ABSTRACT
Ebola viruses (EBOV) cause severe disease in humans and nonhuman primates with high mortality rates and continue to emerge in new geographic locations, including several countries in West Africa, the site of a large ongoing outbreak. Phosphorodiamidate morpholino oligomers (PMOs) are synthetic antisense molecules that are able to target mRNAs in a sequence-specific fashion and suppress translation through steric hindrance. We previously showed that the use of PMOs targeting a combination of VP35 and VP24 protected rhesus monkeys from lethal EBOV infection. Surprisingly, the present study revealed that a PMOplus compound targeting VP24 alone was sufficient to confer protection from lethal EBOV infection but that a PMOplus targeting VP35 alone resulted in no protection. This study further substantiates recent data demonstrating that VP24 may be a key virulence factor encoded by EBOV and suggests that VP24 is a promising target for the development of effective anti-EBOV countermeasures.
IMPORTANCE
Several West African countries are currently being ravaged by an outbreak of Ebola virus (EBOV) that has become a major epidemic affecting not only these African countries but also Europe and the United States. A better understanding of the mechanism of virulence of EBOV is important for the development of effective treatments, as no licensed treatments or vaccines for EBOV disease are currently available. This study of phosphorodiamidate morpholino oligomers (PMOs) targeting the mRNAs of two different EBOV proteins, alone and in combination, demonstrated that targeting a single protein was effective at conferring a significant survival benefit in an EBOV lethal primate model. Future development of PMOs with efficacy against EBOV will be simplified if only one PMO is required instead of a combination, particularly in terms of regulatory approval.
Observation
Ebola virus (EBOV) is a member of the family Filoviridae that causes sporadic outbreaks of severe hemorrhagic fever with high case fatality rates (1). The current outbreak of Ebola virus disease (EVD) in West Africa is the first major outbreak of EVD in this part of the African continent and the largest on record, in terms of both numbers of cases and geographic distribution (2, 3). In addition to natural outbreaks of EVD, the highly virulent nature of EBOV and other viruses from this family raises concerns that filoviruses may be used as biological weapons (4). In spite of many efforts to develop vaccines and antivirals against filoviruses, there are currently no licensed medical countermeasures against these viruses. Phosphorodiamidate morpholino oligomers (PMOs) are synthetic antisense molecules designed to bind to RNA in order to specifically affect the expression of selected genes by steric blockade. Further, PMOs have been used to modulate pre-mRNA splicing or to inhibit mRNA translation (5). We previously showed that a combination of ionized analogues of PMOs (PMOplus), displaying positively charged pendant piperazinyl phosphorodiamidate linkages, protects mice against lethal EBOV challenge when given 4 h prior to viral challenge (5). Furthermore, we showed that a preparation (AVI-6002) containing two PMOplus compounds, one targeting VP24 (AVI-7537) and another targeting VP35 (AVI-7539), protected >60% of rhesus monkeys against lethal EBOV infection when given 30 to 60 min postinfection (p.i.) (6). EBOV VP24 and VP35 genes are of particular interest as therapeutic targets because they are involved in downregulating the host immune response to EBOV infection (7). VP24 functions as an antagonist of both the type I alpha/beta interferon (IFN-α/β) and type II IFN-γ affecting the normal immune response by specifically binding to karyopherin alpha 5 (KPNA) and directly inhibiting the nuclear transport of tyrosine phosphorylated STAT1, thus blocking the effect of exogenous IFN (8). VP35 functions as an antagonist of the type I IFN production (9, 10).
In order to determine the minimum PMO component(s) required for protection against EBOV in a nonhuman primate (NHP) disease model, we evaluated the efficacy of utilizing AVI-7537 or AVI-7539 alone compared to that of the AVI-6002 combination as a postexposure prophylactic against EBOV infection in rhesus monkeys.
Rhesus monkeys (6 to 8 per group) were challenged with an intramuscular target dose of 1,000 PFU of Ebola virus (Homo sapiens-tc/COD/1995/Kikwit) and then administered 40 mg/kg of AVI-7537, 40 mg/kg of AVI-7539, or 40 mg/kg of AVI-6002 intravenously (0.5 ml/kg animal weight in 0.9% sodium chloride) for 1 h ± 30 min after viral challenge once daily for 14 consecutive days (Table 1). Animals in the saline control group were administered sterile saline. Monkeys were randomized at the time of group assignment, and study personnel were blinded to the treatment administered to each group. Research was conducted under an IACUC-approved protocol in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals (11). The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
TABLE 1 .
