Abstract
While the crimp morphology in ligaments and tendons has been described in detail in the literature, its relative distribution within the tissue has not been studied, especially in relation to the complex multi-bundle arrangement as is found in the anterior cruciate ligament (ACL). In this study, the crimp morphology of the ovine ACL was examined topologically and with respect to its double-bundle structure. The crimp morphologies were compared with the knee in three knee positions, namely stance, maximum extension and maximum flexion. As a control, the crimp morphology of the ACL free from its bony attachments was determined. In the control samples, the anterior-medial (AM) bundle contained a combination of coarse and fine crimp, whereas the posterior-lateral (PL) bundle manifested only a coarse crimp. Using the extent of crimp loss observed when subjecting the knee to the respective positions, and comparing with the controls, the crimp morphologies show that the AM bundle of the ACL is most active in the stance position, whereas for the maximum extension and flexion positions the PL bundle is most active. We propose that these differences in crimp morphologies have relevance to ACL design and function.
Keywords: anterior cruciate ligament, double bundle, fibre recruitment, knee posture
Introduction
Ligaments and tendons have unique biomechanical properties that allow them to withstand high loads, facilitate effective load transfer, and stabilize joints (Miller et al. 2011; Woo et al. 2011). The strength of ligaments and tendons relies on the relative amounts of its fibrillar constituents, the tough collagen providing stiffness and elastin the compliance or stretch (Kastelic et al. 1978; Lanir, 1978; Franchi et al. 2007a,b; Woo et al. 2007; Henninger et al. 2013). The structural organisation of the fibres is important, too, as previous studies have shown that any differential response to load arises from differences in the lengths of fibres and the extent of their crimp morphology (Boorman et al. 2006; Franchi et al. 2008).
Crimping is a known feature in ligaments and tendons and its regular periodicity allows it to be visualized using light microscopy and, even more easily, using polarized light microscopy (Rigby et al. 1959; Elliott, 1965; Diamant et al. 1972). This crimp is relatively easy to straighten with small tensile loads (Elliott, 1965; Vidik & Ekholm, 1968), and hence provides a certain degree of initial compliance in a tendon or ligament when stretched from a rest state – referred to as the ‘toe’ region in a typical stress-strain curve (Rigby et al. 1959; Diamant et al. 1972; Atkinson et al. 1999).
When simplified to the shape of a triangle, crimp morphology can be measured in terms of its number, top angle, and base length; typically these values range from 5 to 14 over a 250-μm length span, 120˚–145˚ and 35–75 μm, respectively, depending on the tissue type (Diamant et al. 1972; Gathercole & Keller, 1991; Atkinson et al. 1999; Franchi et al. 2007a). For example, the rectus femoris tendon has half the crimp number compared with the vastus intermedius tendon, the crimp base length in the former being twice that of the latter (Franchi et al. 2009), suggesting that the crimp pattern is related to the mechanical function of the specific tendon or ligament.
Previous studies have used crimp analysis to study variations in strain in a ligament or tendon subjected to mechanical testing (Diamant et al. 1972; Kastelic et al. 1980; Hansen et al. 2002; Boorman et al. 2006; Franchi et al. 2007a) and for the following reasons:
It provides a method for studying recruitment patterns in the tissue during load bearing, thus providing a more detailed understanding of the structure–function relationship for complex tendon-ligament structures. For example, in the ACL with its double-bundle structure, previous studies have shown that in passive extension the PL bundle is taut, while in relative flexion the AM bundle becomes taut with the PL bundle relaxed (Amis & Dawkins, 1991; Petersen & Zantop, 2007). However, the relative extent to which each of these bundles are taut or relaxed, or even co-involved, in providing stability in the different knee positions is still unknown and hence it may be argued that the biomechanical function of each bundle still remains little understood.
It provides the additional geometric information that can simplify complex joint mechanics and thus solve, statically, the indeterminate distribution of strain and stress within tendons and ligaments (Stagni et al. 2004) and help improve the accuracy of current models of muscle and joint mechanics. For example the ACL elongation behaviour has been studied in vivo using non-invasive imaging (Hosseini et al. 2009) and strain gauge implants (Beynnon et al. 1995). However, because the strain values reported do not represent the strain distribution within the ligament tissue, knee joint models based on these strain data tend to simulate the ACL as a relatively homogeneous structure (Hosseini et al. 2009).
Determining the distribution of the crimp pattern in tendons and ligaments allows for a better understanding of the microanatomy and can therefore assist in the development of improved graft designs and engineered tissue replacements. For example, for the ACL, current attempts to use artificial materials (e.g. Dacron and Gortex) to replace the damaged ligament have not proven effective, with failure occurring in artificial ligaments despite their being several times stronger than the natural ligament (Laurencin & Freeman, 2005; Legnani et al. 2010). Further, there still remain many issues relating to the use of auto/allografts and engineered ligaments, one being a limited knowledge of the structural requirements, from the macro to the nano scales – information that is clearly important for effective ligament design and mechanical properties (Petrigliano et al. 2006; Vieira et al. 2009).
