ABSTRACT
A large double-stranded DNA (dsDNA) virus that produces occlusion bodies, typical of baculoviruses, has been described to infect crane fly larvae of the genus Tipula (Diptera, Tipulidae). Because of a lack of genomic data, this virus has remained unclassified. Electron microscopy of an archival virus isolated from Tipula oleracea, T. oleracea nudivirus (ToNV), showed irregularly shaped occlusion bodies measuring from 2 to 5 μm in length and 2 μm in middiameter, filled with rod-shape virions containing single nucleocapsids within a bilayer envelope. Whole-genome amplification and Roche 454 sequencing revealed a complete circular genome sequence of 145.7 kb, containing five direct repeat regions. We predicted 131 open reading frames, including a homolog of the polyhedrin gene encoding the major occlusion body protein of T. paludosa nucleopolyhedrovirus (NPV). BLAST searches demonstrated that ToNV had 21 of the 37 baculovirus core genes but shared 52 genes with nudiviruses (NVs). Phylogenomic analyses indicated that ToNV clearly belongs to the Nudiviridae family but should probably be assigned to a new genus. Among nudiviruses, ToNV was most closely related to the Penaeus monodon NV and Heliothis zea NV clade but distantly related to Drosophila innubia NV, the other nudivirus infecting a Diptera. Lastly, ToNV was found to be most closely related to the nuvidirus ancestor of bracoviruses. This was also reflected in terms of gene content, as ToNV was the only known exogenous virus harboring homologs of the Cc50C22.6 and 27b (Cc50C22.7) genes found in the nudiviral genomic cluster involved in bracovirus particle production.
IMPORTANCE The Nudiviridae is a family of arthropod dsDNA viruses from which striking cases of endogenization have been reported (i.e., symbiotic bracoviruses deriving from a nudivirus and the endogenous nudivirus of the brown planthopper). Although related to baculoviruses, relatively little is known about the genomic diversity of exogenous nudiviruses. Here, we characterized, morphologically and genetically, an archival sample of the Tipula oleracea nudivirus (ToNV), which has the particularity of forming occlusion bodies. Comparative genomic and phylogenomic analyses showed ToNV to be to date the closest known relative of the exogenous ancestor of bracoviruses and that ToNV should be assigned to a new genus. Moreover, we revised the homology relationships of nudiviral genes and identified a new set of 32 core genes for the Nudiviridae, of which 21 were also baculovirus core genes. These findings provide important insights into the evolutionary history of large arthropod dsDNA viruses.
INTRODUCTION
Crane fly larvae, such as Tipula oleracea Linnaeus (cabbage crane fly) or Tipula paludosa Meigen (European crane fly), can cause significant agricultural and horticultural damage (1). The need to control this insect of the order Diptera led to the identification of many infectious agents (1, 2), including viruses inducing polyhedrosis disease. Nuclear polyhedrosis is an arthropod-specific pathology typically associated with the Baculoviridae. Polyhedral occlusion bodies, containing rod-shaped viruses enclosing a large circular supercoiled double-stranded DNA (dsDNA) genome, are produced in the host nuclei. Nuclear polyhedrosis has been reported in a number of families in the order Diptera, including Culicidae (3–7), Chironomidae, Tipulidae, Sciaridae (8, 9), and possibly Phlebotomidae (10). With the exception of mosquito baculoviruses, this disease remains poorly documented in Diptera. Culex nigripalpus Nucleopolyhedrovirus (CuniNPV), infecting mosquito, has been defined as the Deltabaculovirus type species (11). The genome of CuniNPV (12) presents such sequence divergences with the other baculoviruses that until recently, it was difficult to determine the homology of all 37 baculovirus core genes (4, 13, 14).
Following Rennie's brief description (15), Smith and collaborators, in the 1950s, characterized morphologically the polyhedrosis from T. paludosa larvae (TpNPV) as being caused by a rod-shaped occluded virus replicating in the nuclei of blood cells (16–19). Later, Bergoin and collaborators further described TpNPV (20–22). The virus host range appears to be narrow, restricted to T. paludosa and possibly T. oleracea. Infected larvae show a marked pallor and delayed larval development before death (19). TpNPV polyhedra display unusual morphological, biological, and biochemical features compared to those of baculoviruses infecting Lepidoptera and Hymenoptera (19–21). The major occlusion body protein (MOBP) forming TpNPV polyhedra is 25.2 kDa in size (23, 24) and appears to be very distant from baculovirus polyhedrin/granulin based on their respective N-terminal amino acid sequences (24, 25).
Recently, viruses capable of infecting dipteran hosts have been characterized and classified into the Hytrosaviridae and Nudiviridae families (26–29), representing two new families related to the Baculoviridae (29–31). Hytrosaviruses cause salivary gland hypertrophy syndrome (SGH) and replicate within the nucleus of salivary gland cells of their dipteran hosts (26, 31). The genomes of two representative viruses, Glossina pallidipes and Musca domestica SGH viruses (GpSGHV and MdSGHV), were sequenced in 2008 (32, 33). Formerly described as nonoccluded baculoviruses (34), nudiviruses display wider host range than baculoviruses and hytrosaviruses, as they infect more insect orders as well as crustaceans (35, 36). Nudiviruses can infect all developmental stages and have varied tissue tropism. Depending on the host, the infection can be asymptomatic in both larvae and adults, lethal in larvae, and chronic in adults and can also induce malformations and sterility (35). These three viral families, which evolved over 310 million years ago (MYA) (30), share a number of structural and genomic features while retaining lineage specific characteristics (26, 28). In particular, nudiviruses can durably integrate into the genome of their hosts, as has occurred in braconid wasps ∼103 MYA, leading to bracovirus symbiosis (37, 38), a phenomenon that has also occurred independently in the brown planthopper (39).
Given this recent enrichment in insect virus systematics, our aim was to characterize an archival T. oleracea virus (ToNV) preserved in the 1950s by Kenneth M. Smith as nuclear polyhedrosis from the cabbage crane fly. Although resembling TpNPV, it was unclear to which viral family ToNV might belong. Electron microscopy was performed on ToNV occlusion bodies (OBs) for morphological and structural characterization. After whole-genome amplification, the ToNV complete genome sequence was determined by next-generation sequencing (NGS) technology, which allowed the identification of the gene encoding the major occlusion body protein. Phylogenomic analyses on shared genes were used to resolve the relationships between invertebrate large dsDNA viruses and clarify the taxonomic affiliation of ToNV.
MATERIALS AND METHODS
Viral sample.
The occluded ToNV stock solution was obtained from the historical insect virus collection held at the Natural Environment Research Council, Centre for Ecology and Hydrology (NERC-CEH, Wallingford, England). Kenneth M. Smith had deposited the virus referenced as “sample 35—T. oleracea NPV (Diptera).”
Electron microscopy.
A 30-μl aliquot of the archival sample 35 (approximately 2.6 × 107 OBs) was fixed by incubation for 24 h in 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2), washed in 1× phosphate-buffered saline (PBS), postfixed by incubation for 1 h with 2% osmium tetroxide, and dehydrated in a graded series of ethanol solutions. The scanning electron microscopy aliquot was then dried in hexamethyldisilazane. Dry pellets were sprinkled on adhesive carbon discs and sputter coated with platinum. Fixed viruses were examined with a Zeiss Ultra plus scanning electron microscope. For transmission electron microscopy, virus pellets were embedded in Epon resin (Sigma), which was allowed to polymerize for 48 h at 60°C. Ultrathin sections were cut, deposited on electron microscopy grids, and stained with 5% uranyl acetate and 5% lead citrate for examination under a JEOL 1011 transmission electron microscope.
Genome sequencing and assembly.
DNA was purified from a small aliquot of the archival sample 35 using the DNeasy kit (Qiagen). To increase the amount of viral DNA for NGS sequencing, 30 individual reactions of whole-genome amplification were performed overnight from 3 ng of starting DNA material according to the Illustra TempliPhi Amplification kit (GE Healthcare Life Sciences) instructions. Reaction mixtures were then pooled and cleaned with the QIAamp DNA minikit (Qiagen). The DNA library was made from 5 μg amplified viral DNA using the GS FLX Titanium Rapid Library Preparation kit and then sequenced on a 454 GS-FLX Titanium platform (Roche Diagnostic). Over 52,000 single-paired reads were produced. The sequences were cleaned for quality and adaptors with the 454 v2.3 analysis package (Roche Diagnostic) prior to de novo assembly with Newbler v2.6 (40). Overlapping contigs were assembled by using Geneious 6 (41). Genome finishing was performed to resolve sequence ambiguities, like homopolymers and frameshifts, on the bases of PCR and Sanger sequencing (BigDye Terminator kit and ABI Prism 3100-Avant Genetic Analyzer, Life Technologies).
Sequence analyses and genome annotation.
Whole-genome similarities were estimated using TBLASTX (42, 43) against genome sequences of representative baculoviruses, nudiviruses, and hytrosaviruses (Table 1).
TABLE 1.
Baculovirus, nudivirus, and hytrosavirus genome general features and whole-genome comparison with ToNVa
| Virus name | Abbreviation | Accession no. | Genome size (bp) | No. of genes | AT% | CD% | Reference or source | Maximum score | Total score | % Query coverage | E value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Autographa californica MNPV | AcMNPV | NC_001623 | 133,894 | 156 | 59.3 | 97.2 | 85 | 29.9 | 789 | 1 | 0.022 |
| Lymantria dispar MNPV | LdMNPV | NC_001973 | 161,046 | 164 | 42.5 | 87.5 | 86 | 77.6 | 1,307 | 2 | 2e−18 |
| Cydia pomonella GV | CpGV | NC_002816 | 123,500 | 143 | 54.7 | 90.1 | 87 | 88.6 | 2,381 | 3 | 1e−30 |
| Neodiprion sertifer NPV | NeseNPV | NC_005905 | 86,462 | 90 | 66.2 | 84.5 | 88 | 83.1 | 2,681 | 6 | 1e−30 |
| Culex nigripalpus NPV | CuniNPV | NC_003084 | 108,252 | 109 | 49.1 | 91.2 | 12 | 74.4 | 739 | 1 | 1e−13 |
| Heliothis zea NV-1 | HzNV-1 | AF451898 | 228,089 | 155 | 58.2 | 69.4 | 58 | 140 | 10,140 | 17 | 1e−107 |
| Helicoverpa zea NV-2 | HzNV-2 | NC_004156 | 231,621 | 113 | 58.1 | 68.2 | 62 | 140 | 10,223 | 17 | 3e−104 |
| Gryllus bimaculatus NV | GbNV | EF203088 | 96,944 | 98 | 72 | 93.6 | 63 | 126 | 12,781 | 14 | 2e−94 |
| Oryctes rhinoceros NV | OrNV | EU747721 | 127,615 | 139 | 58.4 | 88.5 | 59 | 141 | 5,295 | 9 | 7e−71 |
| Penaeus monodon NV | PmNV | KJ184318 | 119,638 | 115 | 65.5 | 95.6 | 64 | 220 | 10,757 | 18 | 2e−69 |
| Glossina pallidipes SGHV | GpSGHV | NC_010356 | 190,032 | 160 | 72 | 86.5 | 32 | 42.8 | 3,187 | 6 | 8e−04 |
| Musa domestica SGHV | MdSGHV | EU522111 | 124,279 | 108 | 56.5 | 90.9 | 33 | 62.9 | 1,131 | 2 | 5e−10 |
| Tipula oleracea NV | ToNV | KM610234 | 145,704 | 131 | 74.5 | 85.7 | This work |
Maximum score, total score, query coverage, and E value data are those obtained by TBLASTX against genome sequences of the baculovirus, nudivirus, and hytrosavirus representative set presented in the table. MNPV, multiple nucleopolyhedrovirus; NPV, nucleopolyhedrovirus; GV, granulovirus; NV, nudivirus; SGHV, salivary gland hypertrophy virus; CD, coding density.
Open reading frames (ORFs) encoding proteins over 50 amino acids were predicted de novo using ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) combined with FGENESV0 software from the SoftBerry platform. ORFs smaller than 50 amino acids were also taken into account when displaying clear similarity with other known viral gene products. When several ORFs could be predicted within the same region only the largest nonoverlapping ORFs were kept.
