Abstract
The baculum (os penis) has been extensively studied as a taxon-specific character in bats and other mammals but its mechanical function is still unclear. There is a wide consensus in the literature that the baculum is probably a sexually selected character. Using a novel approach combining postmortem manipulation and three-dimensional (3D) imaging, we tested two functional hypotheses in the common noctule bat Nyctalus noctula, the common pipistrelle Pipistrellus pipistrellus, and Nathusius’ pipistrelle Pipistrellus nathusii: (i) whether the baculum can protect the distal urethra and urethral opening from compression during erection and copulation; and (ii) whether the baculum and corpora cavernosa form a functional unit to support both the penile shaft and the more distal glans tip. In freshly dead or frozen and thawed bats, we compared flaccid penises with artificially ‘erect’ penises that were inflated with 10% formalin. Penises were stained with alcoholic iodine and imaged with a lab-based high-resolution x-ray microtomography system. Analysis of the 3D images enabled us to compare the changes in relative positions of the baculum, corpora cavernosa, urethra, and corpus spongiosum with one another between flaccid and ‘erect’ penises. Our results support both functional hypotheses, indicating that the baculum probably performs two different roles during erection. Our approach should prove valuable for comparing and testing the functions of different baculum morphologies in bats and other mammals. Moreover, we have validated an essential component of the groundwork necessary to extend this approach with finite element analysis for quantitative 3D biomechanical modeling of penis function.
Keywords: 3D model, Chiroptera, functional morphology, iodine stain, micro-CT, penis bone, x-ray microtomography
Introduction
Many different mechanical and behavioral hypotheses have been proposed for baculum function in bats, and in mammals more generally. However, the only studies on baculum function based on experimental data were done in rats (Rattus norvegicus) by Kelly (2000) and in mice (Mus musculus domesticus) by Simmons & Firman (2013) and Stockley et al. (2013).
A single mechanical function for all the different baculum shapes is very unlikely (Ruth, 1934; Dixson, 2012), so there are probably various and overlapping mechanical functions (Dyck et al. 2004). The baculum and corpora cavernosa could form a functional unit due to their tight connection and shared envelope of fibrous connective tissue. This functional unit could increase the flexural stiffness of the penis by transferring bending forces from the distal end of the glans penis to the corpora cavernosa during copulation (Long & Frank, 1968; Kelly, 2000). The baculum could increase the stiffness of the corpora cavernosa by being pressed into their distal ends, thus increasing the hydrostatic pressure within the corpora cavernosa during copulation (Long & Frank, 1968; Kelly, 2000). The baculum could affect penile shape during erection, and it could protect the distal end of the urethra from compression, especially in species in which coital locking occurs (Dyck et al. 2004; Dixson, 2012). The baculum may allow males to gain intromission if males are larger then females or if the penis is inserted before it is fully erect (Long & Frank, 1968; Dixson, 2012). It might also serve to expand the female cervix to optimize sperm deposition (Long & Frank, 1968).
In bats of the genera Pipistrellus (Herdina et al. 2014b) and Plecotus (Herdina et al. 2010) our histological data also support the hypotheses that the baculum protects the urethra from compression during copulation (Dyck et al. 2004) and that it forms a functional unit with the corpora cavernosa. During copulation this functional unit can transfer bending forces from the distal end of the glans penis to the corpora cavernosa, thereby increasing the flexural stiffness of the whole erect penis (Long & Frank, 1968; Kelly, 2000; Herdina et al. 2010, 2014b).
In bats, mating systems and songflight behavior have been described for several species, including Nyctalus noctula (McCracken & Wilkinson, 2000), Pipistrellus pipistrellus (Sachteleben & von Helversen, 2006), and Pipistrellus nathusii (Jahelková & Horáček, 2011). In all three species the mating systems have been described as resource defense polygyny with seasonal single-male multi-female groups, but it seems to be more complicated and might share traits with a lek-based mating system (Sachteleben & von Helversen, 2006; Jahelková & Horáček, 2011). However, in bats very little is known about mating behavior, copulation duration, or copulation postures (Glass, 1966; Barclay & Thomas, 1979; O'Brien & Nankervis, 1994; Hosken et al. 2001; Dixson et al. 2004, 2004; Sachteleben & von Helversen, 2006; Jahelková & Horáček, 2011; Liu et al. 2013). For mammals in general, behavioral functions of the baculum have also been proposed. The baculum could enable protracted copulations (Dixson, 1987, 2012) and could stimulate the female reproductive tract (Dyck et al. 2004; Dixson, 2012). It could play a role in eliciting neuroendocrine responses necessary for induced mating postures and ovulation, or the stimulation of corpus luteum function, in species that depend on mating to trigger these events (Patterson & Thaeler, 1982; Dixson, 2012). The baculum could also provide information about male size or quality during intromission (Dyck et al. 2004). This could enable the baculum to function as a reproductive isolating mechanism (Patterson & Thaeler, 1982).
