Abstract
Anthropologists frequently use the shaft bending strength index to infer the physical activity levels of humans living in the past from their lower limb bone remains. This index is typically calculated as the ratio of bone shaft second moments of area about orthogonal principal axes (i.e. Imax/Imin). Individuals with high Imax/Imin values are inferred to have been very active, whereas individuals with low values are inferred to have been more sedentary. However, there is little direct evidence that activity has a causal and predictable effect on the shaft bending strength index. Here, we report the results of two experiments that were designed to test the model within which anthropologists commonly interpret the shaft bending strength index. In the first experiment, mice were treated daily with treadmill exercise for 1 month to simulate a high-activity lifestyle. In the second experiment, in an attempt to simulate a low-activity lifestyle, functional weight-bearing was removed from the hindlimbs of mice for 1 month. Femoral mid-shaft structure was determined with μCT. We found that while exercise resulted in significant enhancement of Imax and Imin compared with controls, it failed to significantly increase the Imax/Imin index. Similarly, stunted bone growth caused by unloading resulted in significantly diminished Imax and Imin compared with controls, but low activity did not lead to significantly decreased Imax/Imin compared with normal activity. Together, these results suggest that caution is required when the bone shaft bending strength index is used to reconstruct the activity levels of past humans.
Keywords: anthropology, bioarchaeology, bone functional adaptation, cortical bone, cross-sectional geometry, diaphyseal shape, physical activity
Introduction
Anthropologists frequently analyze the cross-sectional geometry of lower limb bone shafts to infer the physical activity levels of past humans from their skeletal remains (Ruff, 2008). This strategy is based on the well-documented capacity of bone shafts to adjust their morphology in response to mechanical loading, particularly during the growing years (Lieberman et al. 2001; McKay et al. 2005; Weeks et al. 2008). Of the geometric properties used to infer activity levels, the bending strength index is often considered the most informative (Ruff, 1987; Brock & Ruff, 1988; Larsen et al. 1995; Holt, 2003; Stock, 2006; Lieverse et al. 2011).
The bending strength index can be calculated in two ways, as the ratio of shaft second moments of area in the anteroposterior plane to second moments of area in the mediolateral plane (Ix/Iy) and, more commonly, as the ratio of second moments of area about orthogonal principal axes (Imax/Imin). Shafts with Ix/Iy values close to 1 are roughly circular, whereas shafts with values greater or < 1 are more elliptical. The same logic applies to Imax/Imin, but this ratio is always ≥ 1. In anthropological analyses, individuals with elliptical shafts are inferred to have been more physically active, as doing a lot of walking and running will tend to stress one's lower limb bones in a particular direction (Cavanagh & LaFortune, 1980; Munro et al. 1987; Yang et al. 2014), whereas individuals with circular shafts are inferred to have been more sedentary.
The utility of a relatively simple measure of physical activity level such as the bone shaft bending strength index is readily apparent. However, while there is strong experimental support for a causal link between physical activity level and cross-sectional properties describing overall shaft robusticity (size), direct evidence is currently lacking that activity level has a causal and predictable effect on the shaft bending strength index. Here, we report the results of two experiments involving mice as a model organism that tested whether high activity levels indeed lead to more elliptical limb bone shafts and low activity levels lead to more circular shafts. In the first experiment, animals were treated daily with a treadmill exercise regimen to simulate a high-activity lifestyle. In the second experiment, animals were suspended from their tails to cause prolonged limb unloading and simulate a low-activity lifestyle.
Materials and methods
All experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Stony Brook University.
Experiment 1: exercise
Female Hsd:ICR mice (n = 40) were acquired at 3 weeks old from Harlan Sprague Dawley (Indianapolis, ID, USA). As Hsd:ICR is an outbred stock (as opposed to an inbred strain), genetics was an uncontrolled variable. Mice from this particular stock were chosen for analysis because exercise-loading has previously been shown to have positive effects on their hindlimb bone structure (Kelly et al. 2006). Animals were housed individually and maintained on a 12:12 h light:dark cycle with free access to food and water. At 4 weeks old, animals were divided into runners and sedentary controls (n = 20/group). Groups were matched according to body mass. Runners were treated 5 days per week for 4 weeks with 30 min of exercise on a six-lane treadmill (Columbus Instruments, Columbus, OH, USA) at a rate of 12 m min−1. At 8 weeks old, animals were killed, and right femora were extracted.