ORF similarities were identified using BLASTP, BLASTX, and/or TBLASTN (42, 43) against the NCBI nonredundant protein database (nr, August 2014, at http://blast.ncbi.nlm.nih.gov/Blast.cgi) or against a local database using the BioEdit sequence alignment editor (44). This local database was composed of all the proteins of the previously determined representative baculovirus, nudiviruses, and hytrosaviruses (Table 1). Proteins from partially sequenced nudiviruses, i.e., Penaeus vannamei NV (PvNV, accession number DQ496179), Macrobrachium rosenbergii NV (MrNV, AFP33714), and Drosophila innubia NV (DiNV, JN680861 to JN680871), and the nudiviral proteins from Cotesia congregata bracovirus (BV) (CcBV, FM201559 to FM201576, FM877774, and FM212911 to FM212915), Chelonus inanitus BV (CiBV, FM201579 to FM201597, FN543427 to FN546858, and FN594617), and Microplitis demolitor BV (MdBV, JO913492 to JO979916 and JR139425 to JR139430) were also added. Sequences were further analyzed using probabilistic methods like HMMER (HMMER program using the nr sequence database and default parameters [http://hmmer.janelia.org/search/phmmer]) or HHpred (HHpred program using default parameters [http://toolkit.tuebingen.mpg.de/hhpred], pdb70_26Jul14 database). Finally, gene product sequence alignments were done using the MAFFT plugin in Geneious (best adapted algorithm strategy according to data size and BLOSUM-62 as score matrix) and manually refined if needed.
All ORFs were named according to their putative homologs when possible; otherwise, they were named based on their location in the genome. The final annotation was conducted using ARTEMIS software (45). Sequence coding density (CD) was measured as the ratio between the base number in the coding DNA sequences over the total base number. The presence of protein-specific domains and functional sites, as well as associated patterns and profiles, was defined using InterProScan database (http://www.ebi.ac.uk/Tools/pfa/iprscan/), Motif scan (http://myhits.isb-sib.ch/cgi-bin/motif_scan), and/or CD search (at the NCBI BLAST BLASTP platform).
Early and late baculovirus-like promoters were searched within 300 nucleotides upstream of each predicted ORF translation start codon (46, 47). Early promoters were defined as either a TATA box with the sequence TATA[AT][AT][AT] alone or associated 20 to 40 nucleotides downstream with either one of two initiator motifs, CA[TG]T (E1) or CGTGC (E2). Late promoters were defined as L ([ATG]TAAG) for the baculovirus late promoter and HL (TTATAGTAT) for the HzNV-1-specific late promoter (47, 48).
Repeat regions and imperfect palindromic motifs were searched with the etandem program (http://emboss.bioinformatics.nl/cgi-bin/emboss/etandem) and MEME program suite (49) with a cutoff score at 100 and a minimal size of 20 bp. Consensus motifs were visualized with WebLogo (50). Secondary structure predictions were obtained by using mfold software with default parameters (http://mfold.rna.albany.edu/?q=mfold/DNA-Folding-Form) (51).
Phylogenomic analysis.
A phylogenomic approach was undertaken to position ToNV within the arthropod large dsDNA virus phylogeny (30). Amino acid multiple alignments were performed using the Clustal Omega program (52) on 37 nudivirus-related predicted homologs found in 16 other arthropod large dsDNA viruses, including the following: five nudiviruses, Heliothis zea NV-1 (HzNV-1), Gryllus bimaculatus NV (GbNV), Oryctes rhinoceros NV (OrNV), Penaeus monodon NV (PmNV), and the partially sequenced DiNV; five baculoviruses, Autographa californica MNPV (AcMNPV), Lymantria dispar MNPV (LdMNPV), Cydia pomonella GV (CpGV), Neodiprion sertifer NPV (NeseNPV), and CuniNPV; three bracoviruses (CcBV, CiBV, and MdBV); the endogenous nudivirus from Nilaparvata lugens (Hemiptera) (39); two hytrosaviruses (MdSGHV and GpSGHV); and one nimavirus, the Penaeus monodon white spot syndrome virus (WSSV-TH; AF369029), used as outgroup. As phylogenetic congruence tests did not show any conflicting phylogenetic signal between genes (data not shown), the 37-amino-acid (aa) multiple alignments were concatenated prior to phylogenetic reconstruction. A maximum likelihood (ML) phylogenetic analysis was done using RAxML (53) with the Whelan Goldman model of amino acid substitution and an estimated gamma distribution (WAG+G), selected using ModelGenerator (54) under the Akaike information criterion. Node supports in the ML tree were obtained from 100 bootstrap iterations.
ToNV oral infectivity.
The infectivity of the archival sample 35 was checked by bioassay on T. paludosa. Adult females, identified by DNA barcoding (55, 56), were collected in Tours (France) and allowed to lay eggs individually. Larvae were reared on wet 1.5% agar medium at 25°C and 22°C under a 14-h light (8 μE) and 10-h dark photoperiod, respectively, and 60% relative humidity. They were fed with clean organic lettuce until they reached fourth instar. Then, crane fly larvae were individually infected using 105 or 106 OBs covering 0.5-mm-diameter lettuce leaf discs and monitored until death. To purify viral particles, insect cadavers were individually crushed in 0.5% SDS and then filtered through cheesecloth to remove large cellular debris. Successions of washes using 0.5% SDS, 0.1% SDS, and then water, associated with 13,500 × g centrifugation for 10 min, were applied to the sample until the OB pellet was sufficiently clean. Finally, the pellet was resuspended in 50 μl sterile water. DNA was purified with the QIAamp DNA minikit (Qiagen) from the collected T. paludosa adult females, to check their health status, and from a 5-μl aliquot of collected OBs according to the manufacturer's standard protocol for tissues, with the exception that the proteinase K digestion step was extended (90 min at 70°C) to ensure OB dissolution. T. oleracea nudivirus PCR diagnostic was then performed using p74-specific primers (p74-F, 5′-ACCACCCGTAGACGATAAAT-3′; p74-R, 5′-AACAGAACAGGCTAATGCTG-3′). Amplifications were performed on purified DNA using 0.5 pmol · μl−1 of each primer, 2.25 mM MgCl2, 0.2 mM deoxynucleoside triphosphate (dNTP), and 1.5 unit Diamond Taq polymerase (Eurogentec) under a 35-cycle PCR program (95°C for 5 min; 35 cycles of 95°C for 60 s, 58°C for 60 s, 72°C for 90 s, and 72°C for 10 min).
Nucleotide sequence accession number.
The ToNV genome sequence has been deposited under accession number KM610234 in the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank/) (BioProject PRJNA261283).
RESULTS
General genome features.
The assembly of ToNV sequence raw data produced a 145,704-bp-long contiguous circular genome with an ∼127× average coverage. The ToNV genome size is in the range of other arthropod large dsDNA viruses (Table 1). ToNV base composition is highly AT biased, with AT making up 74.5% of the nucleotide content (Table 1). Whole-genome similarities searches revealed that ToNV is closer to HzNV-1 (E value, 1e−107) and PmNV (coverage, 18%) than to CuniNPV (E value, 1e−13; coverage, 1%) (Table 1). De novo gene prediction revealed that the ToNV genome carries 131 ORFs ranging from 111 to 4,143 bp in size (Fig. 1 and Table 2), representing a 85.7% coding density (one gene per ∼1.11 kb), similar to baculoviruses, nudiviruses, and hytrosaviruses (Table 1). These ORFs were distributed evenly on both DNA strands: 53% clockwise and 47% counterclockwise (Fig. 1 and Table 2).
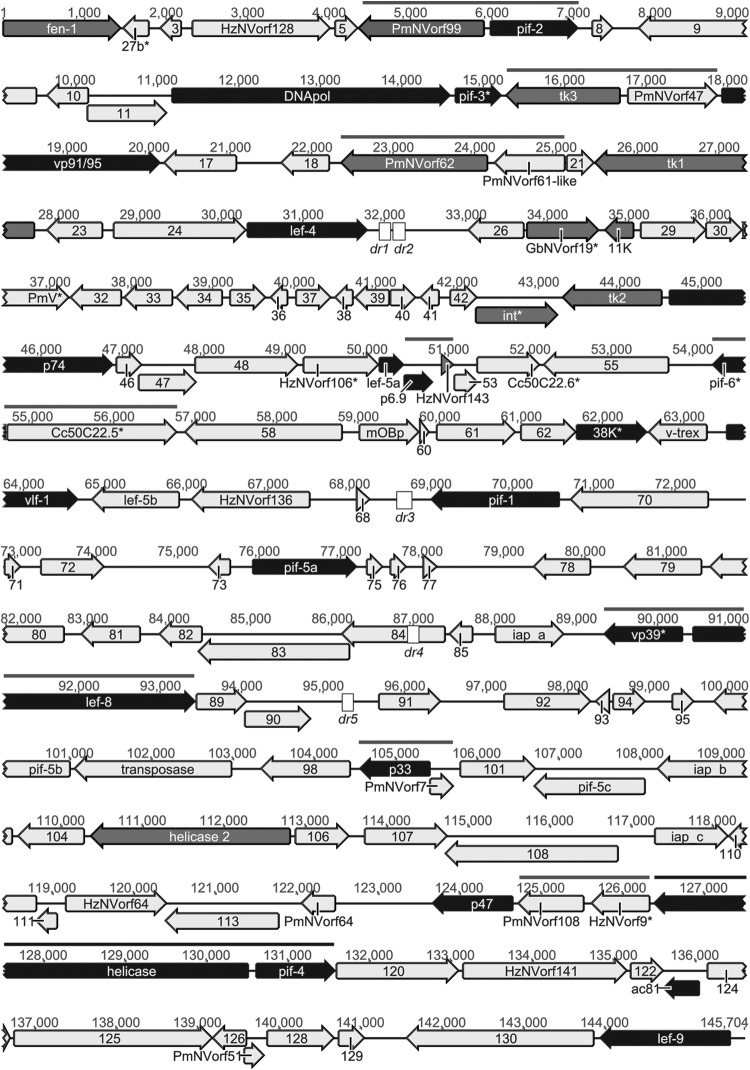
FIG 1.
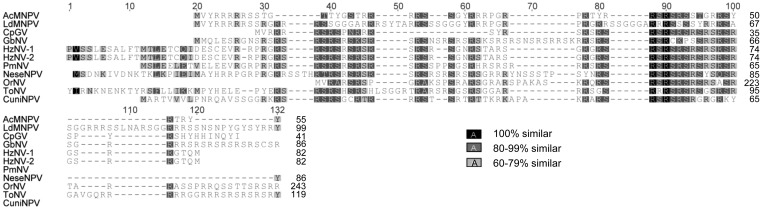
Linear map of the ToNV genome. Genes and their transcriptional directions are indicated as arrows. Black arrows, baculovirus and nudivirus shared core genes; gray arrows, nudivirus core genes. When a core gene belonged to a gene family (i.e., lef-5), only one gene was displayed in accentuated color. Thick black line, gene cluster conserved between baculoviruses and nudiviruses; thick gray lines, gene clusters conserved among several nudiviruses. Conserved genes were named based on their homologs in other virus genomes, but to simplify annotation, all “like” gene name extensions have been removed. ToNV ORFs with unknown function have been designated by their position in the genome (Table 2). *, genes belonging to the nudiviral cluster identified within C. congregata or M. demolitor wasp genomes (37, 38, 67). Direct repeat (dr) region position and name are indicated as boxes (see also Fig. 5 and Tables 3 and 4 for details).
TABLE 2.
