In a randomized clinical trial, needlescopic 3-trocar cholecystectomy was compared with transvaginal/transumbilical hybrid—NOTES—technique for symptomatic cholecystolithiasis. We found significantly less pain despite less analgesics, increased satisfaction with the aesthetic result, and improved postoperative quality of life in the NOTES group. Furthermore, both techniques were equal in terms of safety.
Keywords: cholecystectomy, cholecystolithiasis, natural orifice transluminal endoscopic surgery, needlescopic, NOTES, transvaginal
Abstract
Objective:
For cholecystectomy, both the needlescopic cholecystectomy (NC) 3-trocar technique using 2 to 3 mm trocars and the umbilical-assisted transvaginal cholecystectomy (TVC) technique have found their way into clinical routine. This study compares these 2 techniques in female patients who are in need of an elective cholecystectomy.
Background:
Natural orifice transluminal endoscopic surgery (NOTES) is a surgical concept permitting scarless intra-abdominal operations through natural orifices, such as the vagina. Because of the lack of an adequately powered trial, we designed this first randomized controlled study for the comparison of TVC and NC.
Methods:
This prospective, randomized, nonblinded, single-center trial evaluates the safety and effectiveness of TVC (intervention), compared with NC (control) in female patients with symptomatic cholecystolithiasis. The primary endpoint was intensity of pain until the morning of postoperative day (POD) 2. Secondary outcomes were among others intra- and postoperative complications, procedural time, amount of analgesics used, pain intensity until POD 10, duration of hospital stay, satisfaction with the aesthetic result, and quality of life on POD 10 as quantified with the Eypasch Gastrointestinal Quality of Life Index (GIQLI).
Results:
Between February 2010 and June 2012, 40 patients were randomly assigned to the interventional or control group. All patients completed follow-up. Procedural time, length of postoperative hospital stay, and the rate of intra- and postoperative complications were similar in the 2 groups. However, significant advantages were found for the transvaginal access regarding pain until POD 2, but also until POD 10 (P = 0.043 vs P = 0.010) despite significantly less use of peripheral analgesics (P = 0.019). In the TVC group, patients were significantly more satisfied with the aesthetic result (P < 0.001) and had a significantly better GIQLI (P = 0.028).
Conclusions:
Although comparable in terms of safety, TVC caused less pain, increased satisfaction with the aesthetic result, and improved postoperative quality of life in the short term.
The collateral damage to the abdominal wall for intraperitoneal access accounts for a major part of the operative trauma in abdominal surgery. It is also a source of many intra- and postoperative complications. Minimally invasive surgery was developed to minimize this damage. Laparoscopic cholecystectomy (LC) using two 10-mm and two 5-mm trocars became the gold standard many years ago, when its advantages in terms of postoperative pain, aesthetic results, and duration of hospital stay compared with conventional open cholecystectomy were shown.1–4 Using instruments with a lower diameter for a “needlescopic cholecystectomy” (NC), which was evaluated in several prospective, randomized, sometimes even double-blinded trials, further reduced postoperative pain and improved aesthetics.5,6 The remaining 10-mm trocar incision, which is also used for retrieval, emerged to be the most painful incision.7 However, this abdominal wall trauma for instrument access and specimen retrieval can be avoided by accessing the abdominal cavity through natural orifices, for example, transvaginally via the posterior vault of the vagina [natural orifice transluminal endoscopic surgery (NOTES)]. Admittedly, because of limitations of avoidable instruments, NOTES cholecystectomy has only been introduced into clinical routine as a hybrid procedure [transvaginal cholecystectomy (TVC)].8 It facilitates an additional transumbilical 5-mm trocar resulting in TVC, as described by Zornig et al.9 Our prospective, randomized trial was designed to evaluate TVC in terms of lesser postoperative pain, which was so far only shown in nonrandomized trials.10–13 Even if the advantages of NC against the traditional cholecystectomy are limited, TVC was still supposed to be compared with the least invasive laparoscopic technique. Thus, patients undergoing NC were studied as a control group. The emphasis was put on postoperative pain intensity and the safety of transvaginal access.
METHODS
Study Design and Patients
The needlescopic versus transvaginal cholecystectomy study was a randomized, prospective, single-center, and nonblinded clinical trial comparing transvaginal/transumbilical cholecystectomy with 3-trocar NC. Between February 2010 and June 2012, eligible patients were recruited in the Department of Abdominal, Vascular and Transplant Surgery of the Cologne-Merheim Medical Center. The protocol was approved by the Research Ethics Committee of the Witten/Herdecke University (89/2009). Written and informed consent was obtained from all patients.
Inclusion criteria were as follows: female sex, indication for elective cholecystectomy because of symptomatic cholecystolithiasis, age between 18 and 80 years, and legal competence.
Exclusion criteria were as follows: acute cholecystitis or locally complicated disease (eg, gallbladder empyema, choledocholithiasis, and pancreatitis), liver cirrhosis (Child–Pugh A, B, and C), severe comorbidity, class IV or V as defined by the American Society for Anesthesiologists (ASA), previous malignancy or suspected malignancy in preoperative imaging, a body mass index (BMI) higher than 40 kg/m2, chronic abuse of analgesics or alcohol, neuromuscular disease that could interfere with treatment or measures of pain, history of major abdominal surgery with a high risk of intraperitoneal adhesions (minor operations such as an appendectomy, inguinal hernia repair, and minor gynecological surgery were not considered exclusion criteria), gravidity or breast-feeding, allergy against analgesics, patients who are dependent on or employed by the trial sponsor or physicians, participation in other clinical studies that could interfere with the present trial, and no written informed consent signed.
Enrolled patients were randomly assigned on a 1:1 ratio to either a TVC or an NC. Randomization of the 40 patients was conducted without stratification using numbered, opaque, sealed envelopes.14 Computer-aided randomization of numbers in randomly lined up blocks of 4 or 6 was generated by an independent statistician.