Owing to its frequent involvement in knee injuries the ACL is one of the most studied ligaments (Gianotti et al. 2009; Granan et al. 2009; Janssen et al. 2012; Moses et al. 2012) and much of this research has focused on the anatomical nature and biomechanical significance of its double-bundle structure (Kennedy et al. 1974; Norwood & Cross, 1979; Arnoczky, 1983; Odensten & Gillquist, 1985; Clark & Sidles, 1990; Amis & Dawkins, 1991; Dienst et al. 2002; Zantop et al. 2005, 2006; Duthon et al. 2006; Petersen & Zantop, 2007; Steckel et al. 2007; Stieven-Filho et al. 2011; Amis, 2012). This emphasis is understandable as ACL surgical repair and replacement strategies will always be aimed at reproducing natural knee kinematics and kinetics following injury.
Despite the considerable number of anatomical studies there is little published information on the crimp distribution in the ACL and its functional relevance. This new study therefore provides a detailed description of the pattern of crimp in the ovine ACL, in relation to its double-bundle structure and, as a function of knee posture. We hypothesize that the crimp pattern in the anteromedial and posterolateral bundles will be different than in a neutral knee position, and will vary significantly according to knee flexion position.
Methods
Twelve mature ovine stifle joints (knees) were divided into four test groups of three, namely control, stance position (∼ 50°), maximum extension, and maximum flexion. For the control group, the ACL ligament was excised at the femur-end, and ligament-to-tibia bone samples were isolated and chemically fixed, unconstrained, in 10% formalin for 72 hours. For the other three groups, each intact joint was first positioned in the relevant posture using a 6° of freedom knee-positioning rig and then chemically fixed in situ using 10% formalin for 1 week (Fig.1). Following fixation, the knee was washed and opened, and the ligament was excised at the femur-end, and ligament-to-tibia bone samples extracted. For this study, because of the large area of insertion of the ACL into the tibial plateau, compared to the relatively narrow convergence of the tissue into the femur, only the region of ligament tissue proximal to the tibial insertion was analysed. This region allowed for easy demarcation between bundles and further provided a relatively wide region for studying within-bundle crimp differences.
Fig 1.
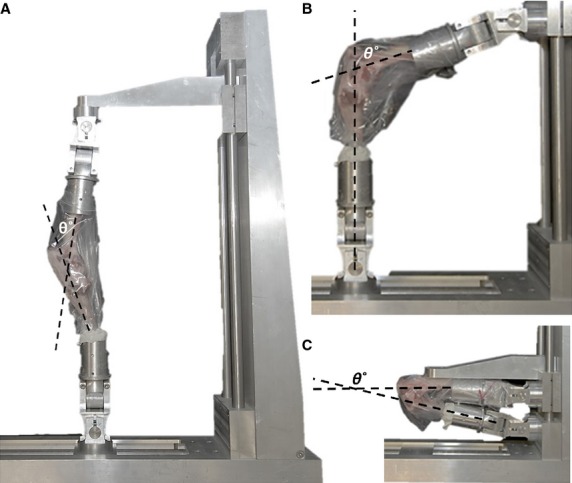
Three test positions were studied: (A) maximum extension, (B) stance position, and (C) maximum flexion. For all tests, the knee and joint capsule were intact and formalin-fixed in position.
All ligament-bone samples were fully decalcified in 10% formic acid and then cryo-sectioned in the sagittal plane to obtain 20-μm serial slices. These slices were then examined in their fully hydrated state using differential inference contrast (DIC) microscopy. The slices from each sample group were morphologically characterized by their degree of crimping, taken as an approximate indicator of elongation and thus of loading (Gathercole & Keller, 1991).
The crimp was classified as substantial, intermediate or minimal and defined as follows (refer also to Fig.2):
Fig 2.
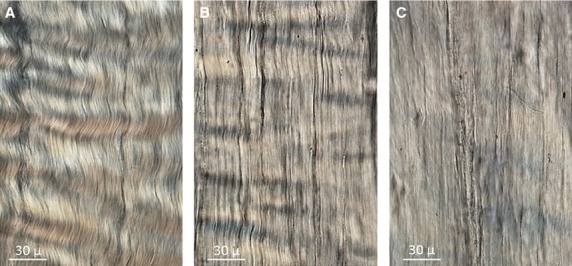
Three levels of fibre crimping: (A) substantial, (B) intermediate, and (C) minimal.
Substantial crimp was characterized by a consistent banding with a clearly visible waveform.
Intermediate crimp had a reduced waveform, missing banding, and/or partially straightened collagen fibres.
Minimal crimp was characterized by extensive areas of straightened collagen fibres with minimal traces of banding.
Quantitative analysis of crimp consisted of two separate measurements, which included the crimp base length and the maximum crimp angle. The crimp base length was obtained from measuring digitally the DIC images and using Image J software. Approximating the morphology of collagen crimp as a wave, the base length was equated to one wave length (Franchi et al. 2009). A wave length was defined as the distance from one crimp apex to its immediate adjacent apex (Fig.3).
Fig 3.
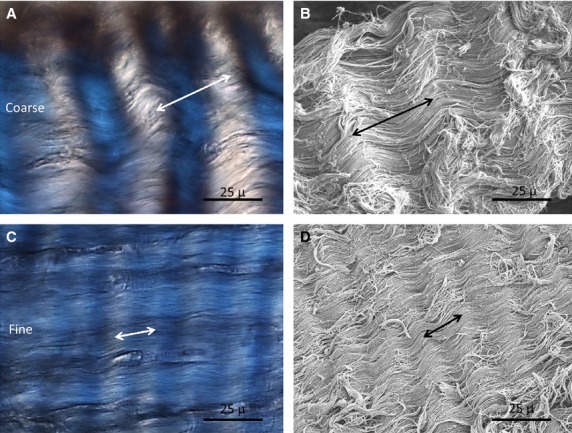
Coarse crimp measurements (A and B) and fine crimp (C and D) using DIC and SEM imaging. Double arrows indicate the breadth of the base length.