ToNV putative ORFs and deduced protein featuresa
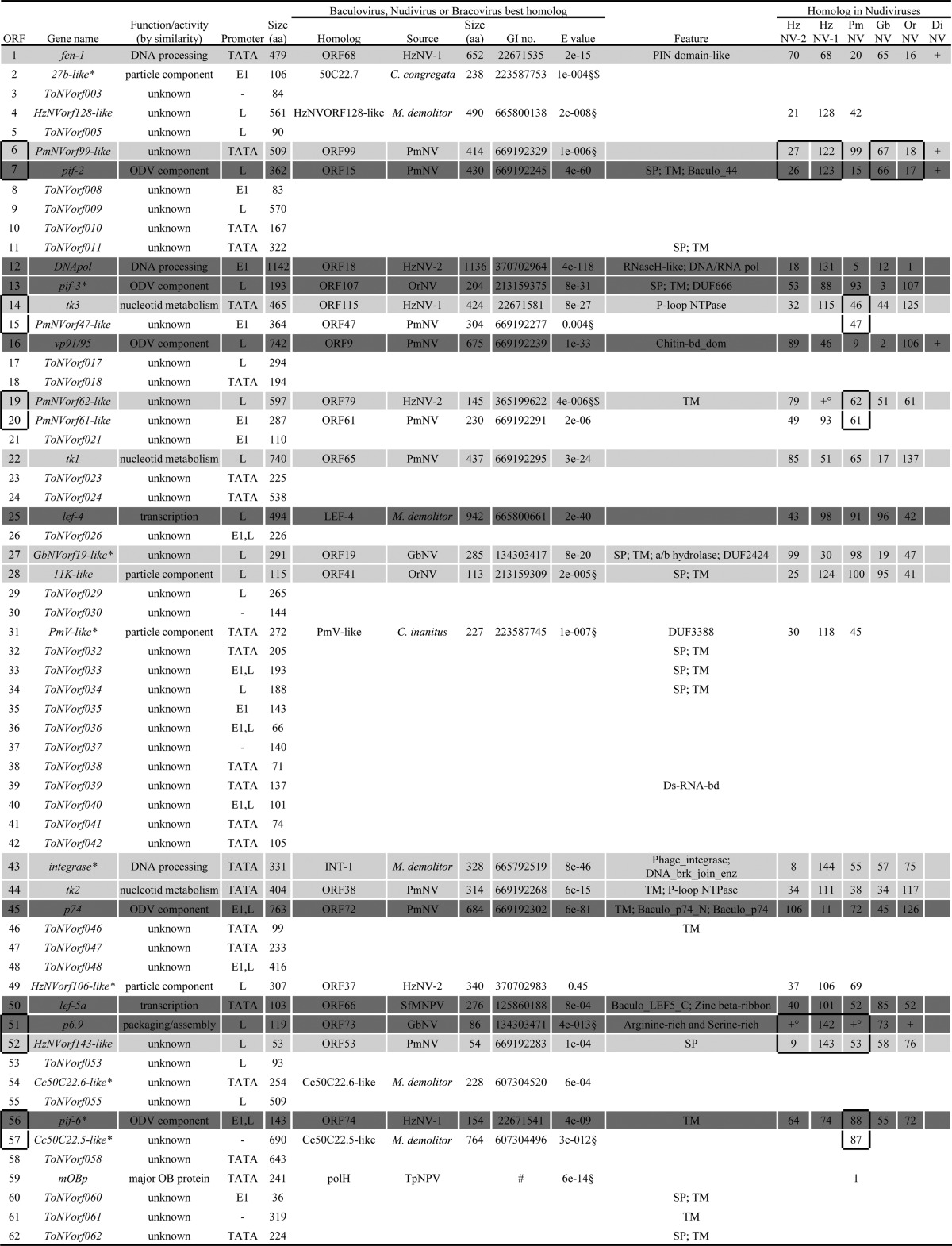
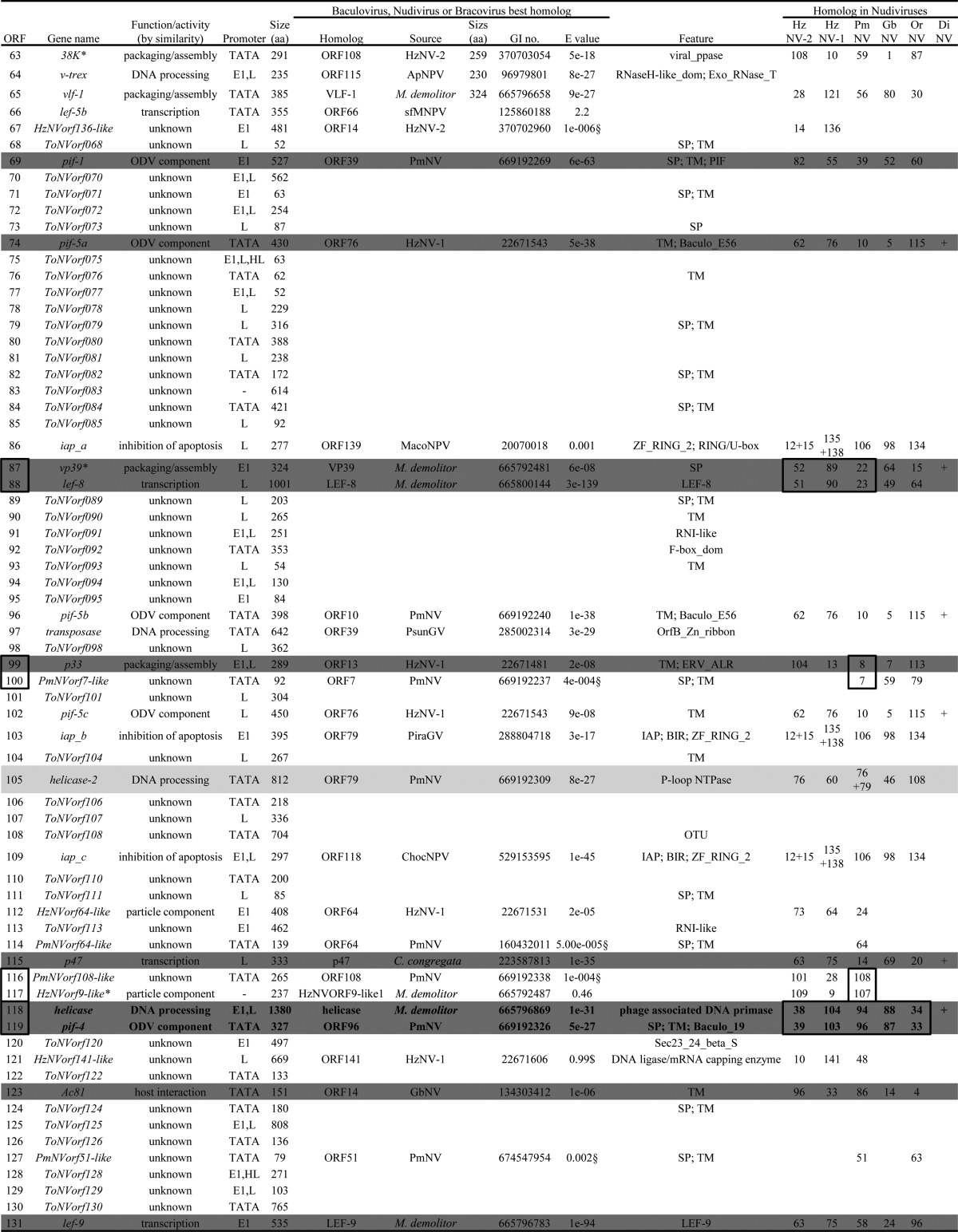
Protein functions were assigned based on protein similarity in baculovirus, nudivirus, bracovirus, and/or conserved domain identification. TATA, early promoter defined as either TATA[AT]T[AT] or TATA[AT]A[AT] alone; E1 and E2, TATA early promoter associated 20 to 40 nucleotides downstream with the initiator motifs CA[TG]T or CGTGC, respectively; L, baculovirus late promoters defined as [ATG]TAAG; HL, HzNV-1 late promoter defined as TTATAGTAT; GI no., GI accession number; “Feature” data include domains and functional sites as well as associated patterns and profiles defined using InterProScan database, Motif scan, or CD search; SP, signal peptide; TM, transmembrane regions; other domains are those defined by the previously determined tools. DiNV, Drosophila innubia NV; SfMNPV, Spodoptera frugiperda MNPV; ApNPV, Antheraea pernyi NPV; MacoNPV, Mamestra configurata NPV; PsunGV, Pseudaletia unipuncta granulovirus; PiraGV, Pieris rapae granulovirus; ChocNPV, Choristoneura occidentalis alphabaculovirus (see Table 1 for definitions of other virus name abbreviations). *, belongs to the nudiviral cluster characterized within C. congregata or M. demolitor wasp genomes (37, 38, 67); §, sequence similarity identified using local BLASTP or BLAST2 sequence comparison against a restricted protein database and global alignment for homolog confirmation (MAFFT under Geneious); $, sequence similarity identified using probabilistic methods (HMMER); +, gene present; °, gene not predicted in the genome of some nudiviruses but present; #, Rohrmann et al., 1981 (24). Boxes surrounding multiple ORF numbers indicate regions of microsynteny between ToNV and the other nudivirus genomes.
Gene content.
Similarity searches in local and public databases were performed to assign a functional annotation to each ToNV predicted ORF. Among the 131 ORF products, ∼24% presented known protein motifs, ∼20% possessed a signal peptide, and ∼30% had one to three transmembrane domains (Table 2). Fifty-seven ORFs displayed similarities with already known viral proteins, including those of baculoviruses, nudiviruses, or bracoviruses (Table 2). Assuming that viral homologs perform the same function, 7 ORFs were predicted to be involved in DNA processing, 3 in nucleotide metabolism, 6 in transcription, 5 in viral packaging, assembly, and release, 17 in viral structural proteins, including per os infectivity factors and other particle components, 1 in host interaction, 3 in apoptosis inhibition, and 15 with unknown function (Table 2). The remaining ORFs displayed either poor or no similarity with sequences available in public database (data not shown).
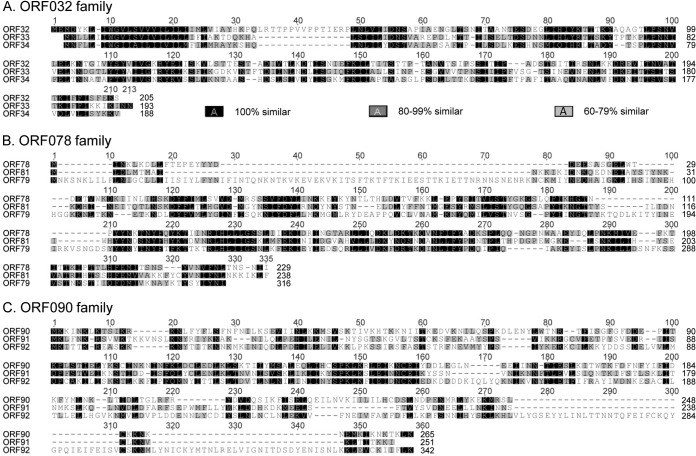
Notably, ToNV harbored several multigenic families (Table 2 and Fig. 2, 3, and 4). We found the following: two homologs of the late expression factor 5 (orf050 = lef-5a and orf066 = lef-5b), both most similar to Spodoptera frugiperda MNPV LEF-5 (Fig. 2A); three homologs of the per os infectivity factors 5 (orf074 = pif-5a, orf096 = pif-5b, and orf102 = pif-5c), most similar to HzNV-1 or PmNV PIF-5 (Fig. 2B); three homologs of the inhibitor of apoptosis IAP-3 (orf086 = iap_a, orf103 = iap_b, and orf109 = iap_c) similar to those of various organisms (Galleria mellonella, Pieris rapae GV, and Aedes aegypti, respectively) (Fig. 2C); two homologs of the viral structural protein VP91/95 (orf016 and orf083), of which ORF016 appeared to be the likely ortholog of VP91/95 and ORF083 a distant paralog (Fig. 3). Moreover, several genes of unknown function could also be gathered in three families (ORF032, ORF078, and ORF090) (Fig. 4).
FIG 2.
Amino acid sequence alignments of ToNV LEF-5 (ORF050 and ORF066), PIF-5 (ORF074, ORF096, and ORF102), and IAP-3 (ORF086, ORF103, and ORF109) multigenic family members with viral or insect homologs. (A) LEF-5 family; SfMNPV, Spodoptera frugiperda MNPV (YP_001036358); (B) PIF-5 family; HzNV-1, Heliothis zea NV-1 (AAN04370), and PmNV, Penaeus monodon NV (YP_009051848); (C) IAP-3 family; PiraGV, Pieris rapae GV (AGS18838); MacoNPV, Mamestra configurata NPV (NP_613222); Gm, Galleria mellonella (ACV04797); ChocNPV, Choristoneura occidentalis NPV (YP_008378620); Aa, Aedes aegypti (EAT39096). Alignments were generated using the MAFFT alignment plugin under Geneious and then eventually manually refined. Each multiple alignment is presented with a global numbering located above the aligned sequences and a specific numbering located on the right side of each considered sequence. The similarity color code shown on the bottom right applies to all parts of the figure.