Diagnosis and Postoperative Course
A thorough history taking and physical examination, sonography, and laboratory values were obtained preoperatively for all patients. A gynecologist examined all patients randomized to the TVC group using a standardized examination form on the day before the operation. Therefore, the sealed envelopes were opened on the preoperative day for randomization, and thus the patients were informed in which group they were. Contraindications for a transvaginal procedure included were as follows: lacking visibility of the cervix; ongoing pregnancy; genital infections; known endometriosis; neoplasms of the vulva, vagina, or cervix; and intact hymen. These criteria did not result in the exclusion of any patients from the TVC procedure. In both groups, a single-shot antibiotic (cefuroxime 1.5 g) was intravenously administered preoperatively. Before every skin incision, a local anesthetic (3 mL of bupivacaine 0.25%) was administered subcutaneously in both groups. Postoperative analgesia in the recovery room was administered only on demand with a peripheral (paracetamol) or a centrally acting analgesic (piritramide, an opioid with a morphine-equivalence factor of 0.7). Postoperative pain medication was standardized and identical for both groups. The following analgesics were offered on the ward: on the day of surgery, 2 × 1 g of paracetamol (Perfalgan) intravenously; on postoperative day (POD) 1, 3 × 2 tablets of paracetamol 500 mg; and from POD 2, 3 × 1 tablet of paracetamol 500 mg. On demand, 7.5 mg of piritramide (Dipidolor) was administered subcutaneously or in a 100-mL short-infusion intravenously. According to hospital standards, low-molecular-weight heparin (nadroparine) was used for thrombosis prophylaxis during the hospital stay. Full oral intake and mobilization were regularly begun on the day of surgery. On POD 2, laboratory values were taken. Regularly on POD 2 or earlier on demand, bandages were taken off and the first wound examination took place. In case of clinical symptoms or atypical laboratory results, an abdominal sonogram was obtained.
Patients were dismissed from POD 2 under the following conditions: complete oral intake, subjective well-being, primary healing of wounds, regular laboratory results, and if applicable, a normal sonogram. All patients were examined in the first ambulatory posthospital checkup on POD 10. On this occasion, the pain and analgesic diary was collected and evaluated. The patient was examined and questioned. The aesthetic result was evaluated by both the examining physician and the patient using an ordinal scale for satisfaction.
Patients from the TVC group were advised against penetrating sexual intercourse for 2 weeks postoperatively. Also, they were examined by a gynecologist 12 days after surgery, again using a standardized examination form.
Surgical Technique
The transvaginal/transumbilical cholecystectomy was performed with rigid reusable instruments in the lithotomy position, as described by Zornig et al.9 The first surgeon was standing on the left of the patient, the second surgeon between the legs. A 12-mm Hg capnoperitoneum was established via an umbilical Veress needle. An umbilical 6-mm trocar (Karl Storz GmbH & Co KG, Tuttlingen, Germany), a transvaginal, curved 5-mm grasping forceps (according to CUSCHIERI O-CON, 43 cm long, Karl Storz GmbH & Co KG, Tuttlingen, Germany), and a transvaginal 11-mm trocar without connector for insufflation (Karl Storz GmbH & Co KG, Tuttlingen, Germany), which were inserted via the posterior vault of the vagina, were used. The dissection of the gallbladder, the cystic duct, and the cystic artery, and clipping (Endo Clip 5-mm clip applier, Covidien, MA) and transecting of them, was done via the umbilical 6-mm trocar while viewing through a transvaginal 10-mm optic (45 degrees, 42 cm long, Karl Storz GmbH & Co KG, Tuttlingen, Germany). The gallbladder was transvaginally extricated through the 11-mm trocar incision in the posterior vault after changing the view to a transumbilical 5-mm optic (45 degrees, 29 cm long, Karl Storz GmbH & Co KG, Tuttlingen, Germany). The 2 small incisions in the posterior vault were closed with resorbable sutures. In difficult cases, an additional 3.9-mm trocar (Karl Storz GmbH & Co KG, Tuttlingen, Germany) was used at the right costal margin. In all cases, a retrieval bag (Endo Catch Gold, Covidien, MA) was used.
The 3-trocar NC was performed in the supine position with splayed legs. The first surgeon was standing on the left of the patient, the second surgeon between the legs. Three trocars were used: one umbilical 11-mm and two 3.9-mm trocars (Karl Storz GmbH & Co KG, Tuttlingen, Germany) in the epigastrium and at the right costal margin. A 12-mm Hg capnoperitoneum was established via the first 11-mm trocar, which was inserted via a minilaparotomy at the umbilicus. Dissection technique of the gallbladder was the same in both groups. After preparation and visual confirmation of the cystic duct and the cystic artery, they were transected after the application of clips at the respective distal and proximal end. This was followed by retrograde dissection of the gallbladder using a cautery hook. The gallbladder was extricated in a retrieval bag (ExBag, Medi-Globe GmbH, Achenmuehle, Germany) through the umbilical trocar incision. For clipping (Lapro-clip, Covidien, MA) the cystic duct and the cystic artery via the umbilical trocar and for retrieving the gallbladder, a 3.3-mm optic (30 degrees, 25 cm long, Karl Storz GmbH & Co KG, Tuttlingen, Germany) was used via the epigastric trocar. In cases with large or multiple concretions, the incision including skin and fascia was extended accordingly. The procedure was completed by obligatory closure of the fascia and intracutaneous resorbable sutures. All trocars in both groups were reusable.
Both techniques allow an intraoperative cholangiogram. It could be done via the cystic duct using a percutaneously inserted catheter with the injection of an contrast agent.
The first surgeon was the same in all procedures of both groups (DB) to eliminate surgeon-related interindividual effects.