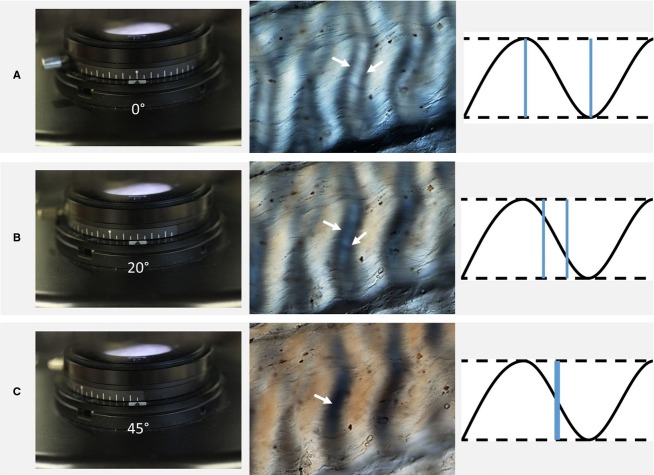
The crimp angle measurements were obtained using the method described by Diamant et al. (1972). The polarizers were orthogonally crossed before mounting fully hydrated between a slide and coverslip on the rotating stage of the microscope. The long-axis of the fibre array was aligned in the direction of one polarizer (Fig.4A). The other polarizer was then rotated until the onset of extinction was detected, which is when the two dark bands merged (Fig.4B,C), this being an indication that the tangent to the crimp wave coincides with the rotating polarizer angle11, and hence yielding the crimp angle.
Fig 4.
Crimp angle measurement using polarized light microscopy following the procedure of Diamant et al. (1972). (A) The initial setting of the polarizer at 0˚, orthogonal to the analyser (second polarizer), with the ligament long axis placed along the x–y axis of the stage, results in the DIC image shown here, as well as the crimp wave illustration. The double band (denoted by the two arrows), gradually comes together with the rotation of the polarizer (B). Finally, when the bands merge to form a single dark band (C), the polarizer rotation angle matches the tangent angle of the crimp wave.
An additional three ovine ACL samples were examined using scanning electron microscopy (SEM). Bone-ligament samples were carefully chemically fixed using 10% formalin for 72 hours. The fixed samples were cut through the sagittal plane using a scalpel blade and samples underwent hexane and graded ethanol treatment, followed by enzymatic digestion for proteoglycan removal. They were then critical point-dried and platinum-coated before imaging using SEM. To ensure accuracy and consistency, the base length measurements were made following a sequence of multiple measurements from the DIC images. For each of the three ACLs, the AM and PL bundles were separated and serial sectioned in the sagittal plane to yield about 180 sections from medial to lateral. The serial sections were grouped and stored systematically from medial to lateral in an 18-well plate. Two serial sections were selected to represent the mid-medial side and two from the mid-lateral side. At magnification 40×, for each of the bundle sections, 40 base-length measurements were made of crimps next to each other. Thus for the four knee position groups, this potentially meant a total of 3840 measurements. The control group measurements were rechecked again by comparing the measurement made from DIC images with those obtained from high resolution SEM images (Fig.3).
Base length and maximum crimp angle measurements were only carried out on sample areas exhibiting substantial crimp (Fig.2A). Reliable measurements were difficult to obtain from regions of any sample exhibiting either an intermediate crimp or containing partially straightened collagen fibres. All the data were analysed statistically using both anova and post hoc analysis (significance, P < 0.05, Bonferroni-corrected).
Results
Crimp base-length and morphology
The different positions tested produced three easily discernible levels of crimp, namely substantial, intermediate and minimal (Fig.2, Table1). The crimp base lengths for the AM and PL bundles (Table1) were mostly within the range typically found in tendons and ligaments (Franchi et al. 2009). Those base lengths in the > 40-μm range were defined as ‘coarse’ compared with those in the < 20-μm range, which appear as ‘fine’ crimp (Fig.3), this difference correlating with their average crimp angles of ∼ 45° and 15°, respectively. The amplitude of the coarse crimp was also greater than that of the fine crimp.
Table 1.