FIG 3.
Amino acid sequence alignment of ToNV VP91 (ORF016 and ORF083) multigenic family members with baculovirus and nudivirus homologs (see the legend of Fig. 2 for details). Accession numbers: AcMNPV, NP_054113; LdMNPV, NP_047728; OrNV, YP_002321417; GbNV, YP_001111269; NeseNPV, YP_025191; HzNV-1, AAM45759; CuniNPV, NP_203339; CpGV, NP_148885 (see Table 1 for definitions of virus name abbreviations).
FIG 4.
Amino acid sequence alignments of ToNV multigenic families with unknown function. (A) ORF032 family (ORF032, ORF033, and ORF034); (B) ORF078 family (ORF078, ORF079, and ORF081); (C) ORF090 family (ORF090, ORF091, and ORF092) (see legend of Fig. 2 for details).
Promoter prediction.
The presence of baculovirus early and late gene promoters (46, 47) was investigated within the 300 bp upstream of each ORF (Table 2). Early promoter motifs either corresponding to a TATA box alone (TATA) or in association with the initiator motif CA[TG]T (E1) were predicted, respectively, for 48 and 38 ORFs. Late baculovirus promoters (L) could be predicted for 57 ORFs. Eighteen ORFs displayed both E1 and L gene promoters. The HzNV-1 late promoter (48) was predicted for two ORFs in combination with E1 and L (orf075) or only with E1 (orf128) promoters (Table 2). Moreover, a minimal TATA box (TATA[AT]) was predicted for five additional genes (orf030, orf037, orf057, orf061, and orf117). The transcriptional initiation small consensus sequence CA[TG]T was also predicted within the regions 300 bp upstream of all ORFs, except for tk1, int, and orf080.
Surprisingly and exactly opposite to what has been shown for AcMNPV (57), three of the four ToNV RNA polymerase subunit-encoding genes (p47, lef-4, and lef-8) were predicted to have a late promoter while the fourth subunit-encoding gene lef-9 was predicted to have an early promoter. Similarly, the well-characterized vp39, which has a late promoter motif in baculoviruses (57), was predicted to have an early promoter in ToNV, a late promoter in HzNV-1 (58), and both early and late promoters in OrNV (59). Thus, prediction suggested that although transcriptional regulation mechanisms might be globally conserved, particular viral genes might be regulated in different manners.
The ToNV genome contains repeat regions with imperfect palindromic motifs.
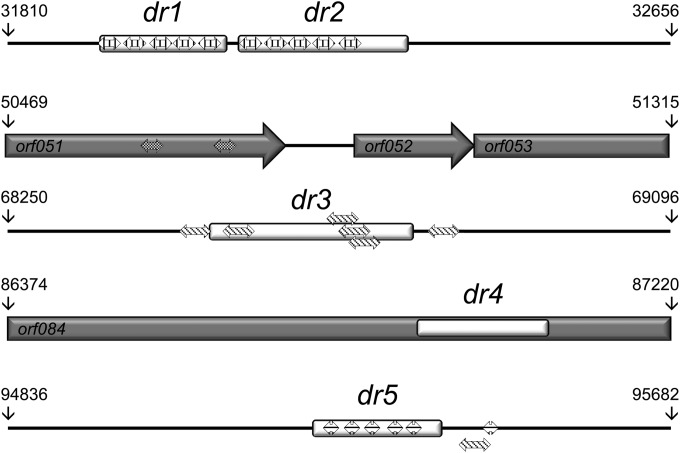
A common genome feature of the Baculoviridae is the presence of clusters of repeated imperfect palindromic AT-rich sequences called homologous repeat (hr) regions that serve as origins of viral DNA replication (60) and as transcriptional enhancers (61). Direct repeats (dr) are also common genome features in Nudiviridae (58, 59, 62–64) and Hytrosaviridae (32, 33). In the genome of ToNV, five different dr regions were detected, mainly outside coding regions, except for dr4, which was found within orf084 (Fig. 1 and 5 and Table 3); all of these were predicted to be able to form hairpin structures (data not shown). Spanning from 160 to 262 bp, the dr regions contained two to seven repeats of specific consensus motifs (Table 3). With the exception of dr4, each dr also contained clusters of two to five small (22- to 41-bp) imperfect palindromic motifs (IPMs) (Table 4 and Fig. 5). The regions dr1 and dr2, which were found located in tandem in the genome (Fig. 5), both contained clusters of five identical IPM2s (Tables 3 and 4). dr3 and dr5 also shared a minimal common 21-bp IPM (TGA[CG]TCAT[AC]G[TA]C[TG]ATGA[GC]TCA) present in six copies in each dr (data not shown). IPMs were not restricted to dr regions since IPM3 was found within orf051 (Table 4 and Fig. 5). Finally, four dr regions (1, 2, 3, and 5) were AT-rich regions composed of direct repeats encompassing clusters of small imperfect, but not all identical, palindromic sequences, reminiscent of baculovirus hr regions.
FIG 5.
Direct repeat (dr) structures and imperfect palindromic motif (IPM) positions within the ToNV genome. The five regions including dr sequences are presented to scale. Positions within the genome are indicated (see Table 3 for details). dr sequences are represented by white boxes (see Table 3 for sequence details). IPMs are represented by small striped double arrows (IPM1, oblique lines; IPM2, vertical lines; IPM3, oblique grid; IPM4, horizontal lines; see Table 4 for details). Genes are represented by gray arrows or boxes, with their ORF numbers inside.
TABLE 3.
Direct repeat identified sequences in the ToNV genomea
| Region name | Start position (bp) | Stop position (bp) | dr size (bp) | MMb size (bp) | No. of occurrences | Identity (%) | Consensus sequence |
|---|---|---|---|---|---|---|---|
| dr1 | 31,922 | 32,081 | 160 | 32 | 5 | 96.9 | ATCACTTCTTATAACTAGCACTTTAATATTTC |
| dr2 | 32,095 | 32,311 | 217 | 31 | 7 | 86.6 | ATATTTCATCACTTCTTATAACTAGCACTTA |
| dr3 | 68,604 | 68,765 | 262 | 131 | 2 | 92 | AGTCTATATGAGTCAGAGTCTATGAGTCAGAGTCTATGAGTCATCGTC |
| TATGAGTCATCGTCTATGAGTCATCGTCTATGAGTCATCATCGTCTATG | |||||||
| AGTCATAAAAATTTCATTTTTTCCAAAAAAACTA | |||||||
| dr4 | 86,896 | 87,063 | 168 | 42 | 4 | 100 | CTGATTCTTGTTCAACTGATGTTTGTTTTGTATTAATTTGTG |
| dr5 | 95,224 | 95,385 | 162 | 27 | 6 | 92 | ATGACTCATAGACAATGACTCATGTAG |
Direct repeat (dr) regions were searched using etandem with a cutoff score of 100 and a minimal size of 20 bp.
MM, minimal motif.
TABLE 4.
Imperfect palindromic motifsa
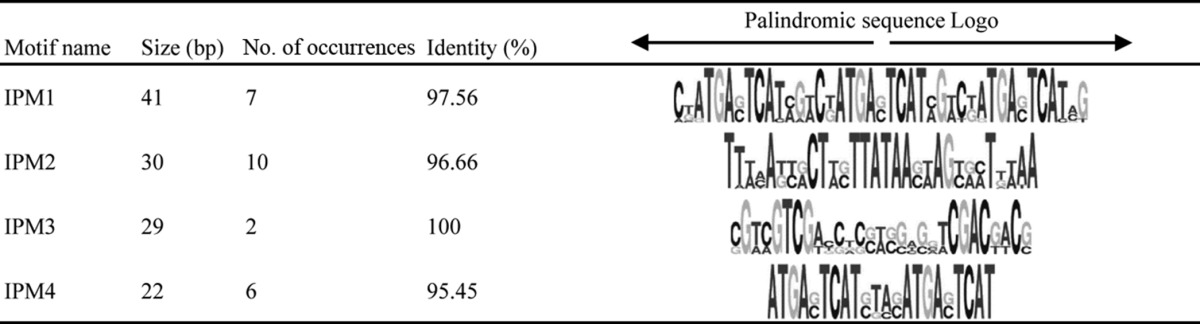
Imperfect palindromic motifs (IPMs) were determined with the MEME program suite and a minimal size of 20 bp. Consensus motifs were visualized with WebLogo.
ToNV shares 21 core genes with baculoviruses and nudiviruses.
Core genes conserved between baculoviruses and nudiviruses were found in the genome of ToNV (Table 2 and Fig. 1). These genes are involved in DNA replication, transcription, virion structure, and infectivity. Twenty genes previously identified (28), including those coding for the DNA polymerase (DNApol) and the helicase, the four RNA polymerase subunits (P47, LEF-4, LEF-8, and LEF-9), and the late expression factor 5 (LEF-5), all the per os infectivity factors (P74, PIF-1, PIF-2, PIF-3, PIF-4 [19 kDa], PIF-5 [ODV-E56], PIF-6 [Ac68], and PIF-7 [VP91/95]), Ac81, the very late factor 1 (VLF-1), the sulfhydryl oxidase (P33), the viral phosphatase (38K), and the major capsid protein (VP39), were found in the ToNV genome. The later gene had been misidentified in PmNV and annotated as the 31K structural protein (64).
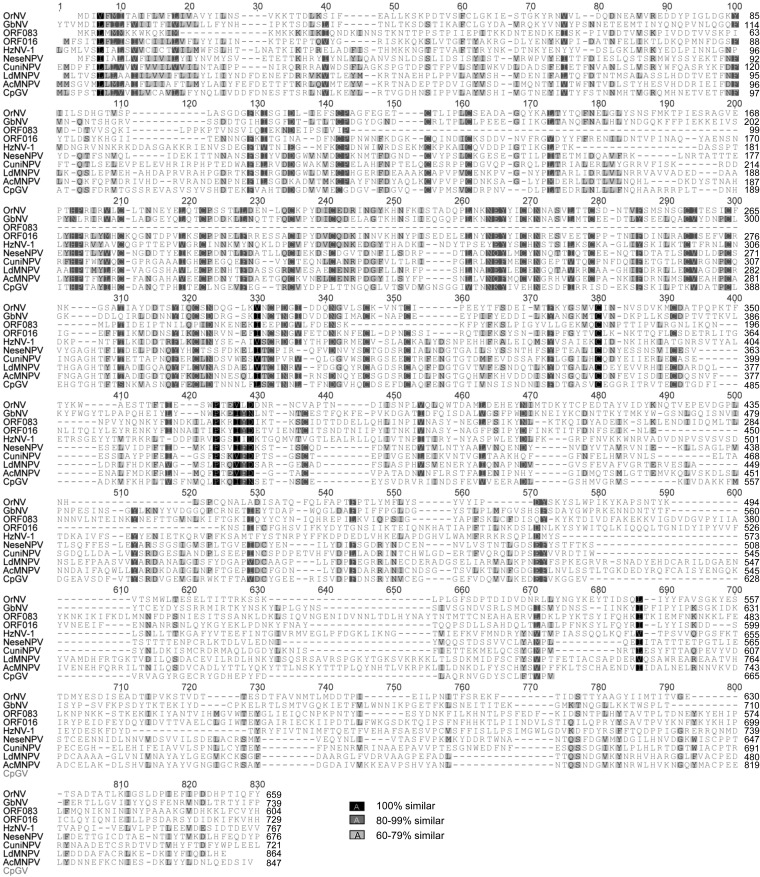
Furthermore, based on thorough comparative genomic analyses involving the reannotation of all available nudivirus genomes, an additional core gene that coded for the DNA-binding protein P6.9, a protein involved in viral packaging, assembly, and release, was identified (Fig. 6). The ORF142 (AAN04434) in HzNV-1 and ORF73 (YP_001111340) in GbNV had been predicted but not identified as P6.9. The OrNV homolog was identified as the C terminus of ORF22 (positions 176 to 243; YP_002321333), and its identification was strengthened by the synteny observed with GbNV (from OrNV ORF22 to ORF27 and from GbNV ORF72 to ORF78). P6.9 homologs were newly identified in HzNV-2 (positions 24,375 to 24,127, between ORF9 and ORF10) and in PmNV (positions 64,881 to 65,078, between ORF52 and ORF53). As ToNV ORF051 was identified as a P6.9 homolog, the set of core genes conserved between baculoviruses and nudiviruses was raised to 21 (shown in dark gray in Table 2 and in black arrows in Fig. 1).