Further studies on baculum function, mating systems, and copulation behavior in bats can also contribute to answering questions on the evolution of bacular diversity and the mechanism of sexual selection on baculum shape and size (Patterson & Thaeler, 1982; Eberhard, 1993; Dixson et al. 2004; Hosken & Stockley, 2004; Lüpold et al. 2004).
Aims
In this study we tested two functional hypotheses in three bat species with similar baculum shapes, the common noctule bat N. noctula, the common pipistrelle P. pipistrellus, and Nathusius’ pipistrelle P. nathusii: (i) whether the baculum can protect the distal urethra and urethral opening from compression during erection and copulation (Dixson, 1995); and (ii) whether the baculum and corpora cavernosa form a functional unit to support both the penile shaft and the more distal glans tip (Kelly, 2000).
Materials and methods
The specimens for this study were made available by the Department of Zoology, Charles University, Prague (N. noctula, n = 15), the Naturalis Biodiversity Center, Leiden (P. pipistrellus, n = 9; P. nathusii, n = 10) and the Natural History Museum Vienna (P. nathusii, n = 1). We chose these three species for this study because of general similarities in their overall baculum morphology (Hill & Harrison, 1987) and mating systems (McCracken & Wilkinson, 2000). The N. noctula specimens were collected approximately 40 h after they died in construction work at their hibernaculum; they were not preserved or frozen during that time. The Pipistrellus specimens were found dead or died in rescue stations and were frozen shortly after death (between 2009 and 2013). We thawed them directly before preparation. All the specimens used in this study will be vouchered in the mammal collection of the Natural History Museum Vienna.
We follow the definition of the glans penis of Meisenheimer (1921), where the corpora cavernosa end in the shaft of the penis and do not extend into the glans, whereas the baculum is always found completely within the glans.
The corpora cavernosa in the penes of 26 bats were inflated with formalin (see Fig.1 and Kelly, 2000). The penis was carefully resected close to the pubic bone. Sewing thread was tied loosely around the penis near its proximal end. The needle (30G, diameter 0.30 mm) of a syringe filled with 10% formalin was inserted into a corpus cavernosum at the proximal (cut off) end. The thread was pulled taut around the penis and needle. The formalin was slowly pushed into the corpora cavernosa, until it started to leak out at the tied-off end. The needle was removed and the thread tied tightly. The penis was then fixed in 10% formalin for at least 7 days. Additionally, the flaccid penes of nine bats were fixed in 10% formalin.
Fig 1.

Photos of inflating the penis of a male Pipistrellus pipistrellus with formalin. Left: the bat with its label and a syringe with 30-gauge needle; top right: the same needle inserted in the resected penis of the same bat with the base tied shut before inflation; bottom right: penis after inflation with formalin leaking out at the base.
After fixation the penes were transferred to 100% ethanol via ascending ethanol concentrations. They were stained with 1% (w/v) elemental iodine in 100% ethanol (I2E; Metscher, 2009a; Herdina et al. 2010) for at least 14 h to several days. Before scanning, the samples were transferred back to 100% ethanol for at least 1 h to improve contrast. Samples were mounted in polypropylene micro-pipette tips (heat-sealed and filled with 100% ethanol; Metscher, 2009b) and sealed with parafilm.
High resolution microCT images were made with the Xradia MicroXCT system in the Theoretical Biology Department at the University of Vienna, using a microfocus tungsten source, secondary optical magnification of the scintillator images, and a 2k × 2k cooled CCD camera (www.xradia.com). Projection images were collected every 0.25° over a rotation of 180° (plus the cone angle; Metscher, 2011) with 2 × 2 pixel binning, 4 × optical magnification, exposure times of 4–10 s, and source voltages of 40–60 kVp at 4–8 W.
Tomographic sections were reconstructed using the XMReconstructor software (version 8.1) supplied with the Xradia system. Reconstructed voxel sizes were 4.0–8.0 μm for the Pipistrellus samples, and 3.4–5.0 μm for the Nyctalus samples. Samples too long to fit within one field of view at the desired magnification were scanned in two overlapping segments along the rotation axis, and the reconstructions were concatenated with the Xradia XMController program stitching function.
The reconstructed images were converted to image stacks of virtual sections and exported to amira 5.4.5 (http://www.fei.com/software/amira-3d-for-life-sciences/). In amira, two penes per species (one flaccid and one inflated) were visualized as volume renderings and the following elements were manually segmented: the baculum, the lacunae of the corpora cavernosa, the tunica albuginea of the corpora cavernosa, the urethral lumen, and the corpus spongiosum (with the urethra). Surface renderings of the penes in their flaccid and ‘erect’ state were compared among species.