Outcome Measures
The primary outcome measure was cumulative intensity of postoperative pain in motion, 6 hours after surgery, on POD 1 (2 measures: in the morning and in the evening), and on POD 2 (in the morning), using the numeric rating scale (NRS-11) ranging from 0 (no pain) to 10 (worst imaginable pain).15
Secondary outcome measures were as follows the aesthetic aspects of the abdominal wall incisions on POD 10 on a 1 (complete satisfaction) to 5 (complete dissatisfaction) scale from patient's and surgeon's point of view, intraoperative complications, conversion rate to classical laparoscopic or open technique, procedural time, assessment of the surgical handling (instrument handling, camera handling, preparation, and gallbladder extraction) by the first and second surgeons on an ordinal scale (from 1 showing “without any problems” to 5 showing “very difficult”), cumulative intensity of postoperative pain in motion from the day of surgery to POD 10 (assessed 6 hours after surgery and at all other days in the morning and in the evening: 21 measurements, using the NRS-11 ranging from 0 to 10), cumulative use of peripheral (paracetamol) and centrally acting analgesics (piritramide) during the first 10 days, postoperative complications, need of reoperation, return to everyday life, and the quality of life on POD 10, assessed using the Gastrointestinal Quality of Life Index (GIQLI; higher score indicates better quality of life) as developed by Eypasch et al.16
The Clavien-Dindo classification was used to assess postoperative complications.17,18 Conversion was defined either as performing a laparotomy (conversion to conventional procedure) or as the necessity to apply additional or 5-mm trocars (conversion to traditional laparoscopic surgery). In the TVC group, using a 3-mm trocar at the right costal margin was permitted, but insertion of 2 additional 3-mm trocars was considered a conversion to the 3-trocar NC.
Studied Data
Preoperative, intraoperative, and early postoperative data of both groups were analyzed. The following parameters were prospectively documented: age, BMI, ASA score, number of gallstones (solitary or multiple), size of the biggest gallstone (mm), previous cholecystitis, pre- and postoperative laboratory values [leukocyte count and C-reactive protein (CRP)], number of percutaneous trocars, histopathologic results, and postoperative duration of hospital stay.
We report the primary outcome measure and related secondary outcome measures, and put these results into context. A separate paper concerning the long-term results of this study is in preparation.
Calculation of Power and Sample Size
For the primary endpoint “postoperative pain,” estimates for standard deviation (SD) and minimal clinically relevant difference were derived from the existing literature. For the sample size calculation, a pain reduction of 1.5 points on the NRS-11 was considered clinically relevant.19 The SD for postoperative pain assessment is known to be about 1.5 points so that the expected difference was about 1 SD.
To prove this difference in a 2-sided superiority testing with an alpha error of 0.05 and a type 2 error of 0.20 (power 80%), a number of 17 patients for each group was calculated. Because of an expected dropout rate of 10% and the intended use of nonparametric statistics, 40 patients (2 × 20) were included.
Statistical Analysis
IBM SPSS Statistics 19 (IBM Corp., Armonk, NY) was used for data processing and statistics of all variables. All analyses were by intention to treat. Normally distributed parameters such as age and BMI were analyzed using a 2-tailed t test. Not normally distributed parameters were analyzed using the Mann-Whitney U test. Dichotomous questions and all yes/no variables were analyzed using the Fisher exact test for categorical variables. The χ2 test for the trend was used to analyze all ordinal parameters. P < 0.05 was considered statistically significant.
This study is registered in the ClinicalTrials.gov Register, ID: NCT01685775, and in the German Clinical Trials Register, ID: DRKS00000341. The Universal Trial Number was U1111-1114-7386.
RESULTS
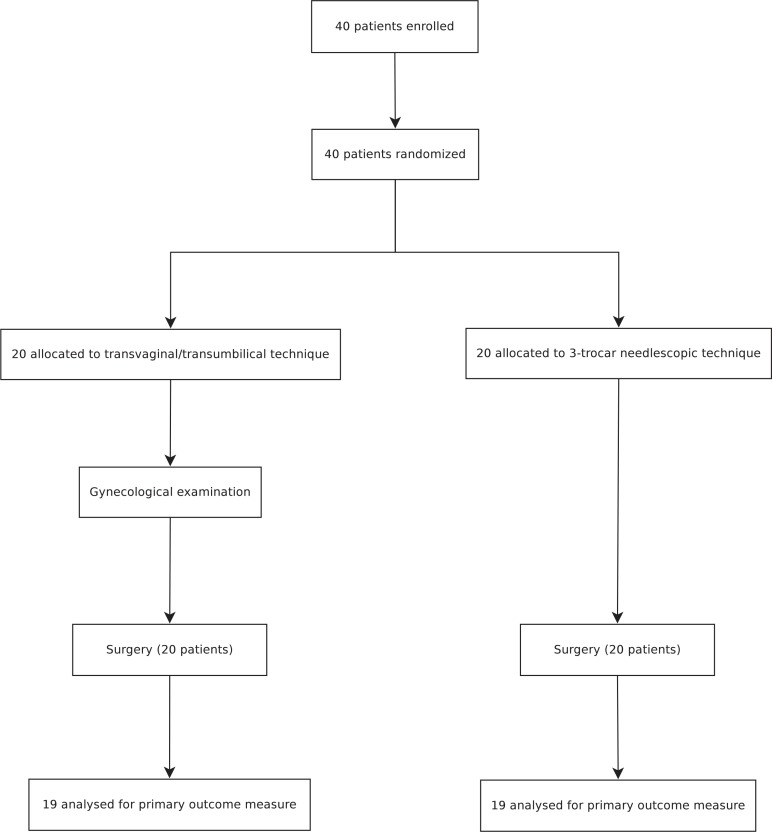
Between February 2010 and June 2012, 40 patients were recruited and randomized (20 in the TVC group and 20 in the NC group). Figure 1 shows the trial profile. Because no conversions were necessary and all patients could be treated according to the study protocol, no further per-protocol analysis was necessary apart from the intention-to-treat analysis.
FIGURE 1.
Trial profile.
Table 1 shows the baseline characteristics of all patients. No statistical differences were observed between the group's demographic characteristics, BMI, ASA scores, number and size of gallstones, previous cholecystitis, and preoperative laboratory values (leukocyte count and CRP). The preoperative gynecological examination was without any pathological finding for all TVC patients and had no influence on further procedures. The gynecological examination of all 20 TVC patients conducted 12 to 14 days postoperatively again showed no pathological findings, especially no wound infections. In all cases, suture material was found in place without irritation.