FIG 6.
Amino acid sequence alignment of ToNV P6.9 (ORF051) with baculovirus and nudivirus homologs (see legend of Fig. 2 for details). Accession numbers: AcMNPV, NP_054130; LdMNPV, NP_047738; CpGV, NP_148870; GbNV, YP_001111340; HzNV-1, AAN04434; HzNV-2 (new prediction from positions 24,375 to 24,127), JN418988; PmNV (new prediction from positions 64,881 to 65,078), KJ184318; NeseNPV, YP_025143; OrNV, YP_002321333; CuniNPV, NP_203327 (see Table 1 for definitions of virus name abbreviations).
ToNV presents nudiviral features, including 11 specific core genes.
Eleven nudivirus core genes were found in the ToNV genome (shown in light gray in Table 2 and in gray arrows in Fig. 1). This included the helicase-2, the integrase (int), and the FEN-1/FLAP endonuclease (fen-1), three nudiviral thymidine kinases (tk1, tk2, and tk3), an 11K-like homolog (62) and four other genes of unknown function, orf006 (PmNVorf99-like), orf019 (PmNVorf62-like), orf027 (GbNVorf19-like), and orf052 (HzNVorf143-like), that were homologous to HzNV-2 orf27, orf79, orf99, and orf9 (62), respectively. Although the HzNV-2 ORF79 homolog had not been predicted in HzNV-1 (58), it was present between HzNV-1 ORF57 and ORF58 from position 79,463 to 79,900.
In contrast, all previously defined nudiviral core genes (28) were not identified in ToNV either because they were absent or because they were not monophyletic in phylogenetic analyses and therefore not orthologous (data not shown). The ribonucleotide reductases rr1 and rr2 as well as the thymidine kinase tk4 (homologous to HzNV-1 orf71 or HzNV-2 orf67) were absent in ToNV. ToNV features three inhibitor of apoptosis genes (iap-3) (i.e., orf083, orf103, and orf109), each deriving from various cellular origins (Fig. 2C), like other nudivirus iap-3 genes. As no group of iap-3 genes derived from a single viral common ancestor, they should not be considered core genes. Similarly, ToNV ORF121 displays a DNA ligase/mRNA capping enzyme domain and was phylogenetically related to HzNV-1 ORF141, HzNV-2 ORF10, and PmNV ORF48 but not to HzNV-1 ORF36, OrNV ORF121, or GbNV ORF38. DNA ligase and IAP-3 thus appear as core functions rather than core genes for nudiviruses.
Another common genome feature of baculoviruses is a conserved cluster of four core genes (helicase, pif-4, 38K, and lef-5) found in synteny in all genomes (13). This cluster was not found in ToNV; only the helicase (orf118) and pif-4 (orf119) were grouped as observed in all fully sequenced nudiviruses (28, 64) (Table 2 and Fig. 1). In addition, other nudivirus-specific gene microsyntenies were found in ToNV (Table 2 and Fig. 1).
Some ToNV genes are more similar to the endogenous nudivirus genes of bracoviruses.
Bracoviruses evolved from the integration of a nudivirus in the genome of an ancestral parasitic wasp (37, 65). The genome of the wasps Cotesia congregata and Microplitis demolitor have retained a locus, comprising at least 10 genes from the original viral genome, termed the nudiviral cluster (37, 66, 67). ToNV displayed 12 of these nudivirus-like genes present in bracoviruses, although dispersed within its genome rather than as a cluster (Fig. 1 and Table 2). Surprisingly, from the 21 core genes shared between baculoviruses and/or nudiviruses and bracoviruses, eight ToNV products showed higher similarity with bracovirus homologs than with their nudivirus or baculovirus next-best homologs (Table 2). Of note, these homologies between ToNV and bracovirus genes demonstrated that two genes found in the nudiviral cluster, 27b (Cc50C22.7) and Cc50C22.6, were genuinely of viral origin. Likewise, a hypothetical protein at the locus tag K425_450 of the nudiviral cluster of the braconid wasp M. demolitor was identified as homologous to ToNV ORF027 and GbNV ORF19, and Cc50C22.5 as homologous to PmNV ORF87. However, so far no ToNV homologs have been identified for HzNVorf94-like or 35a (37, 65–67) or for the three newly identified MdBV putative nudiviral genes (locus tags K425_456, K425_459, and K425_461) (67). ToNV thus appears as an exogenous nudivirus most closely related to the ancestral nudivirus, whose genome integrated into the ancestor of the braconid wasps, thus producing bracoviruses.
ToNV orf059 encodes a functional homolog of the baculovirus polyhedrin.
Sequence alignment revealed that ORF059 displayed 81% sequence similarity (31/38 aa) with the previously published polyhedrin N-terminal sequence from TpNPV (24) (Fig. 7A). The protein encoded by orf059 had a predicted molecular mass of 27.36 kDa, similar to that of its TpNPV homolog (23, 24). Multiple sequence alignments with baculovirus polyhedrin/granulin sequences revealed 43 conserved sites (Fig. 7A). The ToNV ORF059 sequence also displayed 58 conserved sites with two major occlusion body proteins (MOBPs) characterized from shrimp nudiviruses infecting P. monodon (64, 68) and P. vannamei (69) (Fig. 7B).
FIG 7.
Amino acid sequence alignments of baculovirus polyhedrin/granulin sequences and presumed nudivirus homologs (see legend of Fig. 2 for details). (A) Alignment of ToNV ORF059 with TpNPV N-terminal sequence previously determined (24) and AcMNPV, CpGV, and NeseNPV polyhedrin/granulin sequences. (B) Alignment of ToNV ORF059 with Penaeus species nudiviruses major OB protein sequences. (C) Alignment of ToNV ORF001 with OrNV, GbNV, and HzNV-1 nudivirus FEN-1 homologs and AcMNPV, CpGV, and NeseNPV polyhedrin/granulin sequences. Accession numbers: AcMNPV, NP_054037; CpGV, NP_148785; NeseNPV, YP_025108; PmNV, ABY75164; PvNV, DQ496179; OrNV, YP_002321327; GbNV, YP_001111332; HzNV-1, AAN04362. TpNPV, Tipula paludosa NPV (24) (see Table 1 for definitions of other virus name abbreviations).
In contrast, when ToNV ORF059 similarity searches were made against OrNV ORF16, previously identified as the nudivirus polyhedrin (70), or against GbNV ORF65 and HzNV-1 ORF68 homologs, no significant E values (0.5, 0.66, and 0.54, respectively) were obtained. However, these genes were highly similar to ToNV ORF001 (E values between 3e−10 and 9e−23), which in turn was identified as a FEN-1/FLAP endonuclease by the sensitive HHpred method with a 98.7% probability. The GbNV, OrNV, HzNV-1, and PmNV homologs were also identified as FEN-1/FLAP endonucleases with high probability (97.8% to 99.1%). Lastly, when the nudivirus FEN-1/FLAP endonuclease sequences, including that of ToNV ORF001, were multiply aligned with baculovirus polyhedrin/granulin sequences, large gaps had to be introduced in the alignment, which revealed only 13 conserved sites (Fig. 7C), largely contrasting with the 43 sites conserved with ToNV ORF059 (Fig. 7A). Similarity searches and sequence alignments therefore revealed ToNV ORF059 as the most likely nudiviral homolog of baculovirus polyhedrin/granulin.
The ToNV genome encodes infectious occlusion bodies.
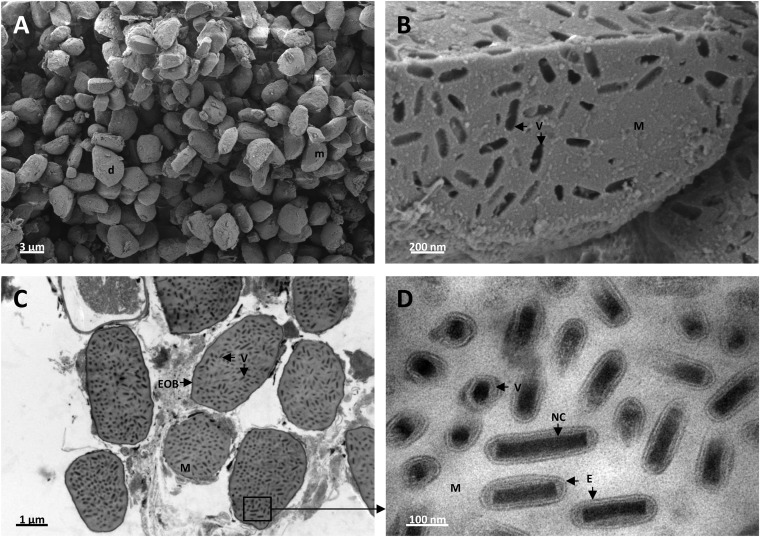
To further characterize ToNV, particles from the archival sample 35 were observed by electron microscopy (Fig. 8) and infectivity was tested by bioassay. Electron microscopy observations showed that OBs measured from 2 to 5 μm in length and 2 μm in middiameter. The OB shape varied from a droplet form to all conceivable ellipsoid forms (Fig. 8A). OBs were filled with protein matrix and many rod-shaped virions (approximately 80 nm by 225 nm) (Fig. 8B to D) containing single nucleocapsids (approximately 40 nm by 160 nm) within a bilayer envelope (Fig. 8D).
FIG 8.
Occlusion bodies, virions, and nucleocapsids from T. oleracea purified nudivirus visualized by scanning (A and B) and transmission (C and D) electron microscopies. (A) Purified ToNV OBs shaped irregularly from droplet (d) to moon (m) by way of all conceivable ovoid and ellipsoid forms. (B) Section of typical OB enlarged image showing rod-shaped virion (V) prints inside the protein matrix (M). (C) Thin cross-section of enveloped occlusion bodies (EOB) filled with numerous virions (V) and protein matrix (M). (D) Enlarged image of protein matrix (M)-embedded virions (V) displaying, mainly in cross-sectional and longitudinal views, nucleocapsids (NC) surrounded by single bilayer membranes forming the envelopes (E).
To check whether the virus characterized in this study was still infectious after nearly 60 years of frozen storage, bioassays on healthy crane fly larvae were performed. T. paludosa larvae exposed with 105 to 106 OBs died between 4 and 15 days postinfection. Visible symptoms corresponded mainly to discoloration of larva epidermis, as previously described for TpNPV (19, 20). PCR diagnostics performed on viral particles purified from individual cadavers detected the presence of ToNV, thus confirming that the historical sample was still per os infectious (data not shown).
Arthropod dsDNA virus phylogenomy.
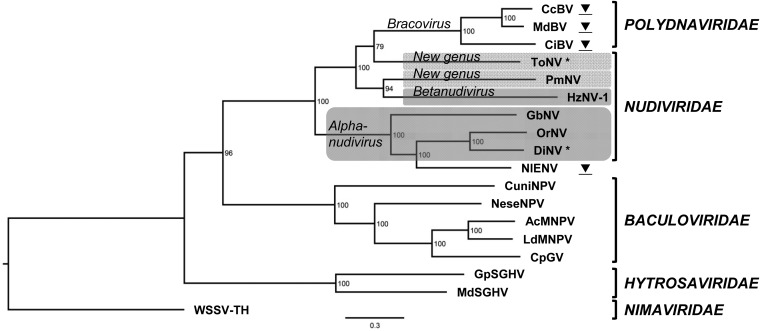
Phylogenetic analyses were performed to determine to which virus ToNV was most closely related. Based on the concatenated alignment of 37 nudivirus-related genes from 18 dsDNA viruses, including four endogenous nudiviruses (three bracoviruses and the endogenous nudivirus of N. lugens [NlENV]) (Table 5), a highly supported phylogenetic tree was obtained by maximum likelihood analyses. The interrelationships between baculovirus, nudivirus, bracovirus, and hytrosavirus families were in accordance with previous results (30, 39, 64). ToNV clearly belonged to the Nudiviridae clade and not to the Baculoviridae (Fig. 9). The tree revealed that ToNV was distant from all other nudiviruses but more closely related to the HzNV-PmNV clade, to which bracoviruses were also related (30). The fruit fly virus DiNV was close to OrNV and belonged to the Alphanudivirus genus, from which NlENV originated (39). This indicated that two nudiviruses infecting the same host order (Diptera) could be distantly related.
