, Schulman JL. 1999. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis 180:579–585. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 48.Reference deleted.
- 49.Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang ZY, Nabel GJ. 2010. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329:1060–1064. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 50.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Tan GS, Krause JC, Moran T, Stein CR, Banach D, Wrammert J, Belshe RB, Garcia-Sastre A, Palese P. 2012. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A 109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Wilson IA, Garcia-Sastre A, Moran TM, Palese P. 2010. Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A 107:18979–18984. doi: 10.1073/pnas.1013387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steel J, Lowen AC, Wang TT, Yondola M, Gao Q, Haye K, Garcia-Sastre A, Palese P. 2010. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 1:e00018–10. doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei CJ, Yassine HM, McTamney PM, Gall JG, Whittle JR, Boyington JC, Nabel GJ. 2012. Elicitation of broadly neutralizing influenza antibodies in animals with previous influenza exposure. Sci Transl Med 4:147ra114. doi: 10.1126/scitranslmed.3004273. [DOI] [PubMed] [Google Scholar]
- 54.Graves PN, Schulman JL, Young JF, Palese P. 1983. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: Unmasking of cross-reactive HA2 determinants. J Virol 126:106–116. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- 55.DiLillo DJ, Tan GS, Palese P, Ravetch JV. 2014. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, Chang CY, Davis CW, McCausland M, Elbein R, Edupuganti S, Spearman P, Andrews SF, Wilson PC, Garcia-Sastre A, Mulligan MJ, Mehta AK, Palese P, Ahmed R. 2014. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A 111:13133–13138. doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nachbagauer R, Wohlbold TJ, Hirsh A, Hai R, Sjursen H, Palese P, Cox RJ, Krammer F. 2014. Induction of broadly-reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J Virol 88:13260–13268. doi: 10.1128/JVI.02133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vono M, Taccone M, Caccin P, Gallotta M, Donvito G, Falzoni S, Palmieri E, Pallaoro M, Rappuoli R, Virgilio FD, Gregorio ED, Montecucco C, Seubert A. 2013. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci U S A 110:21095–21100. doi: 10.1073/pnas.1319784110. [DOI] [PMC free article] [PubMed] [Google Scholar]