Results
The three species studied were chosen to investigate the function of this general baculum shape, with a long slender shaft and bifurcated ends, but we also found certain differences among them. The largest of the species, N. noctula, has the largest baculum absolutely, but also in relation to penis length. In N. noctula, only the distal-most part of the baculum is enveloped by the corpus spongiosum (Fig. 3, gray arrows), whereas in both pipistrelle species more than half of the baculum shaft is enveloped (Figs 4 and 5, gray arrows). Baculum shape is very similar in N. noctula and P. pipistrellus (supporting information Fig. S7) with a dorsoventral curve in lateral view. The baculum of P. nathusii is bent back towards the dorsal surface of the penis in mid-shaft but is curved proximally as in the other species. Soft tissue morphology of the penis is similar all three species, except for the shape of the prepuce and the portion of the baculum covered by corpus spongiosum tissue in the glans penis. Also, in the ‘erect’ penis of P. nathusii the corpora cavernosa seem to show greater expansion than in either of the other species.
Fig 3.
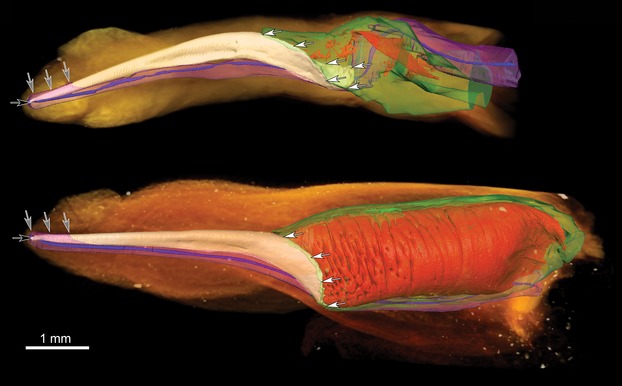
3D models of Nyctalus noctula penes. 3D surface renderings of manually segmented tissues overlaid on volume renderings of a flaccid (top) and an inflated (bottom) N. noctula penis. Gray arrows mark where the corpus spongiosum is enveloping the baculum. White arrows mark the area where the baculum and corpora cavernosa are linked. Black arrow shows the urethral opening. Orange: outer shape of the penis (volume rendering), white: baculum, red: blood lacunae in the corpora cavernosa, green: tunica albuginea of the corpora cavernosa, blue: urethra, pink: corpus spongiosum.
Fig 4.
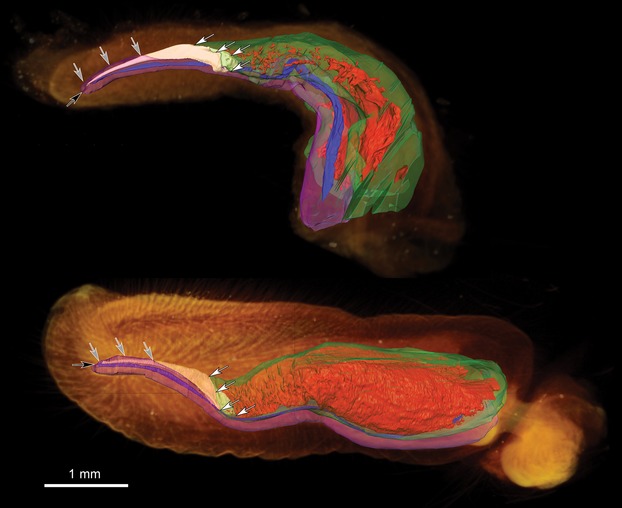
3D models of Pipistrellus pipistrellus penes. 3D surface renderings of manually segmented tissues overlaid on volume renderings of a flaccid (top) and an inflated (bottom) P. pipistrellus penis. Gray arrows mark where the corpus spongiosum is enveloping the baculum. White arrows mark the area where the baculum and corpora cavernosa are linked. Black arrow shows the urethral opening. Orange: outer shape of the penis (volume rendering), white: baculum, red: blood lacunae in the corpora cavernosa, green: tunica albuginea of the corpora cavernosa, blue: urethra, pink: corpus spongiosum.
Fig 5.