TABLE 5.
Sequences used for phylogenetic analysesa
| Gene name | Nudiviruses |
Baculoviruses |
Endogenous nudiviruses |
Hytrosaviruses |
Nimavirus | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ToNV | HzNV-1 | PmNV | GbNV | OrNV | DiNV | AcMNPV | LdMNPV | CpGV | NeseNPV | CuniNPV | CcBV | CiBV | MdBV | NlENV | MdSGHV | GpSGHV | WSSV-TH | |
| DNA polymerase | 12 | 131 | 5 | 12 | 1 | 65 | 83 | 111 | 28 | 91 | + | 1 | 79 | 27 | ||||
| helicase | 118 | 104 | 94 | 88 | 34 | + | 95 | 97 | 90 | 61 | 89 | + | + | |||||
| helicase-2 | 105 | 60 | 79 | 46 | 108 | 50 | 126 | + | 104 | 74 | ||||||||
| integrase | 43 | 144 | 55 | 57 | 75 | + | + | + | ||||||||||
| fen-1 | 1 | 68 | 20 | 65 | 16 | + | + | + | 76 | |||||||||
| tk1 | 22 | 51 | 65 | 17 | 137 | + | ||||||||||||
| guaK | 74 | 23 | + | + | ||||||||||||||
| p47 | 115 | 75 | 14 | 69 | 20 | + | 40 | 48 | 68 | 49 | 73 | + | + | + | ||||
| lef-8 | 88 | 90 | 23 | 49 | 64 | 50 | 51 | 131 | 81 | 26 | + | + | + | + | 70 | 40 | ||
| lef-9 | 131 | 75 | 58 | 24 | 96 | 62 | 64 | 117 | 40 | 59 | + | + | 74 | 33 | ||||
| lef-4 | 25 | 98 | 91 | 96 | 42 | 90 | 93 | 95 | 62 | 96 | + | + | + | 87 | 51 | |||
| vlf-1 | 65 | 121 | 56 | 80 | 30 | 77 | 86 | 106 | 45 | 18 | + | + | + | |||||
| vp91/95 | 16 | 46 | 9 | 2 | 106 | + | 83 | 91 | 101 | 84 | 35 | + | + | + | ||||
| vp39 | 87 | 89 | 22 | 64 | 15 | + | 89 | 92 | 96 | 89 | 24 | + | + | + | + | |||
| p33 | 99 | 13 | 8 | 7 | 113 | 92 | 94 | 93 | 24 | 14 | + | + | ||||||
| 38K | 63 | 10 | 59 | 1 | 87 | 98 | 99 | 88 | 59 | 87 | + | + | + | + | 73 | 44 | ||
| p74 | 45 | 11 | 72 | 45 | 126 | 138 | 27 | 60 | 50 | 74 | + | + | + | + | 39 | 1 | 72 | |
| pif-1 | 69 | 55 | 39 | 52 | 60 | 119 | 155 | 75 | 79 | 29 | + | + | + | 29 | 102 | |||
| pif-2 | 7 | 123 | 15 | 66 | 17 | + | 22 | 119 | 48 | 55 | 38 | + | + | + | 89 | 53 | 41 | |
| pif-3 | 13 | 88 | 93 | 3 | 107 | 115 | 143 | 35 | 69 | 46 | + | + | + | 106 | 76 | |||
| pif-4 | 119 | 103 | 96 | 87 | 33 | 96 | 98 | 89 | 60 | 90 | + | + | + | + | ||||
| pif-6 | 56 | 74 | 88 | 55 | 72 | 68 | 80 | 114 | 41 | 58 | + | + | + | |||||
| Ac81 | 123 | 33 | 86 | 14 | 4 | 81 | 89 | 103 | 48 | 106 | + | 108 | 78 | |||||
| 11K | 28 | 124 | 100 | 95 | 41 | + | + | + | + | |||||||||
| HzNVorf9 | 117 | 9 | 107 | + | + | + | ||||||||||||
| HzNVorf64 | 112 | 64 | 24 | + | + | |||||||||||||
| HzNVorf118 | 31 | 118 | 45 | + | + | + | ||||||||||||
| HzNVorf128 | 4 | 128 | 42 | + | + | + | ||||||||||||
| OrNVorf18 | 6 | 122 | 99 | 67 | 18 | + | + | |||||||||||
| OrNVorf19 | 68 | 19 | + | + | ||||||||||||||
| OrNVorf22 | 72 | 22 | + | + | ||||||||||||||
| OrNVorf25 | 76 | 25 | + | |||||||||||||||
| OrNVorf27 | 78 | 27 | + | + | ||||||||||||||
| OrNVorf44 | 97 | 44 | + | + | ||||||||||||||
| GbNVorf19 | 27 | 30 | 98 | 19 | 47 | + | ||||||||||||
| OrNVorf54 | 83 | 54 | + | |||||||||||||||
| PmNVorf62 | 19 | + | 62 | 51 | 61 | + | ||||||||||||
An ORF number, when available, or a “+” sign indicates sequences used in the analyses. Virus abbreviations and sequence accession numbers are those defined in Table 1, to which must be added the following: DiNV, Drosophila innubia NV (JN680861 to JN680871) (27); NlENV, Nilaparvata lugens endogenous NV (KJ566523 to KJ566588) (39); CcBV, Cotesia congregata BV (FM201559 to FM201576, FM877774, and FM212911 to FM212915) (37); CiBV, Chelonus inanitus BV (FM201579 to FM201597, FN543427 to FN546858, and FN594617) (37); MdBV, Microplitis demolitor BV (JO913492 to JO979916 and JR139425 to JR139430) (67, 81); WSSV-TH, Penaeus monodon white spot syndrome virus (AF369029).
FIG 9.
Arthropod large dsDNA virus phylogeny including ToNV. The tree was obtained by ML inference analysis of concatenated amino acid multiple alignments of 37 nudivirus-related genes (Table 5). Numbers on the nodes indicate ML nonparametric bootstrap supports (100 replicates). The white spot syndrome virus (WSSV-TH) was defined as an outgroup based on genome content. Virus families and genera are those approved by the International Committee on Taxonomy of Viruses (ICTV). The newly proposed and already approved nudivirus genera are shaded in gray. ▼, endogenous viral element derived from two independent nudivirus integration events, which led either to the bracovirus symbiosis or to the Nilaparvata lugens endogenous NV. *, diptera-infecting nudiviruses. Virus abbreviations, sequences, and accession numbers are those defined in Tables 1 and 5.
DISCUSSION
To date, few Nudiviridae have been genetically characterized (27, 58, 59, 62–64). This study presents the first fully sequenced occluded nudivirus from a Diptera, the crane fly T. oleracea.
ToNV is closely related to the T. paludosa NPV based on the amino acid sequence identity between ToNV ORF059 and the N-terminal sequence of TpNPV MOBP (24) (Fig. 7A). Although diverging in sequence, these proteins appear to be both genetic and functional homologs of the baculovirus polyhedrin/granulin. In contrast, no convincing similarity could be identified between ToNV ORF059 and the previously labeled polh/gran of OrNV (ORF16) (70). The genetic bases of OrNV facultative OBs therefore remain to be determined. The relatedness between TpNPV and ToNV led us to redefine the virus TpNPV studied in the 1950s to 1970s (16–23) as a nudivirus (TpNV) instead of a baculovirus. Overall, this points out the phenotypic diversity of Nudiviridae, as within this family some viruses are transmitted as enveloped (Tipula NVs [21]) or nonenveloped (Penaeus NVs [64, 68, 71]) occlusion bodies, others as nonoccluded virions (Penaeus NVs [72], HzNV-1 [73], and GbNV [34, 74]) or as facultatively occluded virions (OrNV [34] and HzNV-2 [75]).
The phylogeny of the nudiviruses highlights two monophyletic clades, the OrNV/GbNV/DiNV clade, corresponding to the Alphanudivirus genus, and a second clade grouping ToNV with HzNVs and PmNV (30) (Fig. 9). As the phylogenetic distances between HzNVs, PmNV and ToNV are much larger than within the Alphanudivirus, their affiliation to a single genus (Betanudivirus) is therefore not as clear. If the proposal that a new genus should be created for PmNV (64) is taken forward, a fourth genus of exogenous nudiviruses would also need to be created for ToNV based on phylogenetic relationships (Fig. 9).
The ToNV data greatly improve comparative genomics analyses of nudiviruses and baculoviruses and allow the revision of core and accessory gene lists for nudiviruses within a new phylogenomic framework for arthropod large dsDNA viruses. Previous studies identify 33 nudivirus core genes (28, 62). The new data show that iap-3, ligase, rr1, rr2, and tk4 should rather be considered nudivirus accessory genes, probably acquired through horizontal transfers like in baculoviruses (76). Although deriving from diverse phylogenetic origins, the DNA ligase and IAP-3 are present in all sequenced nudiviruses and might therefore perform core nudivirus functions. Although the phylogenetic relationships between the different copies of tk genes are not well established, tk1, tk2, and tk3 remain in the list of nudivirus core genes. Three new nudivirus core genes, PmNVorf099-like (orf006), PmNVorf062-like (orf019), and 11K-like (orf028), had previously been overlooked during the annotation of at least one nudivirus genome. Recent developments in genomic comparison tools have improved the detection of gene orthologs, despite relatively distant sequences, and allowed the extension of the baculovirus core genome from 31 to 37 genes (14). However, for optimal results, it is necessary to carefully reannotate the genomes to see the emergence of the true common gene set shared between and within viral lineages. As a case in point, the detection of the p6.9 gene in ToNV as well as in the other nudivirus genomes raises the pool of core genes shared by baculoviruses and nudiviruses to 21. This type of reanalysis could be applied to all large circular dsDNA viruses, which share a more or less restricted common gene set (26).
The genomes of large dsDNA viruses contain multiple gene families (77), particularly of accessory genes, which could reflect virus adaptation (77, 78), but also of some core genes. For example, many baculoviruses harbor two or three copies of Ac66 and up to 16 bro genes (Ac2) (79). Two variants of odv-e66 are described in several baculoviruses (NC_009011, NC_011616, NC_002169, NC_003529, and NC_004117) and in PmNV (ORF34 and ORF36) (64). The inhibitor of apoptosis gene family diversified in five lineages (iap-1 to iap-5) in baculoviruses (79). This is also a diverse gene family in nudiviruses, as two iap-3 genes are present in HzNVs (58, 62) and three in ToNV. All nudiviruses have two nonhomologous helicase genes: DNA helicase, belonging to the baculovirus/nudivirus core genes, and helicase-2, which is a nudivirus core gene (28), also present in both hytrosaviruses (26) and in some baculoviruses (79). The integrase superfamily is also quite diverse in nudiviruses, as it is represented by vlf-1 (HzNV-1 orf121-like), int (HzNV-1 orf144-like), and HzNV-1 orf140-like homologs and found in even greater numbers in bracoviruses (80, 81). Lastly, ToNV is the first virus for which paralogs of the lef-5 and vp91/95 core genes were found (11.1% and 22.1% identity between paralogs, respectively) (Fig. 2A and 3).