Femora were scanned at a 10-μm3 voxel size (70 kVp, 114 μA, 150-ms integration time) using a μCT 40 scanner (Scanco Medical AG, Brüttisellen, Switzerland). The volume of interest was a 600-μm-long region of the mid-shaft. Volumes were segmented using a constrained 3D Gaussian filter to reduce noise (support = 1, sigma = 0.1) and thresholded to extract the bone phase using a single threshold value for all bones. Imax and Imin were computed using the internal imaging code supplied by the scanner manufacturer. These properties were chosen to calculate the bending strength index, rather than Ix and Iy, as they are based on the intrinsic distribution of bone in the shaft and thus provide a better measure of shape (Shaw & Stock, 2009). Values were sized-standardized by the product of body mass and bone length, as recommended when analyzing shaft second moments of area in weight-bearing elements (Ruff et al. 1993; Ruff, 2008).
Experiment 2: unloading
Female F2 mice (n = 29) were bred from a double-cross of female BAL/cByJ and male C3H/HeJ progenitors purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Hindlimb unloading has previously been shown to have a catabolic effect on bone structure in these mice (Judex et al. 2004, 2013). Again, as these mice are genetically heterogeneous, genetics was an uncontrolled variable. Animals were housed individually and kept on a 12:12 h light:dark cycle with food and water freely available. At 16 weeks old, animals were assigned to an unloading group (n = 14) or control group (n = 15), which were also matched by body mass. Hindlimb unloading was induced for 4 weeks by hindlimb elevation through tail suspension (Morey-Holton & Globus, 2002). Briefly, cloth tape was applied to the tail and secured to a cable system connected to a swivel mounted at the top of the cage, which allowed 360° free rotation. The elevation of the cable was adjusted to maintain the animal at roughly 30° head-down tilt, such that its forelimbs contacted the cage floor but its hindlimbs were freed from weight-bearing. Animals were kept in a suspended position 24 h day−1, but could access food and water with their forelimbs. Mice in the control group engaged in normal weight-bearing cage activities.
Immediately prior to and after unloading, all animals underwent in vivo μCT scanning (VivaCT 75, Scanco Medical AG) to track longitudinal changes in shaft structure in the right femur. A 600-μm-long mid-shaft volume was scanned in vivo at a 20.5 μm3 voxel size (45 kVp, 177 μA, 200-ms integration time). During scanning, mice were sedated by inhalation anesthesia with isoflurane. μCT image processing followed the same protocol as in the first experiment.
Statistics
Independent samples t-tests were performed to test for differences in traits between animals treated with treadmill exercise and sedentary controls. In the unloading and control groups, paired samples t-tests were used to assess change in bone properties over the experimental period. To test for treatment effects between the unloading and control groups, ancovas were carried out with post-treatment values as dependent variables and baseline values as the covariate (Vickers, 2001). Analyses were performed in R software (R Core Development Team, 2014). The significance level was P < 0.05, and tests were two-tailed. Descriptive statistics are provided in Tables S1 and S2.
Results
Experiment 1: exercise
Treatment with treadmill exercise resulted in significantly greater femoral mid-shaft Imax (P = 0.004) and Imin (P = 0.005) compared with sedentary controls (Fig.1). However, exercise did not significantly alter the Imax/Imin bending strength index (P = 0.74), indicating that the simulated high-activity lifestyle did not lead to more elliptically shaped femoral mid-shafts.
Fig 1.
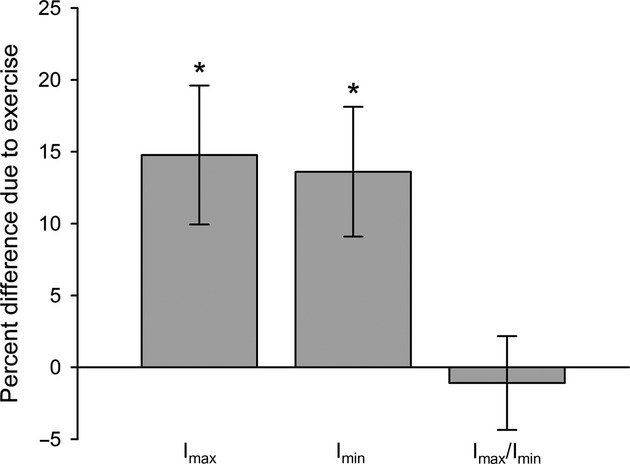
Relative difference in femoral mid-shaft traits between controls and exercised animals. Bars equal the percent difference between the exercise mean relative to the control mean. Whiskers equal the standard deviation of the sampling distribution of the relative difference. Asterisks indicate statistically significant (P < 0.05) differences between groups.