TABLE 1.
Baseline Characteristics of All Patients
| Variable | TVC Group (n = 20) | NC Group (n = 20) | Total (n = 40) | P |
|---|---|---|---|---|
| Age, yr: mean (standard deviation) | 44.8 (15.24) | 47.5 (18.82) | 46.2 (16.67) | 0.615* |
| BMI, kg/m2: mean (standard deviation) | 28.1 (4.21) | 28.5 (4.46) | 28.3 (4.29) | 0.780* |
| ASA scores | 0.358† | |||
| 1 | 2 (10%) | 6 (30%) | 8 (20%) | |
| 2 | 17 (85%) | 12 (60%) | 29 (72.5%) | |
| 3 | 1 (5%) | 2 (10%) | 3 (7.5%) | |
| Gallbladder stones | 0.235‡ | |||
| Solitaire | 2 (10%) | 6 (30%) | 8 (20%) | |
| Multiple | 18 (90%) | 14 (70%) | 32 (80%) | |
| Size of the biggest gallstone, mm: median (Q1–Q3) | 10.5 (7.0–17.3) | 8.0 (4.0–15.0) | 10.0 (5.0–15.0) | 0.367§ |
| Previous cholecystitis | 3 (15%) | 2 (10%) | 5 (12.5%) | 1.000‡ |
| Preoperative laboratory values | ||||
| Leukocyte count, per nL: median (Q1–Q3) | 6.9 (4.6–7.8) | 7.1 (5.9–9.1) | 6.9 (5.3–8.1) | 0.173§ |
| CRP, mg/L: median (Q1–Q3) | 0.0 (0.0–5.6) | 3.3 (0.0–4.3) | 3.0 (0.0–4.4) | 0.684§ |
| Executed procedure | 1.000‡ | |||
| TVC | 20 (100%) | 0 (0%) | ||
| NC | 0 (0%) | 20 (100%) |
*t test.
†χ2 test for the trend.
‡ Fisher exact test.
§Mann-Whitney U test.
Table 2 shows the procedural data and outcomes. No significant differences were found for procedural time, frequency of analgesics in the recovery room, pre- and postoperative difference in CRP and leukocyte count, postoperative hospital stay, postoperative complications, and time to everyday life. As expected, the median amount of percutaneous trocars was significantly less in the TVC group than in the NC group (1 vs 3; P < 0.001). No patient suffered from an intraoperative complication, and there was no case with a need for conversion, drain placement, blood transfusion, or revision surgery. There were neither pre- nor intraoperative findings that suggested choledocholithiasis in any of the patients. Therefore, an intraoperative cholangiogram was not deemed necessary in any patient. Mortality was 0 in both groups. In each group, there were 2 postoperative complications. Two wound infections at the umbilical trocar site, which was also used for the retrieval of the gallbladder, occurred in the NC group. One wound had to be opened and needed regular flushing, whereas the other healed primarily under antibiotic treatment. Contrarily, the 2 postoperative complications in the TVC group were biliary pancreatitis, one of them with postoperative cholestasis and cystic duct leakage. In both cases, an endoscopic retrograde cholangiopancreatography was performed and after the according conservative therapy and prolonged hospital stay, both patients were discharged free of symptoms. Preoperatively, even in these 2 cases, there were no clinical, ultrasonographic, or laboratory (liver function test) findings suspect for choledocholithiasis. Thus, independently of the procedural technique, no indication for intraoperative cholangiogram existed. The resulting Clavien-Dindo classifications are listed in Table 3. Compared with the TVC patients without complications, the 2 patients with the biliary pancreatitis had the highest demand for peripheral and centrally acting analgesics (median, 11.8 g vs 4.0 g; 180 mg vs 6.25 mg) and the highest cumulative pain score from the day of surgery until POD 2 and until POD 10 (median, 5 vs 2; 76.5 vs 17). They also had the lowest GIQLI values (median, 75.5 vs 124).
TABLE 2.
Procedural Data and Outcomes
| Variable | TVC Group (n = 20) | NC Group (n = 20) | Total (n = 40) | P |
|---|---|---|---|---|
| Procedural time, min: median (Q1–Q3) | 50.0 (42.0–66.0) | 54.5 (46.0–62.0) | 52.0 (44.0–62.0) | 0.675* |
| No. of percutaneous trocars: median (Q1–Q3) | 1 (1–1) | 3 (3–3) | 2.5 (1–3) | <0.001* |
| Intraoperative complication | 0 (0%) | 0 (0%) | 0 (0%) | 1.000† |
| Conversion | 0 (0%) | 0 (0%) | 0 (0%) | 1.000† |
| Surgical handling, assessment by the first surgeon, 1/2/3/4/5‡ | ||||