The genome of ToNV displays a number of sequence features that could be implicated in the regulation of DNA replication or gene expression. Five direct AT-rich repeat regions are predicted to form hairpin structures, similar to baculovirus hr and dr regions, which are associated with the functions of replication origin and/or of transcription enhancer (60, 61, 82–84). In the absence of transcriptomic data, gene regulation has to be inferred from comparative data with baculoviruses. Early and late baculovirus promoter motifs could be predicted for most ToNV ORFs. As shown in nudiviruses (58, 59) and in nudivirus-derived bracoviruses (37), the conservation of early, intermediate, and late baculovirus promoter motifs suggests that gene expression regulation has been globally retained during virus evolution and relies on both cellular and viral RNA polymerases (79). Based on promoter prediction, particular genes could be expressed at different times in different viruses, like the nudivirus p51 gene, which displays an HL motif in HzNV-1 (48, 58) and an E1 motif in ToNV. However, a promoter motif, in itself, is not sufficient to predict the timing of gene expression, as shown by the first comprehensive transcriptomic analysis of baculovirus gene expression (57). Gene transcription regulation should therefore not be generalized based on such predictions, and predictions should be viewed with caution until a transcriptomic study is done.
Finally, as more genomic data become available, it appears that nudiviruses have led far more intricate relationships with their hosts than baculoviruses, even though they are roughly the same age (30). ToNV seems to be an intermediary between the Alpha-, Beta- and the newly proposed Gammanudivirus genera (29, 64), as its genome displays genes specific to each genus. ToNV and PmNV are so far the only free nudiviruses with homologs to unknown genes previously identified within the C. congregata nudiviral cluster (37, 64, 66). Overall, this suggests that ToNV could belong to a new nudivirus genus. Surprisingly, two dipteran nudiviruses (ToNV and DiNV) clearly belong to different genera, which contrasts with the Alphanudivirus genus, which includes viruses infecting three insect orders. Nudiviruses also appear to be particularly prone to endogenization as recently shown with the sequences found in the planthopper genome, which derived from an Alphanudivirus (39). In an entirely independent event, the endogenization and domestication of a nudivirus more related to ToNV than to HzNVs or PmNV led to the evolution of bracoviruses (30, 65). The complex evolutionary history of the Nudiviridae will undoubtedly be revealed as their diversity is further explored. In this context, genomic data on the potential nudiviruses reported by Huger and Krieg to infect diverse insect orders would be of particular interest (34). The potential significance of nudivirus diversity will only be better understood when proteomic and functional data become available.
ACKNOWLEDGMENTS
This study was funded by European Research Council starting grant GENOVIR (205206) and supported by CEA-Genoscope (project AP2008: “The role of viruses in parasitoid wasp evolution”).
This work has also been carried out with the technical support of the Genomic and Microscopy Facilities at Université François Rabelais.
We thank Max Bergoin and Aurélien Chateigner for helpful discussion, Karine Musset for her help in Sanger sequencing, and Philippe Roingeard for his expertise in electron microscopy.
REFERENCES
- 1.Blackshaw RP, Coll C. 1999. Economically important leatherjackets of grassland and cereals: biology, impact and control. Integr Pest Manage Rev 4:143–160. doi: 10.1023/A:1009625724013. [DOI] [Google Scholar]
- 2.Taschereau E, Simard L, Brodeur J, Gelhaus J, Bélair G, Dionne J. 2009. Seasonal ecology of the European crane fly (Tipula paludosa) and species diversity of the family Tipulidae on golf courses in Québec, Canada. ITS Res J 11:681–693. [Google Scholar]
- 3.Becnel JJ, White SE, Moser BA, Fukuda T, Rotstein MJ, Undeen AH, Cockburn A. 2001. Epizootiology and transmission of a newly discovered baculovirus from the mosquitoes Culex nigripalpus and C. quinquefasciatus. J Gen Virol 82:275–282. [DOI] [PubMed] [Google Scholar]
- 4.Moser BA, Becnel JJ, White SE, Afonso C, Gerald K, Shanker S, Almira E. 2001. Morphological and molecular evidence that Culex nigripalpus baculovirus is an unusual member of the family Baculoviridae. J Gen Virol 82:283–297. [DOI] [PubMed] [Google Scholar]
- 5.Andreadis TG, Becnel JJ, White SE. 2003. Infectivity and pathogenicity of a novel baculovirus, CuniNPV from Culex nigripalpus (Diptera: Culicidae) for thirteen species and four genera of mosquitoes. J Med Entomol 40:512–517. doi: 10.1603/0022-2585-40.4.512. [DOI] [PubMed] [Google Scholar]
- 6.Perera OP, Green TB, Stevens SM, White S, Becnel JJ. 2007. Proteins associated with Culex nigripalpus nucleopolyhedrovirus occluded virions. J Virol 81:4585–4590. doi: 10.1128/JVI.02391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Araujo Coutinho CJPC, Alves R, Sanscrainte ND, DeBarros Pinto Viviani A, dos Santos PF, de Souza PAVM, de Carvalho-Mello IMVG, Becnel JJ. 2012. Occurrence and phylogenetic characterization of a baculovirus isolated from Culex quinquefasciatus in São Paulo State, Brazil. Arch Virol 157:1741–1745. doi: 10.1007/s00705-012-1372-1. [DOI] [PubMed] [Google Scholar]
- 8.Martignoni ME, Iwai PJ. 1986. A catalogue of viral diseases of insects, mites, and ticks, 4th ed USDA Forest Service General Technical Report PNW-195. USDA Forest Service, Portland, OR. [Google Scholar]
- 9.Adams JR, McClintock JT. 1991. Baculoviridae. Nuclear polyhedrosis viruses, p 87e226 InAdams JR, Bonami JR (ed), Atlas of invertebrate viruses. CRC Press, Boca Raton, FL. [Google Scholar]
- 10.Poinar G Jr, Poinar R. 2005. Fossil evidence of insect pathogens. J Invertebr Pathol 89:243–250. doi: 10.1016/j.jip.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Herniou EA, Arif BM, Becnell JJ, Blissard GW, Bonning B, Harrison R, Jehle JA, Theilmann DA, Vlak JM. 2012. Baculoviridae, p 163–173 InKing AMQ, Adams MJ, Carstens EB, Lafkowitz EJ (ed), Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, London, United Kingdom. [Google Scholar]
- 12.Afonso CL, Tulman ER, Lu Z, Balinsky CA, Moser BA, Becnel JJ, Rock DL, Kutish GF. 2001. Genome sequence of a baculovirus pathogenic for Culex nigripalpus. J Virol 75:11157–11165. doi: 10.1128/JVI.75.22.11157-11165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herniou EA, Olszewski JA, Cory JS, O'Reilly DR. 2003. The genome sequence and evolution of Baculoviruses. Annu Rev Entomol 48:211–234. doi: 10.1146/annurev.ento.48.091801.112756. [DOI] [PubMed] [Google Scholar]
- 14.Garavaglia MJ, Miele SAB, Iserte JA, Belaich MN, Ghiringhelli PD. 2012. The ac53, ac78, ac101, and ac103 genes are newly discovered core genes in the family Baculoviridae. J Virol 86:12069–12079. doi: 10.1128/JVI.01873-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rennie J. 1923. Polyhedral disease in Tipula paludosa MEIG. Proc R Soc Edinb Ser B 20:265–268. [Google Scholar]
- 16.Smith KM, Xeros N. 1954. An unusual virus disease of a dipterous larva. Nature 173:866–867. doi: 10.1038/173866a0. [DOI] [PubMed] [Google Scholar]
- 17.Smith KM. 1955. Intranuclear changes in the polyhedrosis of Tipula paludosa (Diptera). Nature 176:255. doi: 10.1038/176255a0. [DOI] [PubMed] [Google Scholar]
- 18.Smith KM. 1956. The structure of insect virus particles. J Biophys Biochem Cytol 2:301–306. doi: 10.1083/jcb.2.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KM. 1967. The various types of insect viruses and the nuclear polyhedroses, p 8–40 InSmith KM. (ed), Insect virology, 1st ed Academic Press, New York, NY. [Google Scholar]
- 20.Meynardier G, Ricou G, Bergoin M. 1964. Virose à corps d'inclusion chez Tipula paludosa (Diptera) en France. Re Pathol Vég Entomol Agric Fr 43:113–118. [Google Scholar]
- 21.Bergoin M, Guelpa B. 1977. Dissolution des inclusions du virus de la polyhedrose nucléaire du diptère Tipula paludosa MEIG. Etude ultrastructurale du virion. Arch Virol 53:243–254. [DOI] [PubMed] [Google Scholar]
- 22.Revet BMJ, Guelpa B. 1979. The genome of a baculovirus infecting Tipula paludosa (Meig.) (diptera): a high molecular weight closed circular DNA of zero superhelix density. Virology 96:633–639. [DOI] [PubMed] [Google Scholar]
- 23.Croizier G, Croizier L. 1977. Evaluation du poids moléculaire de la protéine des corps d'inclusion de divers baculovirus d'insectes. Arch Virol 55:247–250. [DOI] [PubMed] [Google Scholar]
- 24.Rohrmann GF, Pearson MN, Bailey TJ, Becker RR, Beaudreau GS. 1981. N-terminal polyhedrin sequences and occluded baculovirus evolution. J Mol Evol 17:329–333. [DOI] [PubMed] [Google Scholar]
- 25.Rohrmann GF. 1986. Polyhedrin structure. J Gen Virol 67:1499–1513. [DOI] [PubMed] [Google Scholar]
- 26.Jehle JA, Abd-Alla AMM, Wang Y. 2013. Phylogeny and evolution of Hytrosaviridae. J Invertebr Pathol 112:S62–S67. doi: 10.1016/j.jip.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Unckless RL. 2011. A DNA virus of Drosophila. PLoS One 6:e26564. doi: 10.1371/journal.pone.0026564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Bininda-Emond ORP, Jehle JA. 2012. Nudivirus genomics and phylogeny, p 33–52 InML Garcia, V Romanowski (ed), Viral genomes—molecular structure, diversity, gene expression mechanisms and host-virus interactions. InTech, Rijeka, Croatia. doi: 10.5772/27793. [DOI] [Google Scholar]
- 29.Jehle JA, Burand J, Herniou EA, Harrison R, Arif B, Thielmann D, van Oers M, Becnel J. 2013. Creation of a new Family Nudiviridae including two new genera and three species. Taxonomy Proposals http://talk.ictvonline.org/files/proposals/taxonomy_proposals_invertebrate1/m/inv04/4770.aspx. [Google Scholar]
- 30.Thézé J, Bézier A, Periquet G, Drezen J-M, Herniou EA. 2011. Paleozoic origin of insect large dsDNA viruses. Proc Natl Acad Sci U S A 108:15931–15935. doi: 10.1073/pnas.1105580108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abd-Alla AMM, Vlak JM, Bergoin M, Maruniak JE, Parker A, Burand JP, Jehle JA, Boucias DG, Hytrosavirus Study Group of the ICTV . 2009. Hytrosaviridae: a proposal for classification and nomenclature of a new insect virus family. Arch Virol 154:909–918. doi: 10.1007/s00705-009-0398-5. [DOI] [PubMed] [Google Scholar]
- 32.Abd-Alla AMM, Cousserans F, Parker A, Jehle JA, Parker N, Vlak JM, Robinson A, Bergoin M. 2008. Genome analysis of a Glossina pallidipes salivary gland hypertrophy virus (GpSGHV) reveals a novel large double-stranded circular DNA virus. J Virol 82:4595–4611. doi: 10.1128/JVI.02588-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Maruniak A, Maruniak JE, Farmerie W, Boucias DG. 2008. Sequence analysis of a non-classified, non-occluded DNA virus that causes salivary gland hypertrophy of Musca domestica, MdSGHV. Virology 377:184–196. doi: 10.1016/j.virol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huger AM, Krieg A. 1991. Baculoviridae. Nonoccluded baculoviruses, p 287–319 InAdams JR, Bonami JR (ed), Atlas of invertebrate viruses. CRC Press, Boca Raton, FL. [Google Scholar]
- 35.Burand JP. 1998. Nudiviruses, p 69–90 InMiller LK, Ball LA (ed), The insect viruses. Plenum Press, New York, NY. [Google Scholar]