Study designa
| Group | Treatment | PMOplus dose (mg/kg) | Gene target(s) | No. of monkeys (male/female) |
|---|---|---|---|---|
| 1 | AVI-7537 | 40 | VP24 | 8 (4/4) |
| 2 | AVI-7539 | 40 | VP35 | 8 (4/4) |
| 3 | AVI-6002 | 40 | VP24, VP35 | 8 (4/4) |
| 4 | Saline | 0 | NA | 6 (3/3) |
All animals were challenged with EBOV (Homo sapiens-tc/COD/1995/Kikwit) by the intravenous route and treated daily from day 0 to day 13. NA, not applicable.
Whereas the control animals developed progressive clinical signs consistent with EBOV hemorrhagic fever and succumbed to infection with a median time to death of 8.5 days p.i., five of eight animals (62.5%) receiving the combination compound AVI-6002 survived EBOV infection until the end of the study on day 41 p.i. These results are consistent with our previous finding of 62.5% survival in rhesus monkeys given AVI-6002 (6). While six of eight animals (75%) treated with AVI-7537 alone (targeting VP24) survived until the end of the study, none of the animals treated with AVI-7539 alone (targeting VP35) survived beyond day 10 p.i. (median survival time of 8 days) (Fig. 1A). The survival times of the AVI-6002 and AVI-7537 groups were significantly different (P ≤ 0.0002) from those of the vehicle-treated control groups, as determined using the log-rank Mantel-Cox test.
FIG 1 .
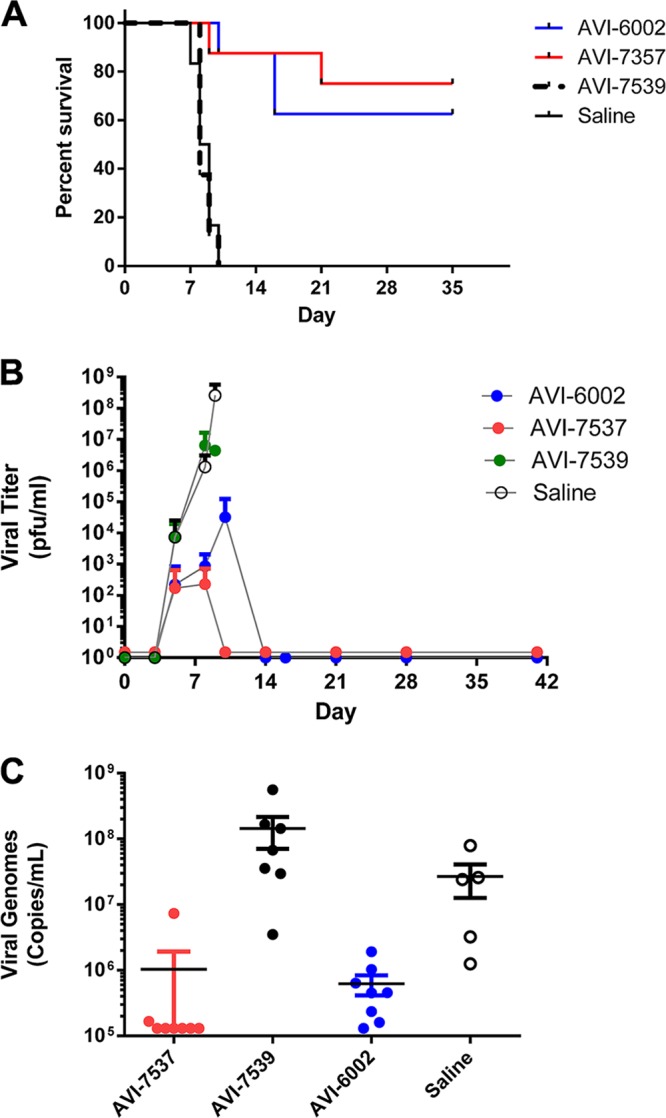
Postexposure protection and viral load determination of EBOV-infected rhesus monkeys treated with PMOplus compounds. (A) Kaplan-Meier survival curves showing statistically significantly (P ≤ 0.0002) higher survival rates for rhesus monkeys treated with either AVI-6002 or AVI-7537 than for those treated with either AVI-7539 or the saline control. (B) Mean serum viral titers (PFU/ml; as measured by plaque assay) on days 0, 3, 5, 8, 10, 14, 21, 28, and 41 p.i. presented as the log10 and standard deviations (vertical bars) (n = 8). (C) Mean serum viral genome copies (genome copies per ml; as measured by qRT-PCR) indicated by a horizontal line for each group on day 8 (day of peak viremia) p.i., with results for each individual represented by a circle. Values below the lower limit of detection for this assay (1.33 × 105 copies/ml) have been converted to 1.3 × 105 for display purposes. Statistical significance determinations by ANOVA (P < 0.030) and Tukey's multiple-comparison test for day 8 comparisons of groups were as follows: AVI-6002 versus AVI-7537, P > 0.9999; AVI-6002 versus AVI-7539, P = 0.042; AVI-6002 versus saline, P = 0.965; AVI-7537 versus AVI-7539, P = 0.043; AVI-7537 versus saline, P = 0.967; and AVI-7539 versus saline, P = 0.198.