| Instrument handling | 2/12/6/0/0 | 4/14/2/0/0 | 6/26/8/0/0 | 0.112§ |
| Camera handling | 2/14/3/1/0 | 0/14/6/0/0 | 2/28/9/1/0 | 0.411§ |
| Preparation | 2/14/3/1/0 | 3/9/8/0/0 | 5/23/11/1/0 | 0.645§ |
| Gallbladder extraction | 17/3/0/0/0 | 3/7/8/2/0 | 20/10/8/2/0 | <0.001§ |
| Surgical handling, assessment by the second surgeon, 1/2/3/4/5‡ | ||||
| Instrument handling | 5/6/7/2/0 | 6/14/0/0/0 | 11/20/7/2/0 | 0.020§ |
| Camera handling | 4/11/4/1/0 | 4/9/7/0/0 | 8/20/11/1/0 | 0.835§ |
| Preparation | 11/5/3/1/0 | 4/13/3/0/0 | 15/18/6/1/0 | 0.311§ |
| Gallbladder extraction | 9/10/1/0/0 | 3/11/4/2/0 | 12/21/5/2/0 | 0.010§ |
| Drainage | 0 (0%) | 0 (0%) | 0 (0%) | 1.000† |
| Blood transfusion | 0 (0%) | 0 (0%) | 0 (0%) | 1.000† |
| Cumulative pain from the day of surgery to day 2 in the morning, NRS-11: median (Q1–Q3) | 8.0 (4–16)¶ | 14.0 (8–19)¶ | 12.0 (5.8–17.5)|| | 0.043* |
| Cumulative pain from the day of surgery to day 10, NRS-11: median (Q1–Q3) | 22.0 (11.5–37.5)¶ | 41.0 (26.0–66.0)¶ | 28.5 (16.3–49.0)|| | 0.010* |
| Frequency of analgesics in the recovery room | 6 (30%) | 7 (35%) | 13 (32.5%) | 1.000† |
| Cumulative paracetamol, g, from the day of surgery to day 10: median (Q1–Q3) | 4.5 (3.0–8.5)¶ | 8.75 (5.0–14.5)¶ | 6.5 (3.5–10.0)|| | 0.019* |
| Cumulative piritramide, mg, from the day of surgery to day 10: median (Q1–Q3) | 7.5 (0.0–22.5)¶ | 0.0 (0.0–17.5)¶ | 6.3 (0.0–21.4)|| | 0.244* |
| Pre- and postoperative difference in CRP, mg/L: median (Q1–Q3) | 12.6 (3.6–26.1) | 10.3 (4.9–22.6) | 12.3 (4.0–24.79) | 0.574* |
| Pre- and postoperative difference in leukocyte count, per nL: median (Q1–Q3) | 0.5 (−0.7–1.3) | 0.1 (−0.7–0.6) | 0.1 (−0.7–1.0) | 0.496* |
| Postoperative complication | 2 (10%) | 2 (10%) | 4 (10%) | 1.000† |
| Revision surgery | 0 (0%) | 0 (0%) | 0 (0%) | 1.000† |
| Postoperative hospital stay, d: median (Q1–Q3) | 2.0 (2–2) | 2.0 (2–2) | 2.0 (2.0–2.0) | 0.892* |
| Time to everyday life, d: median (Q1–Q3) | 5.5 (3.5–12.5) | 6.5 (6.0–11.0) | 6.0 (4.3–12.8) | 0.328* |
| Assessment of aesthetic on postoperative day 10, 1/2/3/4/5** | ||||
| Patient | 20/0/0/0/0 | 6/11/3/0/0 | 26/11/3/0/0 | <0.001§ |
| Investigator | 20/0/0/0/0 | 0/16/4/0/0 | 20/16/4/0/0 | <0.001§ |
| Gastrointestinal Quality of Life Index on postoperative day 10: median (Q1–Q3) | 124 (104.5–132.0) | 107 (89.0–114.0) | 115.5 (99.3–125.5) | 0.028* |
| Mortality | 0 (0%) | 0 (0%) | 0 (0%) | 1.000† |
*Mann-Whitney U test.
†Fisher exact test.
‡Response options: 1, without any problems; 2, easy; 3, moderate; 4, slightly difficult; 5, very difficult.
§χ2 test for the trend.
¶n = 19.
∥n = 38.
**Response options: 1, very satisfied; 2, moderately satisfied; 3, equally satisfied and dissatisfied; 4, moderately dissatisfied; 5, very dissatisfied.
TABLE 3.
Clavien-Dindo Classification of Postoperative Complications
| TVC Group (n = 20) | LC Group (n = 20) | Total (n = 40) | P | |
|---|---|---|---|---|
| 0.852* | ||||
| No complication | 18 (90%) | 18 (90%) | 36 (90%) | |
| Grade I | 0 (0%) | 0 (0%) | 0 (0%) | |
| Grade II | 0 (0%) | 1 (5%) | 1 (2.5%) | |
| Grade III | 2 (10%) | 1 (5%) | 3 (7.5%) | |
| Grade IV | 0 (0%) | 0 (0%) | 0 (0%) | |
| Grade V | 0 (0%) | 0 (0%) | 0 (0%) |
*χ2 test for the trend.
Grade I: no intervention necessary.
Grade II: requiring pharmacological treatment.
Grade III: requiring surgical, endoscopic, or radiological intervention.
Grade IV: life-threatening complication requiring intensive care/intensive care unit management.
Grade V: death of a patient.
One patient of each group did not fill out the pain and analgesic diary prospectively, so they had to drop out of the analysis of postoperative pain.
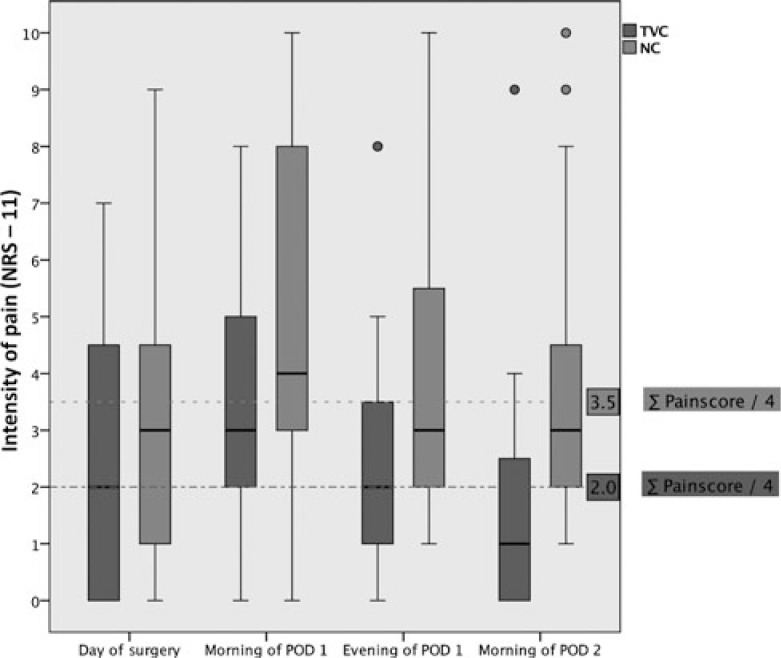
The primary outcome measure, the cumulative intensity of postoperative pain in motion for the first 48 hours postoperatively was significantly lower in the TVC group (Table 2). Figure 2 shows boxplots for the 4 single NRS-11 scores of both groups compared with the respective quarter overall median.