Experiment 2: unloading
In control animals, femoral mid-shaft Imax and Imin both increased significantly (P < 0.0001) over the course of the experimental period (Fig.2). By contrast, in animals subjected to hindlimb unloading, little change occurred in either Imax (P = 0.34) or Imin (P = 0.40). Stunted normal bone growth in the unloading group ultimately led to significantly (P < 0.0001) diminished femoral mid-shaft Imax and Imin compared with controls. During the experiment, the Imax/Imin bending strength index decreased significantly and roughly equally in the control (P = 0.007) and unloading groups (P = 0.002), such that the simulated low-activity lifestyle did not result in greater femoral mid-shaft circularity compared with normal activity (P = 0.64).
Fig 2.
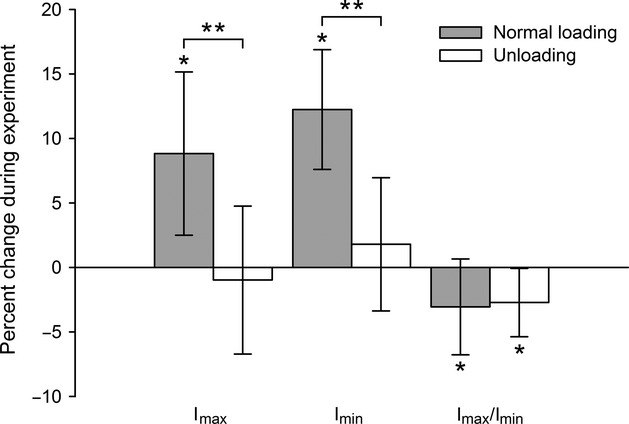
Longitudinal change in femoral mid-shaft traits in controls and animals treated with hindlimb unloading. Bars equal mean percent change relative to baseline values, and whiskers equal the standard deviation. Single asterisks indicate statistically significant (P < 0.05) longitudinal change within groups during the experiment. Double asterisks indicate significant differences between groups at the end of the experiment, determined by statistical analyses that controlled for baseline variation.
Discussion
Extrapolating experimental data from mice to humans requires caution given critical differences between species, including size, locomotor styles and bone tissue composition. Even so, mice are the experimental model of choice in most bone biology research for both logistical reasons and because the genes and molecules influencing the skeleton are highly conserved in mice and humans (Karsenty & Ferron, 2012). Furthermore, the skeletal reaction to alterations in mechanical environment is generally similar between species (Squire et al. 2004; Luu et al. 2009). If extrapolation of mouse data to humans is warranted, then the results of the two experiments presented here suggest that high- and low-activity lifestyles do not have the causal effects on the shaft bending strength index that are often assumed in anthropological analyses of human lower limb bone shaft structure (Ruff, 1987; Brock & Ruff, 1988; Larsen et al. 1995; Holt, 2003; Stock, 2006; Lieverse et al. 2011).
The results of the first experiment are generally consistent with those of previous experiments involving animal models, as well as longitudinal studies involving humans, that measured the effects of exercise on the bone shaft bending strength index. Similar to our experiment, Lieberman et al. (2001) and Plochocki et al. (2008) treated sheep and mice, respectively, with a running regimen and documented significant exercise-related enlargements of hindlimb shaft Imax and Imin but no significant increase in the Imax/Imin index. In a prospective human study by Macdonald et al. (2009), in which growing boys engaged in a school-based exercise program comprised of jumping, skipping, dancing and playground circuits, positive change in tibial Imax during the trial period was significantly greater in the exercise group compared with a control group, yet change in Imax/Imin was not significantly altered by exercise. Vainionpää et al. (2007) conducted a study in which adult women participated regularly in exercise sessions that included step patterns, stamping, jumping, running and walking. In both the femur and tibia, longitudinal changes in Imax, Imin and Imax/Imin were not significantly affected by exercise, perhaps reflecting the diminished responsiveness of the adult skeleton to loading. To our knowledge, no experimental or longitudinal study has ever documented a significant increase in limb bone shaft Imax/Imin resulting from engagement in an exercise regimen.