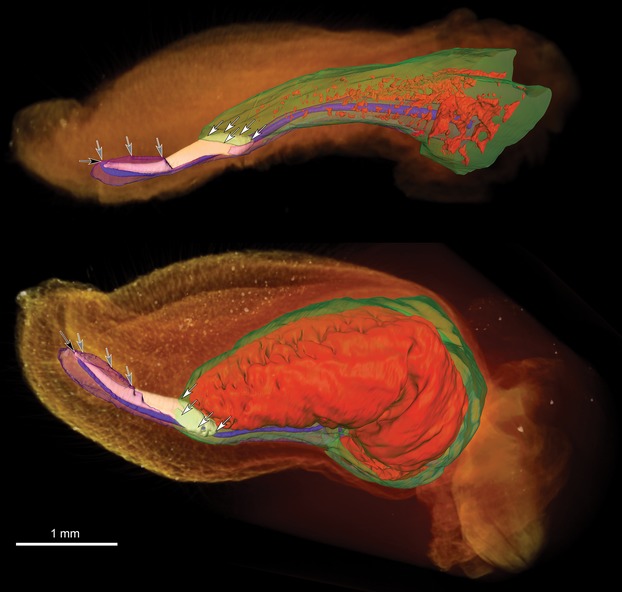
3D models of Pipistrellus nathusii penes. 3D surface renderings of manually segmented tissues overlaid on volume renderings of a flaccid (top) and an inflated (bottom) P. nathusii penis. Gray arrows mark where the corpus spongiosum is enveloping the baculum. White arrows mark the area where the baculum and corpora cavernosa are linked. Black arrow shows the urethral opening. Orange: outer shape of the penis (volume rendering), white: baculum, red: blood lacunae in the corpora cavernosa, green: tunica albuginea of the corpora cavernosa, blue: urethra, pink: corpus spongiosum.
When comparing flaccid penis anatomy with the anatomy of penes with erect corpora cavernosa (Fig.2) a few changes in the position of tissues relative to one another are immediately visible in all three species (Figs 5 and supporting information Figs S1–S6 and Video S1). In the ‘erect’ penes, the lacunae of the corpora cavernosa expand and stretch out the tunica albuginea. Stretching the collagenous tunica albuginea changes its position within the penis and turns it from a thick, folded layer in the flaccid penis to a straight, taut, thin one. The corpora cavernosa expand in length and diameter, keeping their general U-shaped cross-section owing to the network of trabeculae cross-linking the inner walls of the tunica albuginea. In the ‘erect’ penis, the skin is drawn up into a taut surface, indicating that the corpora cavernosa take up most of the available space within the shaft of the penis. With their U-shaped cross-section, they surround the urethra and the corpus spongiosum on the ventral side.
Fig 2.
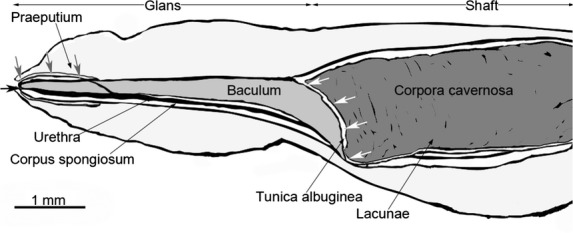
Schematic drawing of an inflated bat penis. The distal tip of the penis is pointing to the left, the proximal base of the penis to the right. Gray arrows mark where the corpus spongiosum is enveloping the baculum. White arrows mark the area where the baculum and corpora cavernosa are linked. Black arrow shows the urethral opening.
When the corpora cavernosa expand, they straighten both the urethra and the corpus spongiosum. In the flaccid penis, the urethra, surrounded for its whole length by the corpus spongiosum, follows the curve of the flaccid penis. When the corpora cavernosa are erect, the urethra and corpus spongiosum are straight, embedded in the ventral surface of the corpora cavernosa in the shaft, and lying directly underneath and partly around the baculum within the glans. The proximal, laterally divided end of the baculum surrounds the urethra laterally and dorsally (Figs6).
Fig 6.
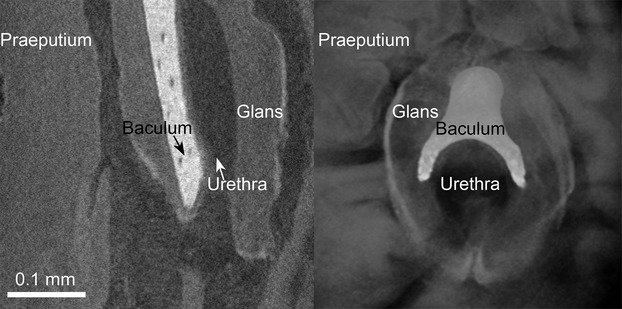
Detail of the penis tip and urethral opening of Pipistrellus pipistrellus. Left: 2D virtual lateral section, right: 3D volume rendering; both depicting the distal part of the glans penis with the distal part of the urethra, the distal tip of the baculum and parts of the praeputium of a P. pipistrellus penis.
The distal end of the baculum is directly dorsal to the urethra, and both are surrounded by the corpus spongiosum. A distal broadening of the corpus spongiosum around the bone can be seen in the distal part of the glans, where the praeputium is distinguishable from the glans (Figs5, gray arrows). The dorsal half of the urethral opening in the glans is directly surrounded by the forked distal tip of the baculum (Fig.6; Figs5, black arrow).
From the three-dimensional (3D) images of this study, we can confirm that the proximal end of the baculum is linked to the corpora cavernosa over a much larger area than we previously thought (Herdina et al. 2010): not only the very end is connected, but also a broad area of the dorsal surface all along the bifurcated part of the proximal base of the baculum (see Herdina, 2008; Herdina et al. 2014b).