FIGURE 2.
Boxplots for the primary outcome measure (single NRS-11 scores of both groups compared with the respective quarter overall median).
Furthermore, the cumulative intensity of pain from the day of surgery to POD 10 was significantly lower for TVC patients, although they needed significantly less analgesics until POD 10. As mentioned earlier, the trend for a higher consumption of centrally acting analgesics can mostly be attributed to the 2 TVC patients with postoperative complications. After exclusion of the patients with postoperative complications of both groups, piritramide consumption in the TVC group even showed a trend to be lower (median/Q1–Q3, 5.0/0–16.5 mg vs 7.5/0–25.0 mg; P = 0.740). In addition, after exclusion of the patients with postoperative complications, the primary outcome measure, the second pain evaluation and the postoperative need for peripheral analgesics, resulted in even more pronounced significance toward TVC (NRS-11, 7.0/3.5–14.0 vs 14.0/7.5–21.5; P = 0.017; NRS-11, 17.0/5.5–30.5 vs 41.0/25.5–55.5; P = 0.002; 4.0/2.5–6.5 g vs 8.5/4.5–15.0 g; P = 0.013). In this analysis, there was also a greater difference in time to everyday life, which, however, was not quite significant (5.0/3.0–10.0 days vs 7.0/6.0–13.5 days; P = 0.075).
Gallbladder extraction in the TVC group was rated to be significantly easier by both surgeons, whereas handling of instruments was considered significantly more difficult by the 2 surgeons for the TVC group. Camera handling and preparation were not rated different between both groups.
Evaluation of the GIQLI by the patient and the aesthetic result on POD 10 by the patient and the investigator was significantly better after TVC. There was no readmission of any patient in either group during follow-up.
DISCUSSION
This trial was designed to compare the intensity of postoperative pain after 2 different techniques of cholecystectomy in patients with symptomatic cholecystolithiasis. Results are significantly reduced postoperative pain in the first 2 days and in the first 10 days after surgery by TVC compared with NC. Need of peripheral analgesics was significantly less in the TVC group. TVC patients were significantly more satisfied with the aesthetic result and had a significantly better GIQLI.
For many years now, a patient-directed aim of operative procedures is the reduction of access-related trauma. Hereby, postoperative pain can be reduced and access-related complications such as wound infections, cutaneous scars, adhesions, and incisional hernias avoided. This leads to a quicker recovery, shorter hospital stay, a better aesthetic result, and an increased postoperative quality of life, as proven for some procedures after the introduction of laparoscopic surgery.20–22 The disadvantages of conventional surgery persist in laparoscopic procedures that require a retrieval incision for the specimen. Therefore, the concept of access to the abdominal cavity through already existing, natural routes, namely NOTES, evolved. After this concept, gallbladders with large concretions were retrieved through a posterior colpotomy during LC in female patients as early as 1993.23 Even for laparoscopic splenectomy, transvaginal retrieval was described early.24 In 2007, several groups published different techniques of TVC for the first time.9,25–27 They used either a flexible endoscope or, like Zornig et al,9 a regular laparoscopic optic. For retraction of the gallbladder or for preparation, one or more percutaneous trocars were used. For example, in the hybrid—NOS—technique of Zornig et al,9 a transumbilical 5-mm trocar, a transvaginal 10-mm –trocar, and a transvaginal rigid 5-mm grasping forceps are used. Meanwhile, pure NOTES techniques exist, but in clinical routine, the hybrid technique is widely used.8 After feasibility and safety of the TVC using rigid instruments and laparoscopes was shown in case series,28–33 it was confirmed in nonrandomized studies comparing TVC and standard LC.34,35 More nonrandomized studies even found advantages for TVC as compared with LC. In a retrospective case-controlled study for TVC, as described by Zornig et al,9 compared with LC in a 3-trocar technique in a total of 93 patients, Hensel et al11 found nausea or vomiting, pain, use of analgesics, and hospital stay significantly reduced. Also, Kilian et al10 found significantly less postoperative pain and shorter hospital stay for 15 TVC patients (Zornig technique) than for 20 LC patients in a 3-armed nonrandomized study. Postoperative pain values for 14 TVC patients were significantly less on POD 1 and 3 than for 22 single-incision cholecystectomy patients and 11 LC patients in the 3-armed study of Solomon et al.36 Borchert et al37 found significantly less pain and consumption of analgesics on POD 3 in their analysis of 77 TVC and 46 LC patients. Santos et al12 compared 7 TVC patients with 7 standard LC patients using a flexible endoscope. Despite the small sample size, they also found significantly less pain on the day of surgery and on POD 1 and significantly less consumption of centrally acting analgesics in the recovery room. The same results were found in our cohort analysis comparing 50 TVC patients (Zornig technique) and 50 LC patients.13 There was significantly less pain on the first 2 days after surgery, and the use of analgesics in the recovery room was significantly less frequent. All cited studies have in common that TVC was performed as a hybrid procedure. However, until now only one prospective randomized clinical trial dealing with the new techniques of cholecystectomy has been published.38 It is a 3-arm pilot study comparing hybrid NOTES transvaginal, pure transumbilical, and conventional LC. No differences in the complication rate, length of hospital stay, and time off from work were found, but the estimated sample size was underpowered, making interpretation of the results limited. Because of the lack of an adequately powered trial, we designed this first randomized controlled study for the comparison of TVC and NC.
In our clinic, we began performing TVC in the year 2008 and that we did it in the manner as described by Zornig et al.9 Analysis of the first 50 TVC patients was followed by a cohort analysis of these patients with 50 LC patients, and it found significantly less postoperative pain despite lower amount of analgesics used.13 We then planned this prospective, randomized clinical trial to confirm the results. Because of the data that show advantages for both needlescopic and 3-trocar techniques concerning postoperative pain and postoperative amount of analgesics,5,6,39 we voted for the NC technique as a control group. Despite a higher conversion rate, this technique had better aesthetic results than traditional LC.4 Thus, the advantages we found for TVC compared with NC are even more pronounced than a comparison with traditional LC would have shown.