Measurements of crimp morphology for all positions tested compared with controls. ‘Not available’ (N/A) is denoted where the crimp was diminished, intermediate or minimal, such that the parameters were not measurable. Length measurements unit: μm
| Base length (averaged value with error, from 20 measurements) |
Calculated side length (assume constant crimp angles) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Maximum extension | Stance | Maximum flexion | Control | Maximum extension | Stance | Deep flexion | ||||||||||
| Base length | Error | Base length | Error | Base length | Error | Base length | Error | Crimp angle (approximate) | Side length | Error | Side length | Error | side length | Error | Side length | Error | |
| AM | |||||||||||||||||
| Anterior Medial | |||||||||||||||||
| S1 | 46.2 | 5.09 | 42.31 | 4.39 | Minimal crimp | 42.28 | 4.79 | 45.00 | 32.67 | 3.60 | 29.92 | 3.10 | N/A | N/A | 29.90 | 3.39 | |
| S2 | 41.92 | 4.5 | 47.63 | 4.17 | 44.07 | 4.35 | 29.64 | 3.18 | 33.68 | 2.95 | N/A | N/A | 31.16 | 3.08 | |||
| S3 | 44.41 | 6.3 | 41.26 | 6.32 | 45.04 | 4.8 | 31.40 | 4.45 | 29.18 | 4.47 | N/A | N/A | 31.85 | 3.39 | |||
| Anterior Lateral | |||||||||||||||||
| S1 | 47.37 | 4.59 | Intermediate crimp | Intermediate crimp | Intermediate crimp | 45.00 | 33.50 | 3.25 | N/A | N/A | N/A | N/A | N/A | N/A | |||
| S2 | 49.43 | 6.14 | 34.95 | 4.34 | N/A | N/A | N/A | N/A | N/A | N/A | |||||||
| S3 | 43.13 | 4.74 | 30.50 | 3.35 | N/A | N/A | N/A | N/A | N/A | N/A | |||||||
| Posterior Medial | |||||||||||||||||
| S1 | 17.45 | 2.48 | Intermediate crimp | Intermediate crimp | 17.86 | 4.56 | 15.00 | 9.03 | 1.28 | N/A | N/A | N/A | N/A | 9.25 | 2.36 | ||
| S2 | 15.61 | 1.73 | 16.37 | 2.1 | 8.08 | 0.90 | N/A | N/A | N/A | N/A | 8.47 | 1.09 | |||||
| S3 | 16.29 | 1.94 | 14.88 | 1.76 | 8.43 | 1.00 | N/A | N/A | N/A | N/A | 7.70 | 0.91 | |||||
| Posterior Lateral | |||||||||||||||||
| S1 | 17.18 | 3.1 | Intermediate crimp | Intermediate crimp | Intermediate crimp | 15.00 | 8.89 | 1.60 | N/A | N/A | N/A | N/A | N/A | N/A | |||
| S2 | 19.69 | 4.36 | 10.19 | 2.26 | N/A | N/A | N/A | N/A | N/A | N/A | |||||||
| S3 | 15.04 | 1.99 | 7.79 | 1.03 | N/A | N/A | N/A | N/A | N/A | N/A | |||||||
| PL | |||||||||||||||||
| Medial | |||||||||||||||||
| S1 | 46.06 | 3.43 | Minimal crimp | 41.94 | 5.94 | Intermediate to minial crimp | 45.00 | 32.57 | 2.43 | N/A | N/A | 29.66 | 4.20 | N/A | N/A | ||
| S2 | 46.23 | 5.4 | 49.23 | 4.28 | 32.69 | 3.82 | N/A | N/A | 34.81 | 3.03 | N/A | N/A | |||||
| S3 | 44.3 | 4.04 | 42.51 | 5.02 | 31.32 | 2.86 | N/A | N/A | 30.06 | 3.55 | N/A | N/A | |||||
| Lateral | |||||||||||||||||
| S1 | 46.54 | 4.8 | 45.56 | 4.12 | 43 | 5.24 | Intermediate to minial crimp | 45.00 | 32.91 | 3.39 | 32.22 | 2.91 | 30.41 | 3.71 | N/A | N/A | |
| S2 | 46.31 | 4.11 | 47.48 | 4.63 | 45.53 | 6.53 | 32.75 | 2.91 | 33.57 | 3.27 | 32.19 | 4.62 | N/A | N/A | |||
| S3 | 43.93 | 3.62 | 47.53 | 5.14 | 42.44 | 7.16 | 31.06 | 2.56 | 33.61 | 3.63 | 30.01 | 5.06 | N/A | N/A | |||
Crimp morphology of the control group
In the isolated ligaments of the control group, both coarse and fine crimp were present in the AM bundle, whereas the PL bundle contained only coarse crimp (Fig.5). In the AM bundle the coarse crimp occurred mostly in the anterior portion proximal to the tibial plateau, with the remaining regions of the bundle containing mostly fine crimp (Fig.5C,D). The uniformly coarse crimp characterising the entire PL bundle was similar to that of the coarse crimp in the AM bundle (Table1).
Fig 5.

Crimping in (A) AM bundle and (B) PL bundle. Coarse crimps were visible in the anterior region of the AM bundle and throughout the PL bundle. (C) A high magnification view of coarse crimping in the AM bundle and (D) fine crimping in the posterior region of the AM bundle.
Comparison of the crimp morphologies in the different positions tested
Representative images of the crimp morphologies in the AM bundle with respect to region within the bundle and for all test groups are shown in Fig.6.
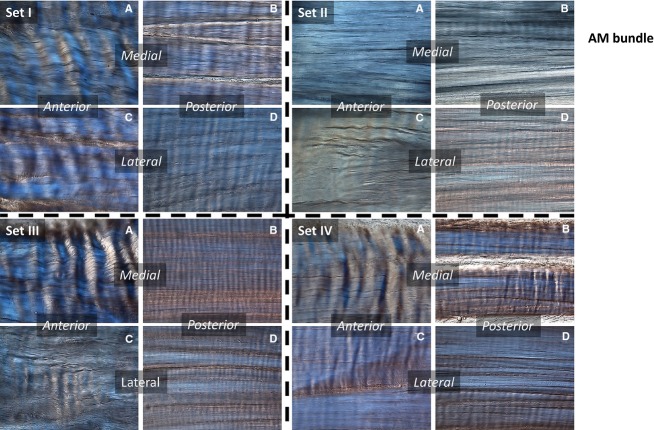
Fig 6.