From our previous histological studies, we know the baculum is tightly linked to the corpora cavernosa via entheses. The tunica albuginea merges into the periosteum of the baculum without a histologically distinguishable border between the tissues (Herdina, 2008; Herdina et al. 2010, 2014b). Thus when the corpora cavernosa expand and stiffen, the baculum is pulled into a straight line with the corpora cavernosa in N. noctula and P. pipistrellus, or into an angle with the tip pointing dorsally in P. nathusii, moving the distal tip up dorsally and pulling the proximal end down (Figs5, white arrows).
Discussion
The shape and size of the baculum show a great variety in bats. Nevertheless, its basic designs are clade-specific and provide key diagnostic criteria often used in taxonomy, particularly in the speciose groups rich in convergent phenotypes. The largest family of bats, Vespertilionidae, is a typical example (compare Hill & Harrison, 1987; Horáček & Hanák, 1985). However, despite numerous biometrical and descriptive comparisons, little is known about functional integration of these structures into penile morphology. Here we have presented a new approach to analyzing function using post-mortem samples. Detailed 3D models based on high-resolution microCT proved valuable for comparing flaccid penes with experimentally inflated ‘erect’ ones on a micromorphological scale, and functional anatomical analysis of the 3D models provided support for two different hypotheses on the mechanical functions of the baculum.
The most widely accepted theory on the evolution of bacular diversity is that the baculum is subject to sexual selection (Eberhard, 1985; Arnqvist, 1998; Danielsson & Askenmo, 1999; House & Simmons, 2003; Hosken & Stockley, 2004; Lüpold et al. 2004; Simmons & Firman, 2013; Stockley et al. 2013). This is supported by the specifically differentiated and sometimes elaborate bacula of many mammals and their persistence in various lineages, and baculum morphology is variable enough within a species to allow sexual selection to take place. At the same time, the difference in baculum morphology between closely related species is usually much more pronounced than differences in baculum morphology within a species (Burt, 1960; Patterson & Thaeler, 1982). In house mice, postcopulatory sexual selection on baculum thickness, through female choice, was shown in breeding experiments by Simmons & Firman (2013) and Stockley et al. (2013). In some primates and carnivores that engage in prolonged intromissions during copulation, the length of the baculum is sexually selected (Dixson, 1987, 2012; Dixson & Anderson, 2004).
In general, the form of the baculum results from integration of three structural units differing in morphology, development, and function: a proximal part interfacing with the corpora cavernosa, a distal part related to the urethral opening, and a medial part responding to prolongation of the structure (Kelly, 2000). Compared with ancestral arrangements, the derived baculum type of the studied taxa shows striking divergences in the morphology of particular parts: a robust and broad proximal unit, an early-developing distal bifurcation (Maeda, 1978), and a quite prolonged stick-like medial unit.
The baculum is directly dorsal to the part of the urethra that goes through the glans, and the distal forked tip of the baculum surrounds the dorsal half of the urethral opening (Fig.6; Herdina, 2008; Herdina et al. 2010, 2014b). One hypothesized function of the baculum is that it protects the distal urethra and urethral opening from compression during erection and copulation (Dixson, 1995). The distal part of the baculum is surrounded by the corpus spongiosum, which anchors the urethra to the bone. The reconstructed and segmented 3D images show that this arrangement is likely to help straighten out the urethra and corpus spongiosum during erection, when the bone is pushed up and forms a straight line with the corpora cavernosa. If we could inflate the corpus spongiosum as well, we might see morphological changes in the shape of the distal tip of the glans. Similar to some rodent species, the glans might have a species-specific bell shape when fully erect (Holmes et al. 1991). Inflating the corpus spongiosum might also help to test other hypotheses on baculum function; e.g. whether the baculum could stimulate the female reproductive tract during copulation, or could serve as an indicator for male quality (Dyck et al. 2004).
The baculum and the corpora cavernosa are enveloped in a common fibrous structure to form a contiguous mechanical unit. Since there is no histologically distinct separation of the tunica albuginea and the baculum periosteum (Herdina, 2008; Herdina et al. 2010, 2014b), the baculum seems to be a stiffening element in the glans. This suggested the hypothesis that the baculum and corpus cavernosum form a functional unit to support both the penile shaft and the more distal glans tip (Kelly, 2000). Here, we observed that the baculum is connected to the corpora cavernosa over a large area along the proximal end and dorsal surface of its bifurcated base (Figs5, gray arrows), and the bone even changes position in relation to the other penis tissues when the corpora cavernosa are inflated. This supports the conclusion that the baculum and corpora cavernosa form a functional unit to stiffen the penis through to the glans during erection.