Similarly to the cited nonrandomized trials, the rate of postoperative complications in our trial, namely 2 cases of postoperative biliary pancreatitis after TVC and 2 cases of wound infections after NC, did not account for a significant difference. However, the existing data confirm the retrieval incision as a source for wound infections and the majority of pain.40 Because a retrieval incision in the abdominal wall is not required in TVC, we would expect a significant difference in the rate of wound infections in a study with a higher sample size. On the one hand, our study was not designed and sized for this parameter as a primary outcome, so further investigation is needed to confirm this trend. On the other hand, the primary outcome parameter “reduction of postoperative pain” by avoiding specimen retrieval through the abdominal wall with the TVC technique is markedly confirmed in our trial. For evaluation of a theoretically lower rate of port-site hernias after TVC, the sample size of our study is too small as well and a follow-up of several years is required. In our study there are 2 cases of postoperative biliary pancreatitis in the TVC group. Whether this is a technique-specific complication or a coincidence in a study collective cannot be determined because of the lack of significance. An accumulation of this complication after TVC has not been described in the literature. In our own evaluation of the first 50 TVC patients compared with traditional LC patients, that complication did not occur.13 In contrast to our cohort analysis, no in pre- and postoperative difference in CRP and in postoperative hospital stay between the 2 groups was found in our randomized trial. However, the surgical technique for the control groups differs, which limits comparability between the 2 studies. The missing difference might be attributed to the reduced invasiveness of NC. Interestingly, there is a pronounced difference in mean procedural time. Although procedural time was 77.8 minutes in our cohort analysis, it was 53.6 minutes in this trial. This impressively reflects the procedural learning curve, as the cohort analysis consisted of the first 50 TVC patients who were observed in our clinic.
CONCLUSIONS
In a randomized clinical trial, needlescopic 3-trocar cholecystectomy was compared to transvaginal/transumbilical hybrid-NOTES-technique for symptomatic cholecystolithiasis. Although comparable in terms of safety, we found in the NOTES-group significantly less pain despite less use analgesics, increased satisfaction with the aesthetic result, and improved postoperative quality of life in the short term.
ACKNOWLEDGMENT
The authors thank Dr S. Sauerland of the Institute for Research in Operative Medicine, University of Witten/Herdecke, Cologne, Germany, for assistance with the statistical analysis of this study.
Footnotes
Disclosure: Supported in part by the German Ministry of Research and Education (CHIR-Net Grant, BMBF No. 01-GH-0605). The authors declare no conflicts of interest.
REFERENCES
- 1.Barkun JS, Barkun AN, Sampalis JS, et al. Randomised controlled trial of laparoscopic versus mini cholecystectomy. The McGill Gallstone Treatment Group. Lancet. 1992;340:1116–1119. [DOI] [PubMed] [Google Scholar]
- 2.Majeed AW, Troy G, Nicholl JP, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet. 1996;347:989–994. [DOI] [PubMed] [Google Scholar]
- 3.Neugebauer E, Troidl H, Spangenberger W, et al. Conventional versus laparoscopic cholecystectomy and the randomized controlled trial. Cholecystectomy Study Group. Br J Surg. 1991;78:150–154. [DOI] [PubMed] [Google Scholar]
- 4.McCloy R, Randall D, Schug SA, et al. Is smaller necessarily better? A systematic review comparing the effects of minilaparoscopic and conventional laparoscopic cholecystectomy on patient outcomes. Surg Endosc. 2008;22:2541–2553. [DOI] [PubMed] [Google Scholar]
- 5.Hosono S, Osaka H. Minilaparoscopic versus conventional laparoscopic cholecystectomy: a meta-analysis of randomized controlled trials. J Laparoendosc Adv Surg Tech A. 2007;17:191–199. [DOI] [PubMed] [Google Scholar]
- 6.Gurusamy KS, Samraj K, Ramamoorthy R, et al. Miniport versus standard ports for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2010;17:CD006804. [DOI] [PubMed] [Google Scholar]
- 7.Bisgaard T, Klarskov B, Trap R, et al. Microlaparoscopic vs conventional laparoscopic cholecystectomy: a prospective randomized double-blind trial. Surg Endosc. 2002;16:458–464. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann KS, Ritz JP, Wibmer A, et al. The German registry for natural orifice translumenal endoscopic surgery: report of the first 551 patients. Ann Surg. 2010;252:263–270. [DOI] [PubMed] [Google Scholar]
- 9.Zornig C, Emmermann A, von Waldenfels HA, et al. Laparoscopic cholecystectomy without visible scar: combined transvaginal and transumbilical approach. Endoscopy. 2007;39:913–915. [DOI] [PubMed] [Google Scholar]
- 10.Kilian M, Raue W, Menenakos C, et al. Transvaginal-hybrid vs. single-port-access vs. “conventional” laparoscopic cholecystectomy: a prospective observational study. Langenbecks Arch Surg. 2011;396:709–715. [DOI] [PubMed] [Google Scholar]
- 11.Hensel M, Schernikau U, Schmidt A, et al. Comparison between transvaginal and laparoscopic cholecystectomy: a retrospective case-control study. Zentralbl Chir. 2012;137:48–54. [DOI] [PubMed] [Google Scholar]