- 36.Wang Y, Jehle JA. 2009. Nudiviruses and other large, double-stranded circular DNA viruses of invertebrates: new insights on an old topic. J Invertebr Pathol 101:187–193. doi: 10.1016/j.jip.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Bézier A, Annaheim M, Herbinière J, Wetterwald C, Gyapay G, Bernard-Samain S, Wincker P, Roditi I, Heller M, Belghazi M, Pfister-Wilhem R, Periquet G, Dupuy C, Huguet E, Volkoff AN, Lanzrein B, Drezen J-M. 2009. Polydnaviruses of braconid wasps derive from an ancestral nudivirus. Science 323:926–930. doi: 10.1126/science.1166788. [DOI] [PubMed] [Google Scholar]
- 38.Bézier A, Louis F, Jancek S, Periquet G, Thézé J, Gyapay G, Musset K, Lesorbe J, Lenoble P, Dupuy C, Gundersen-Rindal D, Herniou EA, Drezen J-M. 2013. Functional endogenous viral elements in the genome of the parasitoid wasp Cotesia congregata: insights into the evolutionary dynamics of bracoviruses. Phil Trans R Soc B 368:20130047. doi: 10.1098/rstb.2013.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng R-L, Lou Y-H, Gu L-Z, Wang Z, Xu J-Y, Xu H-J, Zhang C-X. 2014. The brown planthopper nudivirus DNA integrated in its host genome. J Virol 88:5310–5318. doi: 10.1128/JVI.03166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, Dewell SB, Du L, Fierro JM, Gomes XV, Godwin BC, He W, Helgesen S, Ho CH, Irzyk GP, Jando SC, Alenquer MLI, Jarvie TP, Jirage KB, Kim JB, Knight JR, Lanza JR, Leamon JH, Lefkowitz SM, Lei M, Li J, Lohman KL, Lu H, Makhijani VB, McDade KE, McKenna MP, Myers EW, Nickerson E, Nobile JR, Plant R, Puc BP, Ronan MT, Roth GT, Sarkis GJ, Simons JF, Simpson JW, Srinivasan M, Tartaro KR, Tomasz A, Vogt KA, Volkmer GA, Wang SH, Wang Y, Weiner MP, Yu P, Begley RF, Rothberg JM. 2005. Genome sequencing in microfabricated high-density picolitre reactors. Nature 437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. 2012. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altschul SF, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schäffer AA, Yu YK. 2005. Protein database searches using compositionally adjusted substitution matrices. FEBS J 272:5101–5109. doi: 10.1111/j.1742-4658.2005.04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser (Oxf) 41:95–98. [Google Scholar]
- 45.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 46.Xing K, Deng R, Wang J, Feng J, Huang M, Wang X. 2005. Analysis and prediction of baculovirus promoter sequences. Virus Res 113:64–71. doi: 10.1016/j.virusres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 47.Passarelli AL, Guarino LA. 2007. Baculovirus late and very late gene regulation. Curr Drug Targets 8:1103–1115. doi: 10.2174/138945007782151324. [DOI] [PubMed] [Google Scholar]
- 48.Guttieri MC, Burand JP. 2001. Location, nucleotide sequence, and regulation of the p51 late gene of the Hz-1 insect virus: identification of a putative late regulatory element. Virus Genes 23:17–25. doi: 10.1023/A:1011166926225. [DOI] [PubMed] [Google Scholar]
- 49.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME Suite: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zuker M. 2003. Mfold web server for Nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 54.Keane TM, Creevey CJ, Pentony MM, Naughton TJ, Mclnerney JO. 2006. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol Biol 6:29. doi: 10.1186/1471-2148-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299. [PubMed] [Google Scholar]
- 56.Ratnasingham S, Hebert PDN. 2007. BOLD: the Barcode of Life Data System. Mol Ecol Notes 7:355–364. doi: 10.1111/j.1471-8286.2007.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y-R, Zhong S, Fei Z, Hashimoto Y, Xiang JZ, Zhang S, Blissard GW. 2013. The transcriptome of the baculovirus Autographa californica multiple nucleopolyhedrovirus in Trichoplusia ni cells. J Virol 87:6391–6405. doi: 10.1128/JVI.00194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng CH, Liu SM, Chow TY, Hsiao YY, Wang DP, Huang JJ, Chen HH. 2002. Analysis of the complete genome sequence of the Hz-1 virus suggests that it is related to members of the Baculoviridae. J Virol 76:9024–9034. doi: 10.1128/JVI.76.18.9024-9034.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, van Oers MM, Crawford AM, Vlak JM, Jehle JA. 2007. Genomic analysis of Oryctes rhinoceros virus reveals genetic relatedness to Heliothis zea virus 1. Arch Virol 152:519–531. doi: 10.1007/s00705-006-0872-2. [DOI] [PubMed] [Google Scholar]
- 60.Pearson M, Bjornson R, Pearson G, Rohrmann G. 1992. The Autographa californica baculovirus genome: evidence for multiple replication origins. Science 257:1382–1384. [DOI] [PubMed] [Google Scholar]
- 61.Guarino LA, Summers MD. 1986. Interspersed homologous DNA of Autographa californica nuclear polyhedrosis virus enhances delayed-early gene expression. J Virol 60:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burand JP, Kim W, Afonso CL, Tulman ER, Kutish GF, Lu Z, Rock DL. 2012. Analysis of the genome of the sexually transmitted insect virus Helicoverpa zea nudivirus 2. Viruses 4:28–61. doi: 10.3390/v4010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Kleespies RG, Huger AM, Jehle JA. 2007. The genome of Gryllus bimaculatus nudivirus indicates an ancient diversification of baculovirus-related nonoccluded nudiviruses of insects. J Virol 81:5395–5406. doi: 10.1128/JVI.02781-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang YT, Lee DY, Wang Y, Hu JM, Li WH, Leu JH, Chang GD, Ke HM, Kang ST, Lin SS, Kou GH, Lo CF. 2014. The genome and occlusion bodies of marine Penaeus monodon nudivirus (PmNV, also known as MBV and PemoNPV) suggest that it should be assigned to a new nudivirus genus that is distinct from the terrestrial nudiviruses. BMC Genomics 15:628. doi: 10.1186/1471-2164-15-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herniou EA, Huguet H, Thézé J, Bézier A, Periquet G, Drezen J-M. 2013. When parasitic wasps hijacked viruses: genomic and functional evolution of polydnaviruses. Phil Trans R Soc B 368:20130051. doi: 10.1098/rstb.2013.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bézier A, Herbinière J, Lanzrein B, Drezen J-M. 2009. Polydnavirus hidden face: the genes producing virus particles of parasitic wasps. J Invertebr Pathol 101:194–203. doi: 10.1016/j.jip.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Burke GR, Walden KKO, Whitfield JB, Robertson HM, Strand MS. 2014. Widespread genome reorganization of an obligate virus mutualist. PLoS Genet 9:e1004660. doi: 10.1371/journal.pgen.1004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaivisuthangkura P, Tawilert C, Tejangkura T, Rukpratanporn S, Longyant S, Sithigorngul W, Sithigorngul P. 2008. Molecular isolation and characterization of a novel occlusion body protein gene from Penaeus monodon nucleopolyhedrovirus. Virology 381:261–267. doi: 10.1016/j.virol.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 69.Bonami JR, Bruce LD, Poulos BT, Mari J, Lightner DV. 1995. Partial characterization and cloning of the genome of PvSNPV (=BP-type virus) pathogenic for Penaeus vannamei. Dis Aquat Org 23:59–66. doi: 10.3354/dao023059. [DOI] [Google Scholar]
- 70.Wang Y, Bininda-Emonds ORP, van Oers MM, Vlak JM, Jehle JA. 2011. The genome of Oryctes rhinoceros nudivirus provides novel insight into the evolution of nuclear arthropod-specific large circular double-stranded DNA viruses. Virus Genes 42:444–456. doi: 10.1007/s11262-011-0589-5. [DOI] [PubMed] [Google Scholar]
- 71.Bonami JR, Aubert H, Mari J, Poulos BT, Lightner DV. 1997. The polyhedral of the occluded baculoviruses of marine decapod crustacean: a unique structure, crystal organization, and proposed model. J Struct Biol 120:134–145. [DOI] [PubMed] [Google Scholar]
- 72.Couch JA. 1974. Free and occluded virus, similar to Baculovirus, in hepatopancreas of pink shrimp. Nature 247:229–231. [Google Scholar]
- 73.Granados RR, Nguyen T, Cato B. 1978. An insect cell line persistently infected with baculovirus-like particle. Intervirology 10:309–317. [DOI] [PubMed] [Google Scholar]
- 74.Huger AM. 1985. A new virus disease of crickets (Orthoptera: Gryllidae) causing macronucleosis of fatbody. J Invertebr Pathol 45:108–111. [Google Scholar]
- 75.Raina AK, Adams JR, Lupiani B, Lynn DE, Kim W, Burand JP, Dougherty EM. 2000. Further characterization of the Gonad-Specific Virus of corn eatworm, Helicoverpa zea. J Invertebr Pathol 76:6–12. doi: 10.1006/jipa.2000.4942. [DOI] [PubMed] [Google Scholar]
- 76.Hughes AL, Friedman R. 2003. Genome-wide survey for genes horizontally transferred from cellular organisms to baculoviruses. Mol Biol Evol 20:979–987. doi: 10.1093/molbev/msg107. [DOI] [PubMed] [Google Scholar]
- 77.Thézé J, Takatsuka J, Li Z, Gallais J, Doucet D, Arif B, Nakai M, Herniou EA. 2013. New insights into the evolution of Entomopoxvirinae from the complete genome sequences of four entomopoxviruses infecting Adoxophyes honmai, Choristoneura biennis, Choristoneura rosaceana, and Mythimna separata. J Virol 87:7992–8003. doi: 10.1128/JVI.00453-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elde NC, Child SJ, Eickbush MT, Kitzman JO, Rogers KS, Shendure J, Geballe AP, Malik HS. 2012. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell 150:831–841. doi: 10.1016/j.cell.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rohrmann GF. 2013. Baculovirus molecular biology, 3rd ed National Library of Medicine (US), National Center for Biotechnology Information, Bethesda, MD. [PubMed] [Google Scholar]
- 80.Drezen J-M, Herniou EA, Bézier A. 2012. Evolutionary progenitors of bracoviruses, p 15–31 InBeckage NE, Drezen JM (ed), Parasitoid viruses symbionts and pathogens. CRC Press-Elsevier, San Diego, CA. [Google Scholar]
- 81.Burke GR, Strand MR. 2012. Deep sequencing identifies viral and wasp genes with potential roles in replication of Microplitis demolitor bracovirus. J Virol 86:3293–3306. doi: 10.1128/JVI.06434-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee H, Krell PJ. 1994. Reiterated DNA fragments in defective genomes of Autographa californica nuclear polyhedrosis virus are competent for AcMNPV-dependent DNA replication. Virology 202:418–429. [DOI] [PubMed] [Google Scholar]
- 83.Habib S, Hasnain SE. 2000. Differential activity of two non-hr origins during replication of the baculovirus Autographa californica nuclear polyhedrosis virus genome. J Virol 74:5182–5189. doi: 10.1128/JVI.74.11.5182-5189.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cvetic C, Walter JC. 2005. Eukaryotic origins of DNA replication: could you please be more specific? Semin Cell Dev Biol 16:343–353. doi: 10.1016/j.semcdb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 85.Ayres MD, Howard SC, Kuzio J, Lopez-Ferber M, Possee RD. 1994. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology 202:586–605. [DOI] [PubMed] [Google Scholar]
- 86.Kuzio J, Pearson MN, Harwood SH, Funk CJ, Evans JT, Slavicek JM, Rohrmann GF. 1999. Sequence and analysis of the genome of a baculovirus pathogenic for Lymantria dispar. Virology 253:17–34. [DOI] [PubMed] [Google Scholar]
- 87.Luque T, Finch R, Crook N, O'Reilly DR, Winstanley D. 2001. The complete sequence of the Cydia pomonella granulovirus genome. J Gen Virol 82:2531–2547. [DOI] [PubMed] [Google Scholar]
- 88.Garcia-Maruniak A, Maruniak J, Zanotto PMA, Doumbouya AE, Liu J-C, Meritt TM, Lanoie JS. 2004. Sequence analysis of the genome of the Neodiprion sertifer nucleopolyhedrovirus. J Virol 78:7036–7051. doi: 10.1128/JVI.78.13.7036-7051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]