AM bundle. Sets refer to the positions tested – Control (I), Stance (II), Maximum Flexion (III), Maximum Extension (IV). (A–D) Region in the bundle: anterior-medial (A), posterior-medial (B), anterior-lateral (C), and posterior-lateral (D).
In the stance position, relative to the controls, the fibres in the anterior portion of the AM bundle exhibited a significant loss of crimp. This loss of crimp also occurred in the posterior regions, but to a lesser extent.
In maximum flexion, crimp in the lateral portion of fibres in the AM bundle was eliminated, both in the anterior and posterior regions, whereas the medial fibres were largely unaffected. In maximum extension, there was a significant loss of crimp in the anterior-lateral portion of the AM bundle compared with the controls. For the posterior-outer aspect of the AM bundle, there was crimp loss in all positions tested. For the inner portion, this was also the case except in the deep flexion position.
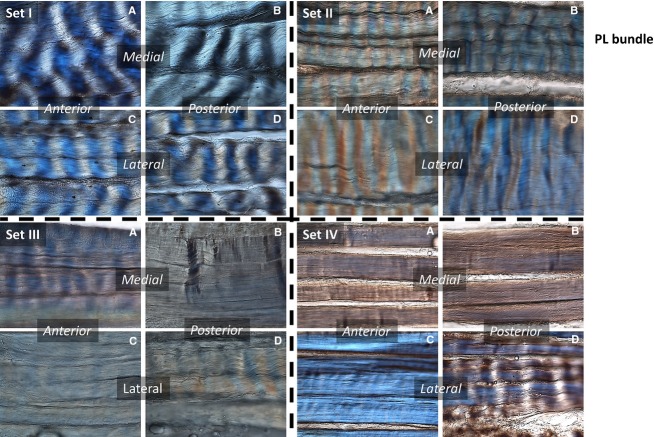
Representative crimp morphologies for the PL bundle from all test groups are shown in Fig.7. In the stance position, there were no significant differences in crimp morphology in any region relative to the controls. In maximum flexion, the fibres in all portions of the ligament tended to lose their crimp, with the greatest loss occurring in the anterior-lateral and posterior-medial portions. In maximum extension, there was a significant loss of crimp in all portions of the PL bundle except the posterior-lateral region.
Fig 7.
PL bundle. Sets refer to the positions tested – Control (I), Stance (II), Maximum Flexion (III), Maximum Extension (IV). (A–D) Region in the bundle: anterior-medial (A), posterior-medial (B), anterior-lateral (C), and posterior-lateral (D).
Discussion
This study has shown that the crimp morphology in the ovine ACL differs between the AM and PL bundles, and for different knee positions. For example, our results show that in the extreme (non-physiological) positions, the crimp in the PL bundle is mostly lost but is retained in the AM bundle. By contrast, in the stance position (∼ 50° in sheep; Tapper et al. 2004, 2008), this difference in crimp loss between the AM and PL bundles is reversed. The schematic in Fig.8 summarizes the relative crimp responses regionally in the bundles and as a function of the three positions studied. Our extreme positions were obtained by applying large changes in flexion angle but only in a single plane. In real life there would also be coupled rotations and translations in other planes which, while not considered in the present study, would clearly contribute to the complex loading in the ligaments (Tapper et al. 2004, 2008; Atarod et al. 2014). In addition, the static positions adopted in the present study do not represent a physiologically realistic loading of the joint where muscle forces would also be involved in stabilising the joint. However, by comparing three very different positions, and an idealized control where the ligament is free from the joint, the variation in crimp morphology responses provided insight into the potential recruitment patterns of the ligament in negotiating the demand for a diverse range of joint motions. Further, as ligaments are passive stabilizers, their mechanical response with the joint in different positions regardless of muscle activity would still be largely dependent on the resulting joint position. Thus by studying the effect of knee position on ligament crimp distribution, we believe these experiments can still yield valuable information concerning ligament function.
Fig 8.
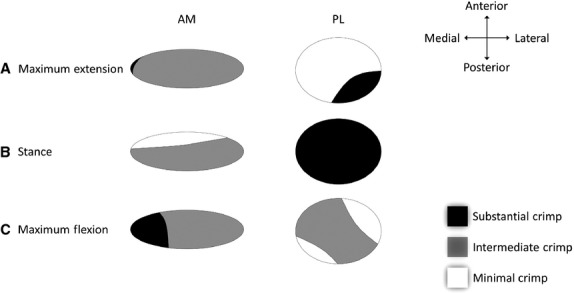
Cross-section of each bundle highlighting the difference in the degree of crimping in (A) maximum-extension, (B) stance, and (C) maximum flexion. Substantial crimping indicates relatively less loading, whereas minimal crimping indicates most loading. The interesting pattern of crimping shown in the PL bundle in Maximum flexion (C) may be related to the control of knee rotation.