Of course, many mammalian species without a baculum to take on these functions have penes that function adequately for copulation. In humans, the corpus spongiosum seems to be responsible for protecting the urethral opening during copulation (Hatzichristou et al. 2003). The corpus spongiosum is a low-pressure system (Purohit & Beckett, 1976; Kelly, 2000) but it expands the glans significantly in many species, so in species lacking a baculum the glans could take over the function of the baculum as a stiffening element (Holmes et al. 1991).
In this first application of our new approach to three species of vespertilionid bats, it was not possible to draw conclusions about intraspecific variation from our rather small sample. In related studies, we have described intraspecific variation in baculum shape in P. pipistrellus (Herdina et al. 2014a) and compared P. pipistrellus and P. nathusii baculum histomorphology and size (Herdina et al. 2014b). There is little interspecific variation, as we specifically chose the species for the similarities they show in overall baculum morphology and mating systems. The shared features of the bacula in all three species are the bifurcated, sturdy base, the long, slender shaft with its forked tip, and the dorsoventral curve in lateral view. The main difference in baculum shape is the way the baculum of P. nathusii bends back towards the dorsal surface of the penis in mid-shaft, while curved proximally as in the other species. The larger species N. noctula has a larger baculum than both of the pipistrelle species. Soft tissue morphology is very similar, except for the shape of the prepuce and the portion of the baculum covered by corpus spongiosum tissue in the glans penis. Also, in the ‘erect’ penis the corpora cavernosa seem to show greater expansion in P. nathusii than in either of the other species.
In this study only the corpora cavernosa were inflated, not the corpus spongiosum or the accessory swelling tissue within the praeputium. This limited the functional hypotheses we were able to test with this experiment. Even with morphological structures this small, it should be possible to inflate all of the swelling tissues in the penis, possibly using a chiropteran species larger than a pipistrelle. All the accessory swelling tissue within the large praeputium, together with the tiny distal end of the glans, also brings up the question of whether the praeputium can even be pulled back during copulation. According to Matthews (1937), in vespertilionids the minute glans can be exposed by retracting the prepuce in specimens examined immediately after death. Inflating all the swelling tissues would also be an important step towards an even more anatomically complete 3D model of an important reproductive organ and its function.
This new methodological approach to an old problem helps to accurately follow the changes of tissue positions relative to one another, even in post-mortem samples. The efficacy of generating and analyzing anatomically accurate 3D models also opens up the possibility of using microCT-based models to create finite element models for quantitative testing of more complex functional hypotheses, such as the effects of the increasing hydrostatic pressure within the penis during copulation (Kelly, 2000). To test hypotheses on baculum function such as stimulating the female reproductive tract (Dyck et al. 2004), an extended approach involving female reproductive anatomy and behavioral studies would be necessary.
Conclusion
Both functional hypotheses tested – that the baculum protects the urethral opening and that it forms a functional unit with the corpora cavernosa to provide stiffness within the glans – are supported by our data. The novel approach of combining experimental designs such as inflating penes with high resolution microCT will help in the comparison and testing of the functions of different baculum morphologies, and paves the way for finite element modeling to test biomechanical hypotheses in non-living specimens.
Acknowledgments
We would like to thank the staff of the Mammal collection of the Natural History Museum Vienna, especially Frank Zachos and Barbara Herzig, for providing samples and vouchering all the samples after this study, the Naturalis Biodiversity Center, especially Steven van der Mije, and the Charles University in Prague for providing samples for this study. We are grateful to Elisabeth R. Dumont and her team, to Ian Grosse, to Patricia Brennan and Teri Orr, and to Helge Hilgers and Adrienne Hilgers, for valuable discussions on the subject. We thank Friederike Spitzenberger and the two reviewers for their helpful suggestions and comments on this manuscript. Finally, we would like to thank Gerd B. Müller of the Department of Theoretical Biology, University of Vienna, for providing resources of his Department, the Dean's Office of the Faculty of Life Sciences, University of Vienna, for awarding a PhD Completion Grant to A.N.H., and the Austrian Federal Ministry of Science and Research and the OeAD for awarding a Marietta Blau Fellowship to A.N.H.
Conflict of interest
The authors have no conflict of interest.
Authors’ contributions
I.H. and P.H.C.L. provided the samples for this study. A.N.H. inflated the bat penes with formalin and took standard measurements of the bats together with D.A.K., H.J., and P.H.C.L. B.D.M. stained and microCT scanned the samples. A.N.H. and B.D.M. reconstructed and manually segmented the microCT images to create 3D models. All authors evaluated and discussed the results. A.N.H. and B.D.M. wrote the manuscript and prepared the figures, and all authors added valuable comments and corrections and approved the final manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Video S1.Movie of an inflated Nyctalus noctula penis.
Fig. S1–S6. Pictures of 3D surface rendering of inflated Nyctalus noctula penis, with the tissues added step by step. Orange: outer shape of the penis (volume rendering), white: baculum, red: blood lacunae in the corpora cavernosa, green: tunica albuginea of the corpora cavernosa, blue: urethra, pink: corpus spongiosum.