- 12.Santos BF, Teitelbaum EN, Arafat FO, et al. Comparison of short-term outcomes between transvaginal hybrid NOTES cholecystectomy and laparoscopic cholecystectomy. Surg Endosc. 2012;26:3058–3066. [DOI] [PubMed] [Google Scholar]
- 13.Bulian DR, Trump L, Knuth J, et al. Less pain after transvaginal/transumbilical cholecystectomy than after the classical laparoscopic technique: short-term results of a matched-cohort study. Surg Endosc. 2013;27:580–586. [DOI] [PubMed] [Google Scholar]
- 14.Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet. 2002;359:614–618. [DOI] [PubMed] [Google Scholar]
- 15.Hartrick CT, Kovan JP, Shapiro S. The numeric rating scale for clinical pain measurement: a ratio measure? Pain Practice. 2003;3:310–316. [DOI] [PubMed] [Google Scholar]
- 16.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. [DOI] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 19.Cepeda MS, Africano JM, Polo R, et al. What decline in pain intensity is meaningful to patients with acute pain? Pain. 2003;105:151–157. [DOI] [PubMed] [Google Scholar]
- 20.Keus F, Gooszen HG, van Laarhoven CJ. Open, small-incision, or laparoscopic cholecystectomy for patients with symptomatic cholecystolithiasis. An overview of Cochrane Hepato-Biliary Group reviews. Cochrane Database Syst Rev. 2010;20:CD008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwenk W, Haase O, Neudecker J, et al. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;20:CD003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters MJ, Mukhtar A, Yunus RM, et al. Meta-analysis of randomized clinical trials comparing open and laparoscopic anti-reflux surgery. Am J Gastroenterol. 2009;104:1548–61; quiz 1547, 1562. [DOI] [PubMed] [Google Scholar]
- 23.Delvaux G, Devroey P, De Waele B, et al. Transvaginal removal of gallbladders with large stones after laparoscopic cholecystectomy. Surg Laparosc Endosc. 1993;3:307–309. [PubMed] [Google Scholar]
- 24.Emmermann A, Zornig C, Peiper M, et al. Laparoscopic splenectomy. Technique and results in a series of 27 cases. Surg Endosc. 1995;9:924–927. [PubMed] [Google Scholar]
- 25.Marescaux J, Dallemagne B, Perretta S, et al. Surgery without scars: report of transluminal cholecystectomy in a human being. Arch Surg. 2007;142:823–826; discussion 826–827. [DOI] [PubMed] [Google Scholar]
- 26.Bessler M, Stevens PD, Milone L, et al. Transvaginal laparoscopically assisted endoscopic cholecystectomy: a hybrid approach to natural orifice surgery. Gastrointest Endosc. 2007;66:1243–1245. [DOI] [PubMed] [Google Scholar]
- 27.Dolz C, Noguera JF, Martin A, et al. [Transvaginal cholecystectomy (NOTES) combined with minilaparoscopy]. Rev Esp Enferm Dig. 2007;99:698–702. [DOI] [PubMed] [Google Scholar]
- 28.Ramos AC, Murakami A, Galvao Neto M, et al. NOTES transvaginal video-assisted cholecystectomy: first series. Endoscopy. 2008;40:572–575. [DOI] [PubMed] [Google Scholar]
- 29.Zornig C, Mofid H, Siemssen L, et al. Transvaginal NOTES hybrid cholecystectomy: feasibility results in 68 cases with mid-term follow-up. Endoscopy. 2009;41:391–394. [DOI] [PubMed] [Google Scholar]
- 30.Linke GR, Tarantino I, Hoetzel R, et al. Transvaginal rigid-hybrid NOTES cholecystectomy: evaluation in routine clinical practice. Endoscopy. 2010;42:571–575. [DOI] [PubMed] [Google Scholar]
- 31.Federlein M, Borchert D, Muller V, et al. Transvaginal video-assisted cholecystectomy in clinical practice. Surg Endosc. 2010;24:2444–2452. [DOI] [PubMed] [Google Scholar]
- 32.Hensel M, Schernikau U, Schmidt A, et al. Surgical outcome and midterm follow-up after transvaginal NOTES hybrid cholecystectomy: analysis of a prospective clinical series. J Laparoendosc Adv Surg Tech A. 2011;21:101–106. [DOI] [PubMed] [Google Scholar]
- 33.Nijhawan S, Barajas-Gamboa JS, Majid S, et al. NOTES transvaginal hybrid cholecystectomy: the United States human experience. Surg Endosc. 2013;27:514–517. [DOI] [PubMed] [Google Scholar]
- 34.Noguera JF, Cuadrado A, Dolz C, et al. [Non-randomised, comparative, prospective study of transvaginal endoscopic cholecystectomy versus transparietal laparoscopic cholecystectomy]. Cir Esp. 2009;85:287–291. [DOI] [PubMed] [Google Scholar]
- 35.Zornig C, Siemssen L, Emmermann A, et al. NOTES cholecystectomy: matched-pair analysis comparing the transvaginal hybrid and conventional laparoscopic techniques in a series of 216 patients. Surg Endosc. 2011;25:1822–1826. [DOI] [PubMed] [Google Scholar]
- 36.Solomon D, Shariff AH, Silasi DA, et al. Transvaginal cholecystectomy versus single-incision laparoscopic cholecystectomy versus four-port laparoscopic cholecystectomy: a prospective cohort study. Surg Endosc. 2012;26:2823–2827. [DOI] [PubMed] [Google Scholar]
- 37.Borchert D, Federlein M, Ruckbeil O, et al. Prospective evaluation of transvaginal assisted cholecystectomy. Surg Endosc. 2012;26:3597–3604. [DOI] [PubMed] [Google Scholar]
- 38.Noguera JF, Cuadrado A, Dolz C, et al. Prospective randomized clinical trial comparing laparoscopic cholecystectomy and hybrid natural orifice transluminal endoscopic surgery (NOTES) (NCT00835250). Surg Endosc. 2012;26:3435–3441. [DOI] [PubMed] [Google Scholar]
- 39.Al-Azawi D, Houssein N, Rayis AB, et al. Three-port versus four-port laparoscopic cholecystectomy in acute and chronic cholecystitis. BMC Surg. 2007;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monkhouse SJ, Court EL, Beard LA, et al. A retrospective wound review of standard four-port laparoscopic cholecystectomy: is there need for single-port laparoscopic surgery? Surg Endosc. 2012;26:255–260. [DOI] [PubMed] [Google Scholar]