The AM bundle was observed to be most active in the stance position and this was more concentrated in the medial portion. Conversely, the AM bundle was least active in both the maximum flexion and extension positions. Whereas the anterior-lateral portion of the AM bundle was relatively active in all positions, the PL bundle was least active in the stance position, and most active in the maximum extension and flexion positions. By using crimp morphology to determine the extent of activity in the different regions of the ligament as a function of knee position, the differential function and mechanical significance of the double-bundles in the ovine ACL can be explored. Further, regional variations in the intensity and extent of crimp may be used as an indicator of which portions of the ligament are ‘primed for action’. This idea is supported by well-established biomechanical studies showing that in the tensile response of ligaments there is an initial toe-region displaying a high degree of compliance corresponding to the straightening of fibre crimp (Vidik & Ekholm, 1968; Stromberg & Wiederhielm, 1969; Shah et al. 1977; Hansen et al. 2002). Hence less crimping would result in a quicker stiff response (smaller toe-region) when the ligament is stretched. The reduced crimp in the AM bundle, in the stance position, therefore suggests that this bundle is relatively ‘primed’ in this position. The simulated stance position in the present study is similar to the stance position dominant during ovine gait (Tapper et al. 2004), for which it has been shown that the ACL is important in stabilising the knee and preventing anterior tibial translation (Atarod et al. 2014). That the PL bundle, and not the AM bundle, lost its crimp in both the maximum flexed and extended positions further suggests that the PL bundle is designed to stabilize the joint differentially.
We propose that one of the roles of the PL bundle is to provide stability during subtle out-of-plane rotations such as internal-external rotation, and this is further supported by a recent study showing that in the AM bundle the extent of fibre-rooting into bone is deeper than that for the PL bundle (Zhao et al. 2014). In the study of Zhao et al. (2014) the deeper fibre rooting of the AM bundle was interpreted as being better designed to resist the force lines of action in anterior tibial translation, compared with the shallower pocket-insertions of the PL bundle, twisted about the AM bundle axis, which appear to resist more torsional motions.
On the issue of coarse vs. fine crimping, consider again the toe-region of a typical load-displacement curve. The ease with which crimp unravelling can occur is mostly dependent on the height of the crimp (denoted by ‘a’ in Fig.9) as well as defined by the base length and crimp angle. A smaller height would imply a reduced toe-region, and that the finer crimp with its smaller height would be more ‘primed for action’. However, in reference to a single coarse crimp, several fine crimp configurations would be possible within it, as shown by a simple triangle analogy where smaller triangles are made to fit in one single larger one (Fig.9). The data from the morphometric measurements (Table1) indicate that the fine crimp had a much smaller angle than the coarse crimp. The schematic (Fig.9) shows that within the scale of a coarse crimp, several smaller finer crimps could fit, but by trigonometric interpretation, and the ratio of the heights of the coarse crimp to the fine crimp (a/a’), we can show that by a relatively smaller reduction of the crimp angle in the fine crimps, the shallowness is increased many times over. The crimp angle could therefore be an important means of optimising the morphological state of a ligament region in readiness for direct load bearing. In addition, the ratio of heights between two different sized crimps, a/a’ (Fig.9), could be a potentially useful index to characterize the ‘pre-stiffness’ mechanical properties of load-bearing tissue when this ratio is correlated with mechanical data and a range of crimp morphologies.
Fig 9.
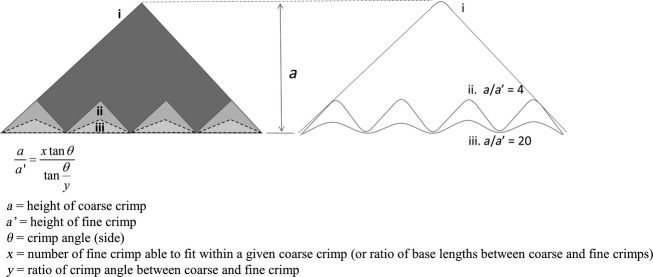
Schematic of coarse crimp (i) and how a fine crimping configuration could either have the same crimp angle as the coarse crimp (ii) or a smaller crimp angle (iii – dotted outline), as is the case in the fine crimps observed in the present study. The effect of different crimp angles was calculated using the equation shown, which was derived from a standard trigonometric interpretation. The ratio of coarse crimp height to fine crimp height, given the same number of fine crimps, is very sensitive to the crimp angle. Using θ = 45˚ and y = 3 (i.e. fine crimp angle 15˚), the ratio a/a’ increases five-fold from when y = 1 (i.e. if fine crimp angle is the same as coarse crimp angle).
The significance of having coarse vs. fine crimping, or both, may well have important functional implications, as the AM bundle contains both but the PL bundle only coarse crimping. The fine crimping in the AM bundle was largely confined to the posterior portion, that is, closer to the hypothetical joint centre. Could it be that this portion of the AM bundle, with its more readily stiff, finely crimped structure, is designed to provide an ‘immediate’ intrinsic stability? Conversely, the anterior portion with its coarsely crimped bundles would appear to allow for a certain degree of compliance before stiffening, and thus resist more eccentric loading further from the joint centre. This would in turn create a stiffness gradient along the anterior to posterior regions.
Conclusion
From the different passive constraints applied to the ovine knee joint, the micro-level crimp morphologies of the ACL observed in this study show that the AM bundle is most responsive in the stance position, whereas the PL bundle is most responsive at the maximum extension and flexion position. Further, two morphologically different crimps, coarse crimp and fine crimp, are found in the ACL. We propose that these differences in crimp morphologies have relevance to ACL design and function.
Acknowledgments
The authors are grateful to the New Zealand Wishbone Trust (http://www.nzoa.org.nz/wishbone-trust) for funding this study.