The results of the second experiment are novel, and thus they are unlikely to be accepted outright without replication. Therefore, it is instructive to compare these results with those obtained from separate but similar experiments performed in our laboratory. In an unpublished study, we treated growing female mice with hindlimb unloading for 2 weeks starting at 9 weeks old and, consistent with our initial results, normal development of femoral Imax and Imin was found to be significantly retarded by unloading but the shaft bending strength index was not affected (Fig. S1). In another study, skeletally mature adult male mice were tail-suspended for 2 weeks beginning at 28 weeks old, and instead of stunting normal bone growth, unloading led to significant loss of femoral Imax and Imin compared with morphological stasis among controls (Fig. S2; see also Gupta et al. 2012). Yet, once again, the femoral shaft bending strength index was not altered by unloading. At present, all experimental data of which we are aware indicate that unloading does not lead to more circular shafts, regardless of whether it occurs early or later in life (but, see Lanyon, 1980).
What evidence exists then for the link between physical activity level and the bone shaft bending strength index that is assumed in many anthropological analyses of human skeletal remains? Two types of indirect evidence are frequently cited. First, differences in the shaft bending strength index have been observed among living human populations with distinct activity patterns. For example, Shaw & Stock (2009) analyzed tibial mid-shaft shape among university cross-country runners, field hockey players and non-athletic controls, and found that, relative to both hockey players and controls, runners exhibited more elliptical shafts, primarily due to enhanced bending rigidity in the anteroposterior plane. The authors speculated that the elliptical shape of the runners' shafts was caused by the repetitive anteroposterior tibial loading that characterizes their training regimen, although this could not be demonstrated conclusively as this was a cross-sectional study and not a longitudinal study. Second, in anthropological studies of humans living in the past, changes in archaeological measures of settlement patterns that are concomitant with changes in the bone shaft bending strength index may suggest a connection between physical activity level and shaft shape. For example, Holt (2003) has shown that during the European Upper Paleolithic, femoral shafts became more circular at roughly the same time that changes in the archaeological record point to increasing population density and sedentism. Of these two types of indirect evidence, we find the former to be somewhat more persuasive as the activity patterns of living groups are actually known, while those of ancient populations are not.
There are also two lines of direct evidence that are often cited to support the idea of a close relationship between physical activity level and the bone shaft bending strength index. First, the hypothesis that elliptically shaped bone shafts reflect high levels of physical activity is based on the premise that bone formation primarily takes place in the plane that bones engender peak mechanical stresses and strains; and the results of certain experiments with animal models in which in vivo strains were directly related to patterns of bone growth support this assumption. For example, peak magnitude strains have been associated with sites of increased bone formation in experiments involving external loading of rodent limbs (Mosley et al. 1997; Warden et al. 2004) and natural physiological limb loading in goats (Main, 2007). In contrast, however, other experiments involving weight-bearing exercise in chickens and sheep (Judex et al. 1997; Wallace et al. 2014) and exogenous limb loading in turkeys (Gross et al. 1997) found no significant associations between bone formation stimulated by loading and local strain magnitude. Ultimately, the disparity between results from different experiments suggests to us that a universal relationship between bone growth and local strain magnitude probably does not exist and, therefore, that loading cannot be assumed to have a predictable effect on shaft shape in every instance. Second, in the aforementioned longitudinal human study by Vainionpää et al. (2007), although engagement in the exercise regimen did not significantly alter the lower limb bone shaft bending strength index, across the entire study population (i.e. exercise participants and controls), weak but significant positive correlations were detected between the number of daily impacts (measured by accelerometer) and changes in femoral and tibial Imax/Imin throughout the course of the study. This is arguably the most compelling evidence for a causal relationship between physical activity level and human lower limb bone shaft shape. Nevertheless, until additional direct evidence is available from longitudinal and/or experimental studies, we suggest that much prudence is necessary when the shaft bending strength index is used to infer the activity levels of past humans.
Acknowledgments
The funding for this research was provided by the National Aeronautics and Space Administration (grants NNX08BA35G and NNX12AL25G) and the L.S.B. Leakey Foundation (grant 49357).