Fig. S7. 3D PDF of a Pipistrellus pipistrellus baculum.
References
- Arnqvist G. Comparative evidence for the evolution of genitalia by sexual selection. Nature. 1998;393:784–786. [Google Scholar]
- Barclay RMR, Thomas DW. Copulation call of Myotis lucifugus: a discrete situation-specific communication signal. J Mammal. 1979;60:632–634. [Google Scholar]
- Burt WH. Bacula of North American mammals. Miscellaneous Publications of the Museum of Zoology, University of Michigan. 1960;113:1–76. [Google Scholar]
- Danielsson I, Askenmo C. Male genital traits and mating interval affect male fertilisation success in the water strider Gerris lacustris. Behav Ecol Sociobiol. 1999;46:149–156. [Google Scholar]
- Dixson AF. Observations on the evolution of the genitalia and copulatory behaviour in male primates. J Zool. 1987;213:423–443. [Google Scholar]
- Dixson AF. Baculum length and copulatory behaviour in carnivores and pinnipeds (Grand Order Ferae) J Zool. 1995;235:67–76. [Google Scholar]
- Dixson AF. Primate Sexuality: Comparative Studies of the Prosimians, Monkeys, Apes, and Humans. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- Dixson AF, Anderson MJ. Sexual behavior, reproductive physiology and sperm competition in male mammals. Physiol Behav. 2004;83:361–371. doi: 10.1016/j.physbeh.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Dixson A, Nyholt J, Anderson M. A positive relationship between baculum length and prolonged intromission patterns in mammals. Acta Zool Sinica. 2004;50:490–503. [Google Scholar]
- Dyck MG, Bourgeois JM, Miller EH. Growth and variation in the bacula of polar bears (Ursus maritimus) in the Canadian Arctic. J Zool. 2004;264:105–110. [Google Scholar]
- Eberhard WG. Sexual Selection and Animal Genitalia. Cambridge, MA: Harvard University Press; 1985. [Google Scholar]
- Eberhard WG. Evaluating models of sexual selection: genitalia as a test case. Am Nat. 1993;142:564–571. doi: 10.1086/285556. [DOI] [PubMed] [Google Scholar]
- Glass BP. 1966. pp. 40–41. Some notes on reproduction in the red bat, Lasiurus borealis. In Proceedings of the Oklahoma Academy of Science.
- Hatzichristou DG, Tzortzis V, Hatzimouratidis K, et al. Protective role of the glans penis during coitus. Int J Impot Res. 2003;15:337–342. doi: 10.1038/sj.ijir.3901039. [DOI] [PubMed] [Google Scholar]
- Herdina AN. 2008. Master thesis, University of Vienna 74Light microscopy of the penis bone (baculum) in the Plecotus species (Chiroptera) in Austria Department for Theoretical Biology, Morphology Section, Faculty of Life Sciences,
- Herdina AN, Herzig-Straschil B, Hilgers H, et al. Histomorphology of the penis bone (baculum) in the gray long-eared bat Plecotus austriacus (Chiroptera, Vespertilionidae) Anat Rec. 2010;293:1248–1258. doi: 10.1002/ar.21148. [DOI] [PubMed] [Google Scholar]
- Herdina AN, Hulva P, Horáček I, et al. MicroCT imaging reveals morphometric baculum differences for discriminating the cryptic species Pipistrellus pipistrellus and P. pygmaeus. Acta Chiropterol. 2014a;16:157–168. [Google Scholar]
- Herdina AN, Plenk H, Jr, Benda P, et al. Correlative 3D imaging of Pipistrellus penis micromorphology: validating quantitative microCT images with undecalcified serial ground section histomorphology. J Morphol. 2014b doi: 10.1002/jmor.20372. in press. [DOI] [PubMed] [Google Scholar]
- Hill JL, Harrison DL. The baculum in the Vespertilioninae (Chiroptera: Vespertilionidae) with a systematic review, a synopsis of Pipistrellus and Eptesicus, and the descriptions of a new genus and subgenus. Bull br Mus nat Hist Zool. 1987;52:225–305. [Google Scholar]
- Holmes GM, Chapple WD, Leipheimer RE, et al. Electromyographic analysis of male rat perineal muscles during copulation and reflexive erections. Physiol Behav. 1991;49:1235–1246. doi: 10.1016/0031-9384(91)90357-t. [DOI] [PubMed] [Google Scholar]
- Horáček I, Hanák V. Generic status of Pipistrellus savii and comments on classification of the genus Pipistrellus (Chiroptera, Vespertilionidae) Myotis. 1985;23–24:9–16. [Google Scholar]
- Hosken DJ, Stockley P. Sexual selection and genital evolution. Trends Ecol Evol. 2004;19:87–93. doi: 10.1016/j.tree.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Hosken DJ, Jones KE, Chipperfield K, et al. Is the bat os penis sexually selected? Behav Ecol Sociobiol. 2001;50:450–460. [Google Scholar]