References
- Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20:613–620. doi: 10.1007/s00167-011-1864-7. [DOI] [PubMed] [Google Scholar]
- Amis AA, Dawkins GPC. Functional anatomy of the anterior cruciate ligament. J Bone Joint Surg. 1991;73-B:8. doi: 10.1302/0301-620X.73B2.2005151. [DOI] [PubMed] [Google Scholar]
- Arnoczky SP. Anatomy of the anterior cruciate ligament. Clin Orthop Relat Res. 1983;172:19–25. [PubMed] [Google Scholar]
- Atarod M, Frank CB, Shrive NG. Decreased posterior cruciate and altered collateral ligament loading following ACL transection: a longitudinal study in the ovine model. J Orthop Res. 2014;32:431–438. doi: 10.1002/jor.22529. [DOI] [PubMed] [Google Scholar]
- Atkinson T, Ewers BJ, Haut RC. The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J Biomech. 1999;32:907–914. doi: 10.1016/s0021-9290(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Fleming BC, Johnson RJ, et al. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sport Med. 1995;23:24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- Boorman RS, Norman T, Matsen FA, 3rd, et al. Using a freeze substitution fixation technique and histological crimp analysis for characterizing regions of strain in ligaments loaded in situ. J Orthop Res. 2006;24:793–799. doi: 10.1002/jor.20081. [DOI] [PubMed] [Google Scholar]
- Clark JM, Sidles JA. The interrelation of fiber bundles in the anterior ruciate ligament. J Orthop Res. 1990;8:180–188. doi: 10.1002/jor.1100080205. [DOI] [PubMed] [Google Scholar]
- Diamant J, Keller A, Baer E, et al. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci. 1972;180:293–315. doi: 10.1098/rspb.1972.0019. [DOI] [PubMed] [Google Scholar]
- Dienst M, Burks RT, Greis PE. Anatomy and biomechanics of the anterior cruciate ligament. Orthop Clin North Am. 2002;33:605–620. doi: 10.1016/s0030-5898(02)00010-x. [DOI] [PubMed] [Google Scholar]
- Duthon VB, Barea C, Abrassart S, et al. Anatomy of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14:204–213. doi: 10.1007/s00167-005-0679-9. [DOI] [PubMed] [Google Scholar]
- Elliott DH. Structure and function of mammalian tendon. Biol Rev. 1965;40:392–421. doi: 10.1111/j.1469-185x.1965.tb00808.x. [DOI] [PubMed] [Google Scholar]
- Franchi M, Fini M, Quaranta M, et al. Crimp morphology in relaxed and stretched rat Achilles tendon. J Anat. 2007a;210:1–7. doi: 10.1111/j.1469-7580.2006.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi M, Trire A, Quaranta M, et al. Collagen structure of tendon relates to function. ScientificWorldJournal. 2007b;7:404–420. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi M, Raspanti M, Dell'Orbo C, et al. Different crimp patterns in collagen fibrils relate to the subfibrillar arrangement. Connect Tissue Res. 2008;49:85–91. doi: 10.1080/03008200801913635. [DOI] [PubMed] [Google Scholar]
- Franchi M, Quaranta M, Macciocca M, et al. Structure relates to elastic recoil and functional role in quadriceps tendon and patellar ligament. Micron. 2009;40:370–377. doi: 10.1016/j.micron.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Gathercole LJ, Keller A. Crimp morphology in the fibre-forming collagens. Matrix. 1991;11:214–234. doi: 10.1016/s0934-8832(11)80161-7. [DOI] [PubMed] [Google Scholar]
- Gianotti SM, Marshall SW, Hume PA, et al. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12:622–627. doi: 10.1016/j.jsams.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Granan LP, Forssblad M, Lind M, et al. The Scandinavian ACL registries 2004–2007: baseline epidemiology. Acta Orthop. 2009;80:563–567. doi: 10.3109/17453670903350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KA, Weiss JA, Barton JK. Recruitment of tendon crimp with applied tensile strain. J Biomech Eng. 2002;124:72. doi: 10.1115/1.1427698. [DOI] [PubMed] [Google Scholar]
- Henninger HB, Underwood CJ, Romney SJ, et al. Effect of elastin digestion on the quasi-static tensile response of medial collateral ligament. J Orthop Res. 2013;31:1226–1233. doi: 10.1002/jor.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini A, Gill TJ, Li G. In vivo anterior cruciate ligament elongation in response to axial tibial loads. J Orthop Sci. 2009;14:298–306. doi: 10.1007/s00776-009-1325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen KW, Orchard JW, Driscoll TR, et al. High incidence and costs for anterior cruciate ligament reconstructions performed in Australia from 2003–2004 to 2007–2008: time for an anterior cruciate ligament register by Scandinavian model? Scand J Med Sci Sports. 2012;22:495–501. doi: 10.1111/j.1600-0838.2010.01253.x. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:13. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Palley I, Baer E. A structual mechanical model for tendon crimping. J Biomech. 1980;13:887–893. doi: 10.1016/0021-9290(80)90177-3. [DOI] [PubMed] [Google Scholar]
- Kennedy JC, Weinberg HW, Wilson AS. The anatomy and function of the anterior cruciate ligament. J Bone Joint Surg. 1974;56-A:223–235. [PubMed] [Google Scholar]