Conflict of interest
The authors declare no conflict of interest.
Author contributions
I.J.W., S.G., J.S., B.D. and S.J. conceived, designed and performed the experiments; I.J.W., S.G. and J.S. analyzed the data; I.J.W. wrote the paper.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Change in femoral mid-shaft traits in growing controls and animals treated with hindlimb unloading.
Fig. S2. Longitudinal change in femoral mid-shaft traits in skeletally mature adult controls and animals treated with hindlimb unloading.
Table S1. Femoral mid-shaft trait values of controls and animals treated with treadmill exercise at the end of the experiment.
Table S2. Femoral mid-shaft trait values of controls and animals treated with hindlimb unloading at baseline and after the treatment period.
References
- Brock SL, Ruff CB. Diachronic patterns of change in structural properties of the femur in the prehistoric American Southwest. Am J Phys Anthropol. 1988;75:113–127. doi: 10.1002/ajpa.1330750113. [DOI] [PubMed] [Google Scholar]
- Cavanagh PR, LaFortune MA. Ground reaction forces in distance running. J Biomech. 1980;13:397–406. doi: 10.1016/0021-9290(80)90033-0. [DOI] [PubMed] [Google Scholar]
- Gross TS, Edwards JL, McLeod KJ, et al. Strain gradients correlate with sites of periosteal bone formation. J Bone Miner Res. 1997;12:982–988. doi: 10.1359/jbmr.1997.12.6.982. [DOI] [PubMed] [Google Scholar]
- Gupta S, Vijayaraghavan S, Uzer G, et al. Multiple exposures to unloading decrease bone's responsivity but compound skeletal losses in C57BL/6 mice. Am J Physiol Regul Intergr Comp Physiol. 2012;303:R159–R167. doi: 10.1152/ajpregu.00499.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt BM. Mobility in Upper Paleolithic and Mesolithic Europe: evidence from the lower limb. Am J Phys Anthropol. 2003;122:200–215. doi: 10.1002/ajpa.10256. [DOI] [PubMed] [Google Scholar]
- Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- Judex S, Gross TS, Zernicke RF. Strain gradients correlate with sites of exercise-induced bone-forming surfaces in the adult skeleton. J Bone Miner Res. 1997;12:1737–1745. doi: 10.1359/jbmr.1997.12.10.1737. [DOI] [PubMed] [Google Scholar]
- Judex S, Garman R, Squire M, et al. Genetically linked site-specificity of disuse osteoporosis. J Bone Miner Res. 2004;19:607–613. doi: 10.1359/JBMR.040110. [DOI] [PubMed] [Google Scholar]
- Judex S, Zhang W, Donahue LR, et al. Genetic loci that control the loss and regain of trabecular bone during unloading and reambulation. J Bone Miner Res. 2013;28:1537–1549. doi: 10.1002/jbmr.1883. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481:314–320. doi: 10.1038/nature10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SA, Czech PP, Wight JT, et al. Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J Morphol. 2006;267:360–374. doi: 10.1002/jmor.10407. [DOI] [PubMed] [Google Scholar]
- Lanyon LE. The influence of function on the development of bone curvature. An experimental study on the rat tibia. J Zool Lond. 1980;192:457–466. [Google Scholar]
- Larsen CS, Ruff CB, Kelly RL. Structural analysis of the Stillwater postcranial human remains: behavioral implications of articular joint pathology and long bone diaphyseal morphology. Anthropol Pap Am Mus. 1995;77:107–133. [Google Scholar]
- Lieberman DE, Devlin MJ, Pearson OM. Articular area responses to mechanical loading: effects of exercise, age, and skeletal location. Am J Phys Anthropol. 2001;116:266–277. doi: 10.1002/ajpa.1123. [DOI] [PubMed] [Google Scholar]
- Lieverse AR, Stock JT, Katzenberg MA, et al. The bioarchaeology of habitual activity and dietary change in the Siberian Middle Holocene. In: Pinhasi R, Stock JT, editors. Human Bioarchaeology of the Transition to Agriculture. New York: Wiley-Blackwell; 2011. pp. 265–291. [Google Scholar]
- Luu YK, Capilla E, Rosen CH, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald HM, Cooper DML, McKay HA. Anterior-posterior bending strength at the tibial shaft increases with physical activity in boys: evidence for non-uniform geometric adaptation. Osteoporos Int. 2009;20:61–70. doi: 10.1007/s00198-008-0636-9. [DOI] [PubMed] [Google Scholar]