- House CM, Simmons LW. Genital morphology and fertilization success in the dung beetle Onthophagus taurus: an example of sexually selected male genitalia. Proc Biol Sci. 2003;270:447–455. doi: 10.1098/rspb.2002.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahelková H, Horáček I. Mating system of a migratory bat, Nathusius’ pipistrelle (Pipistrellus nathusii): different male strategies. Acta Chiropt. 2011;13:123–137. [Google Scholar]
- Kelly DA. Anatomy of the baculum-corpus cavernosum interface in the Norway rat (Rattus norvegicus), and implications for force transfer during copulation. J Morphol. 2000;244:69–77. doi: 10.1002/(SICI)1097-4687(200004)244:1<69::AID-JMOR7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Liu Y, Metzner W, Feng J. Vocalization during copulation behavior in greater horseshoe bats, Rhinolophus ferrumequinum. Chin Sci Bull. 2013;58:2179–2184. [Google Scholar]
- Long CA, Frank T. Morphometric variaton and function in the baculum, with comments on correlation of parts. J Mammal. 1968;49:32–43. [Google Scholar]
- Lüpold S, McElligott AG, Hosken DJ. Bat genitalia: allometry, variation and good genes. Biol J Linn Soc. 2004;83:497–507. [Google Scholar]
- Maeda K. Baculum of the Japanese large noctule, Nyctalus lasiopterus aviator Thomas, 1911. Acta Anat Nippon. 1978;53:447–453. [PubMed] [Google Scholar]
- Matthews LH. The form of the penis in the British rhinolophid bats, compared with that in some of the vespertilionid bats; and the female sexual cycle in the British horse-shoe bats, Rhinolophus ferrum-equinum insulanus Barrett-Hamilton and R. hipposideros minutus Montagu. Trans Zool Soc London. 1937;23(Part 4):213–266. [Google Scholar]
- McCracken GF, Wilkinson GS. Bat mating systems. In: Zubaid A, editor. Functional and Evolutionary Ecology of Bats. New York: Oxford University Press; 2000. [Google Scholar]
- Meisenheimer J. Geschlecht und Geschlechter im Tierreiche. Jena: Verlag von Gustav Fischer; 1921. [Google Scholar]
- Metscher BD. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 2009a;9:11. doi: 10.1186/1472-6793-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metscher BD. Micro-CT for developmental biology: a versatile tool for high-contrast 3D imaging at histological resolutions. Dev Dyn. 2009b;238:632–640. doi: 10.1002/dvdy.21857. [DOI] [PubMed] [Google Scholar]
- Metscher BD. X-ray microtomographic imaging of intact vertebrate embryos. Cold Spring Harb Protoc. 2011;12:1462–1471. doi: 10.1101/pdb.prot067033. [DOI] [PubMed] [Google Scholar]
- O'Brien GM, Nankervis RF. Coital behavior of male Pteropus scapulatus (little red flying foxes) in captivity. Physiol Behav. 1994;56:471–477. doi: 10.1016/0031-9384(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Patterson BD, Thaeler CS., Jr The mammalian baculum: hypothesis on the nature of bacular variability. J Mammal. 1982;63:1–15. [Google Scholar]
- Purohit R, Beckett S. Penile pressures and muscle activity associated with erection and ejaculation in the dog. Am J Physiol. 1976;231:1343–1348. doi: 10.1152/ajplegacy.1976.231.5.1343. [DOI] [PubMed] [Google Scholar]
- Ruth EB. The os priapi: a study in bone development. Anat Rec. 1934;60:231–249. [Google Scholar]
- Sachteleben J, von Helversen O. Songflight behaviour and mating system of the pipistrelle bat (Pipistrellus pipistrellus) in an urban habitat. Acta Chiropt. 2006;8:391–401. [Google Scholar]
- Simmons LW, Firman RC. Experimental evidence for the evolution of the mammalian baculum by sexual selection. Evolution. 2013;68:276–283. doi: 10.1111/evo.12229. [DOI] [PubMed] [Google Scholar]
- Stockley P, Ramm S, Sherborne A, et al. Baculum morphology predicts reproductive success of male house mice under sexual selection. BMC Biol. 2013;11:66. doi: 10.1186/1741-7007-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1.Movie of an inflated Nyctalus noctula penis.
Fig. S1–S6. Pictures of 3D surface rendering of inflated Nyctalus noctula penis, with the tissues added step by step. Orange: outer shape of the penis (volume rendering), white: baculum, red: blood lacunae in the corpora cavernosa, green: tunica albuginea of the corpora cavernosa, blue: urethra, pink: corpus spongiosum.
Fig. S7. 3D PDF of a Pipistrellus pipistrellus baculum.