- Lanir Y. Structure-strength relations in mammalian tendon. Biophys J. 1978;24:541–554. doi: 10.1016/S0006-3495(78)85400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurencin CT, Freeman JW. Ligament tissue engineering: an evolutionary materials science approach. Biomaterials. 2005;26:7530–7536. doi: 10.1016/j.biomaterials.2005.05.073. [DOI] [PubMed] [Google Scholar]
- Legnani C, Ventura A, Terzaghi C, et al. Anterior cruciate ligament reconstruction with synthetic grafts. A review of literature. Int Orthop. 2010;34:465–471. doi: 10.1007/s00264-010-0963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Hsu JE, Soslowsky LJ. In: Materials in Tendon and Ligament Repair Comprehensive Biomaterials. Ducheyne P, editor. Oxford, United Kingdom: Elsevier; 2011. pp. 257–279. [Google Scholar]
- Moses B, Orchard J, Orchard J. Systematic review: annual incidence of ACL injury and surgery in various populations. Res Sports Med. 2012;20:157–179. doi: 10.1080/15438627.2012.680633. [DOI] [PubMed] [Google Scholar]
- Norwood LA, Cross MJ. Anterior cruciate ligament: functional anatomy of its bundles in rotatory instabilities. Am J Sport Med. 1979;7:23–26. doi: 10.1177/036354657900700106. [DOI] [PubMed] [Google Scholar]
- Odensten M, Gillquist J. Functional anatomy of the anterior cruciate ligament and a rationale for reconstruction. J Bone Joint Surg. 1985;67-A:257–262. [PubMed] [Google Scholar]
- Petersen W, Zantop T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin Orthop Relat Res. 2007;454:35–47. doi: 10.1097/BLO.0b013e31802b4a59. [DOI] [PubMed] [Google Scholar]
- Petrigliano FA, McAllister DR, Wu BM. Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy. 2006;22:10. doi: 10.1016/j.arthro.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Rigby BJ, Hirai N, Spikes JD, et al. The mechanical properties of rat tail tendon. J Gen Physiol. 1959;43:265–283. doi: 10.1085/jgp.43.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah JS, Jayson IV, Hampson WGJ. Low tension studies of collagen fibres from ligaments of the human spine. Ann Rheum Dis. 1977;36:139–145. doi: 10.1136/ard.36.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagni R, Leardini A, Ensini A. Ligament fibre recruitment at the human ankle joint complex in passive flexion. J Biomech. 2004;37:1823–1829. doi: 10.1016/j.jbiomech.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Steckel H, Starman JS, Baums MH, et al. The double-bundle technique for anterior cruciate ligament reconstruction: a systematic overview. Scand J Med Sci Sports. 2007;17:99–108. doi: 10.1111/j.1600-0838.2006.00600.x. [DOI] [PubMed] [Google Scholar]
- Stieven-Filho E, Garschagen ET, Namba M, et al. Anatomic study of the double-bundle of the ACL with the knee in 90° flexion. Rev Col Bras Cir. 2011;38:5. [PubMed] [Google Scholar]
- Stromberg DD, Wiederhielm CA. Viscoelastic description of a collagenous tissue in simple elongation. J Appl Physiol. 1969;26:857–862. doi: 10.1152/jappl.1969.26.6.857. [DOI] [PubMed] [Google Scholar]
- Tapper JE, Ronsky JL, Powers MJ, et al. In vivo measurement of the dynamic 3-D kinematics of the ovine stifle joint. J Biomech Eng. 2004;126:301. doi: 10.1115/1.1695576. [DOI] [PubMed] [Google Scholar]
- Tapper JE, Fukushima S, Azuma H, et al. Dynamic in vivo three-dimensional (3D) kinematics of the anterior cruciate ligament/medial collateral ligament transected ovine stifle joint. J Orthop Res. 2008;26:660–672. doi: 10.1002/jor.20557. [DOI] [PubMed] [Google Scholar]
- Vidik A, Ekholm R. Light and electron microscopic studies of collagen fibers under strain. Z Anat Entwicklungsgesch. 1968;127:154–164. doi: 10.1007/BF00521981. [DOI] [PubMed] [Google Scholar]
- Vieira AC, Guedes RM, Marques AT. Development of ligament tissue biodegradable devices: a review. J Biomech. 2009;42:2421–2430. doi: 10.1016/j.jbiomech.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Woo SL, An KN, Frank CB. Anatomy, biology, and biomechanics of tendon and ligament. In: Buckwalter JA, Einhorn TA, Simon SR, et al., editors. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. Rosemont, USA: American Academy of Orthopaedic Surgeons; 2007. pp. 581–616. [Google Scholar]
- Woo SLY, Almarza AJ, Karaoglu S, Liang R, Fisher MB. Chapter 54 - Functional Tissue Engineering of Ligament and Tendon Injuries. In: Nerem AALAT, editor. Principles of Regenerative Medicine (Second edition) San Diego: Academic Press; 2011. pp. 997–1021. [Google Scholar]
- Zantop T, Petersen W, Fu FH. Anatomy of the anterior cruciate ligament. Oper Tech Orthop. 2005;15:20–28. [Google Scholar]
- Zantop T, Petersen W, Sekiya JK, et al. Anterior cruciate ligament anatomy and function relating to anatomical reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14:982–992. doi: 10.1007/s00167-006-0076-z. [DOI] [PubMed] [Google Scholar]
- Zhao L, Thambyah A, Broom ND. A multi-scale structural study of the porcine anterior cruciate ligament tibial enthesis. J Anat. 2014;224:624–633. doi: 10.1111/joa.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]