Viral titer (PFU/ml) was determined in serum by plaque assay on days 0, 3, 5, 8, 10, 14, 21, and 41 p.i. (Fig. 1B). Analysis of variance (ANOVA) using the Mann-Whitney test on cumulative viral titers through day 8 (peak viremia day) revealed no significant difference between AVI-6002 and AVI-7537 results, but the results for both AVI-6002 and AVI-7537 were significantly different (P < 0.001) from those for AVI-7539 and the saline control group. Quantitative reverse transcriptase PCR (qRT-PCR) analysis indicated that AVI-6002 and AVI-7537 were similar in ability to reduce the number of viral genome copies in sera, in agreement with the plaque assay data. Six out of eight animals treated with AVI-7537 had no detectable viral RNA in their sera on day 8 (day of peak viremia) p.i., while all of the surviving animals in the AVI-7539-treated and saline control groups had at least 106 viral genome copies per ml in their sera on day 8 (Fig. 1C). Comparisons of individual animals’ viral genome levels on day 8 showed that animals treated with AVI-7537 alone had significantly lower viral RNA than those treated with AVI-7539 alone (P = 0.043), similar to levels observed with the combination treatment (Fig. 1C).
As liver and kidney damage are hallmarks of filovirus infection in humans and nonhuman primates, we examined the serum liver enzymes aspartate amino transferase (AST) and alanine aminotransferase (ALT) and blood urea nitrogen (BUN) in these animals (Fig. 2). On day 8, serum BUN levels were significantly reduced (P < 0.05) in animals treated with AVI-7537 or the AVI-6002 combination (i.e., targeting both VP24 and VP35) compared to treatment with AVI-7539 alone or the saline controls (Fig. 2A). Similar to the BUN levels, both liver enzymes were significantly reduced (P < 0.05) in animals treated with AVI-7537 alone or the combination AVI-6002 compared to treatment with AVI-7539 alone (Fig. 2B and C).
FIG 2 .
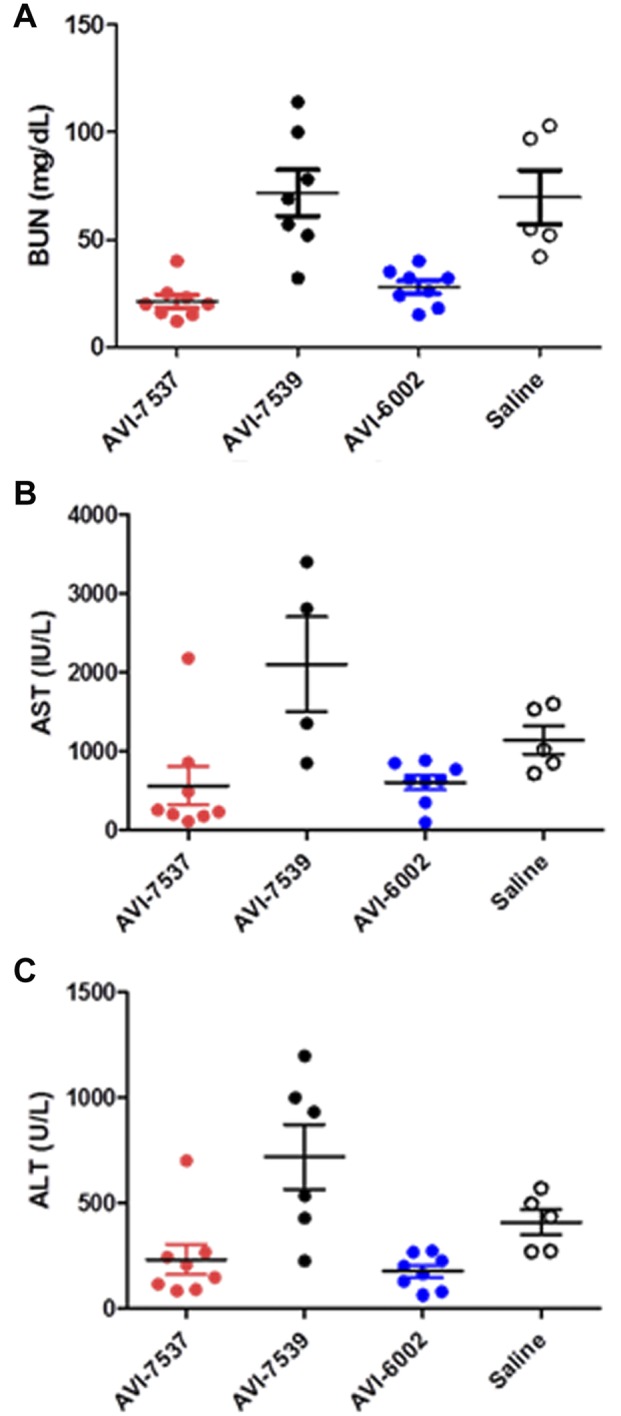
Individual animal serum chemistry values indicative of liver (AST or ALT) or renal (BUN) function. Data are from day 8 p.i., mean values are indicated by a horizontal bar with vertical standard deviation bars, and individual animals are represented by filled circles. (A) Blood urea nitrogen (BUN) levels are given in mg/dl. BUN levels in animals treated with AVI-7537 were significantly different from those treated with AVI-7539, and BUN levels in animals treated with AVI-6002 or AVI-7539 were significantly different from those treated with AVI-6002 (P < 