- Main RP. Ontogenetic relationships between in vivo strain environment, bone histomorphometry and growth in the goat radius. J Anat. 2007;210:272–293. doi: 10.1111/j.1469-7580.2007.00696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay HA, MacLean L, Petit M, et al. “Bounce at the Bell”: a novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br J Sports Med. 2005;39:521–526. doi: 10.1136/bjsm.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley JR, March BM, Lynch J, et al. Strain magnitude related changes in whole bone architecture in growing rats. Bone. 1997;20:191–198. doi: 10.1016/s8756-3282(96)00385-7. [DOI] [PubMed] [Google Scholar]
- Munro CF, Miller DI, Fuglevand AJ. Ground reaction forces in running: a re-examination. J Biomech. 1987;20:147–155. doi: 10.1016/0021-9290(87)90306-x. [DOI] [PubMed] [Google Scholar]
- Plochocki JH, Rivera JP, Zhang C, et al. Bone modeling response to voluntary exercise in the hindlimb of mice. J Morphol. 2008;269:313–318. doi: 10.1002/jmor.10587. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Ruff CB. Sexual dimorphism in human lower limb bone structure: relationships to subsistence strategy and sexual division of labor. J Hum Evol. 1987;16:391–416. [Google Scholar]
- Ruff CB. Biomechanical analyses of archaeological human skeletons. In: Katzenberg MA, Saunders SR, editors. Biological Anthropology of the Human Skeleton. New York: Alan R. Liss; 2008. pp. 183–206. [Google Scholar]
- Ruff CB, Trinkaus E, Walker A, et al. Postcranial robusticity in Homo. I: temporal trends and mechanical interpretation. Am J Phys Anthropol. 1993;91:21–53. doi: 10.1002/ajpa.1330910103. [DOI] [PubMed] [Google Scholar]
- Shaw CN, Stock JT. Intensity, repetitiveness, and directionality of habitual adolescent mobility patterns influence the tibial diaphysis morphology of athletes. Am J Phys Anthropol. 2009;140:149–159. doi: 10.1002/ajpa.21064. [DOI] [PubMed] [Google Scholar]
- Squire M, Donahue LR, Rubin C, et al. Genetic variations that regulate bone morphology in the male mouse skeleton do not define its susceptibility to mechanical unloading. Bone. 2004;35:1353–1360. doi: 10.1016/j.bone.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Stock JT. Robusticity relative to patterns of mobility, climatic adaptation, and selection for tissue economy. Am J Phys Anthropol. 2006;131:194–204. doi: 10.1002/ajpa.20398. [DOI] [PubMed] [Google Scholar]
- Vainionpää A, Korpelainen R, Sievänen H, et al. Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone. 2007;40:604–611. doi: 10.1016/j.bone.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace IJ, Demes B, Mongle C, et al. Exercise-induced bone formation is poorly linked to local strain magnitude in the sheep tibia. PLoS ONE. 2014;9:e99108. doi: 10.1371/journal.pone.0099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden SJ, Fuchs RK, Turner CH. Steps for targeting exercise towards the skeleton to increase bone strength. Eur Med Phys. 2004;40:223–232. [PubMed] [Google Scholar]
- Weeks BK, Young CM, Beck BR. Eight months of regular in-school jumping improves indices of bone strength in adolescent boys and girls: the POWER PE study. J Bone Miner Res. 2008;23:1002–1011. doi: 10.1359/jbmr.080226. [DOI] [PubMed] [Google Scholar]
- Yang P-F, Sanno M, Ganse B, et al. Torsion and antero-posterior bending in the in vivo human tibia loading regimes during walking and running. PLoS ONE. 2014;9:e94525. doi: 10.1371/journal.pone.0094525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Change in femoral mid-shaft traits in growing controls and animals treated with hindlimb unloading.
Fig. S2. Longitudinal change in femoral mid-shaft traits in skeletally mature adult controls and animals treated with hindlimb unloading.
Table S1. Femoral mid-shaft trait values of controls and animals treated with treadmill exercise at the end of the experiment.
Table S2. Femoral mid-shaft trait values of controls and animals treated with hindlimb unloading at baseline and after the treatment period.