0.05). (B) Aspartate transaminase (AST) levels are given in IU/ml. AST levels in animals treated with AVI-7537 were significantly different from those treated with AVI-7539 (P < 0.05), and AST levels in animals treated AVI-7539 were significantly different from those treated with AVI-6002 (P < 0.05). (C) Alanine transaminase (ALT) levels are given in U/ml. ALT levels in animals treated with AVI-7537 were significantly different from those treated with AVI-7539 (P < 0.05), and ALT levels in animals treated with AVI-7539 were significantly different from those treated with AVI-6002 (P < 0.05).
These data show that no antiviral synergy exists between AVI-7537 and AVI-7539 and, more importantly, reveal that targeting VP24 alone is sufficient to confer protection against lethal EBOV infection. This result is quite surprising, as VP35 is often viewed as an attractive therapeutic target due to its many critical roles in viral infection, and targeting VP35 in previous work conferred efficacy in vitro and in intraperitoneally infected rodent survival studies. Specifically, treatments with 20 mg/kg of AVI-7539, AVI-7537, and AVI-6002 led to 38%, 49%, and 83% survival, respectively (12). In contrast, in the present study, no protection against lethal EBOV infection was seen in the NHPs treated with AVI-7539. We postulate that these differences may be explained by increased sensitivity of mouse-adapted EBOV to IFN inhibition (13). In addition to its critical roles in immune evasion and host adaptation, recent studies have indicated that VP24 plays a larger role in the viral cycle than previously thought (7, 14, 15), indicating that VP24 is a viable therapeutic target. Indeed, the results of the present study suggest that impairment of VP24 alone is enough to protect against EBOV infection and that targeting VP24 may lead to the development of more effective countermeasures against this important viral pathogen. Furthermore, AVI-7537 has recently been shown to be safe and well tolerated in humans (16) and should be further developed as an effective EBOV therapeutic.
ACKNOWLEDGMENTS
This work was conducted under a contract (W9113M-10-C-0056) with the Joint Product Management Office of BioDefense Therapeutics (BD-Tx). BD-Tx is a component of the Medical Countermeasure Systems Joint Project Management Office (JPM-MCS) within the U.S. Department of Defense’s Joint Program Executive Office for Chemical and Biological Defense.
A.E.H., J.S.C., P.S., and P.L.I. are/were employed by Sarepta Therapeutics, Inc.
Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
Footnotes
Citation Warren TK, Whitehouse CA, Wells J, Welch L, Heald AE, Charleston JS, Sazani P, Reid SP, Iversen PL, Bavari S. 2015. A single phosphorodiamidate morpholino oligomer targeting VP24 protects rhesus monkeys against lethal Ebola virus infection. mBio 6(1):e02344-14. doi:10.1128/mBio.02344-14.
REFERENCES
- 1.Feldmann H, Geisbert TW. 2011. Ebola haemorrhagic fever. Lancet 377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keita S, De Clerck H, Tiffany A, Dominguez G, Loua M, Traore A, Kolie M, Malano ER, Heleze E, Bocquin A, Mely S, Raoul H, Caro V, Cadar D, Gabriel M, Pahlmann M, Tappe D, Schmidt-Chanasit J, Impouma B, Diallo AK, Formenty P, Van Herp M, Gunther S. 2014. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 371:1418–1425. doi: 10.1056/NEJMoa1404505. [DOI] [PubMed] [Google Scholar]
- 3.Gostin LO, Lucey D, Phelan A. 2014. The Ebola epidemic: A global health emergency. JAMA 312:1095–1096. doi: 10.1001/jama.2014.11176. [DOI] [PubMed] [Google Scholar]
- 4.Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O’Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM Jr., Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K, Working Group on Civilian Biodefense . 2002. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA 287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 5.Swenson DL, Warfield KL, Warren TK, Lovejoy C, Hassinger JN, Ruthel G, Blouch RE, Moulton HM, Weller DD, Iversen PL, Bavari S. 2009. Chemical modifications of antisense morpholino oligomers enhance their efficacy against Ebola virus infection. Antimicrob Agents Chemother 53:2089–2099. doi: 10.1128/AAC.00936-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren TK, Warfield KL, Wells J, Swenson DL, Donner KS, Van Tongeren SA, Garza NL, Dong L, Mourich DV, Crumley S, Nichols DK, Iversen PL, Bavari S. 2010. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat Med 16:991–994. doi: 10.1038/nm.2202. [DOI] [PubMed] [Google Scholar]
- 7.Ramanan P, Shabman RS, Brown CS, Amarasinghe GK, Basler CF, Leung DW. 2011. Filoviral immune evasion mechanisms. Viruses 3:1634–1649. doi: 10.3390/v3091634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, Alinger JB, Berry KN, Yen B, Hamilton J, Brett TJ, Pappu RV, Leung DW, Basler CF, Amarasinghe GK. 2014. Ebola virus VP24 targets a unique NLS binding site on karyopherin alpha 5 to selectively compete with nuclear import of phosphorylated STAT1. Cell Host Microbe 16:187–200. doi: 10.1016/j.chom.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basler CF, Wang X, Mühlberger E, Volchkov V, Paragas J, Klenk HD, García-Sastre A, Palese P. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci U S A 97:12289–12294. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. 2006. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol 80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC. [Google Scholar]
- 12.Iversen PL, Warren TK, Wells JB, Garza NL, Mourich DV, Welch LS, Panchal RG, Bavari S. 2012. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses 4:2806–2830. doi: 10.3390/v4112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradfute SB, Bavari S. 2011. Correlates of immunity to filovirus infection. Viruses 3:982–1000. doi: 10.3390/v3070982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mateo M, Carbonnelle C, Reynard O, Kolesnikova L, Nemirov K, Page A, Volchkova VA, Volchkov VE. 2011. VP24 is a molecular determinant of Ebola virus virulence in guinea pigs. J Infect Dis 204(Suppl 3):S1011–S1020. doi: 10.1093/infdis/jir338. [DOI] [PubMed] [Google Scholar]
- 15.Watt A, Moukambi F, Banadyga L, Groseth A, Callison J, Herwig A, Ebihara H, Feldmann H, Hoenen T. 2014. A novel life cycle modeling system for Ebola virus shows a genome length-dependent role of VP24 in virus infectivity. J Virol 88:10511–10524. doi: 10.1128/JVI.01272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heald AE, Iversen PL, Saoud JB, Sazani P, Charleston JS, Axtelle T, Wong M, Smith WB, Vutikullird A, Kaye E. 2014. Safety and pharmacokinetic profiles of phosphorodiamidate morpholino oligomers with activity against Ebola virus and Marburg virus: results of two single-ascending-dose studies. Antimicrob Agents Chemother 58:6639–6647. doi: 10.1128/AAC.03442-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


