Abstract
Several β cell antigens recognized by T cells in the non-obese diabetic (NOD) mouse model of type 1 diabetes (T1D) are also T cell targets in the human disease. While numerous antigen-specific therapies prevent diabetes in NOD mice, successful translation of rodent findings to patients has been difficult. A human leucocyte antigen (HLA)-transgenic mouse model incorporating human β cell-specific T cells might provide a better platform for evaluating antigen-specific therapies. The ability to study such T cells is limited by their low frequency in peripheral blood and the difficulty in obtaining islet-infiltrating T cells from patients. We have worked to overcome this limitation by using lentiviral transduction to ‘reprogram’ primary human CD8 T cells to express three T cell receptors (TCRs) specific for a peptide derived from the β cell antigen islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP265–273) and recognized in the context of the human class I major histocompatibility complex (MHC) molecule HLA-A2. The TCRs bound peptide/MHC multimers with a range of avidities, but all bound with at least 10-fold lower avidity than the anti-viral TCR used for comparison. One exhibited antigenic recognition promiscuity. The β cell-specific human CD8 T cells generated by lentiviral transduction with one of the TCRs released interferon (IFN)-γ in response to antigen and exhibited cytotoxic activity against peptide-pulsed target cells. The cells engrafted in HLA-A2-transgenic NOD-scid IL2rγnull mice and could be detected in the blood, spleen and pancreas up to 5 weeks post-transfer, suggesting the utility of this approach for the evaluation of T cell-modulatory therapies for T1D and other T cell-mediated autoimmune diseases.
Keywords: autoimmunity, CD8 T cells, type 1 diabetes
Introduction
Type 1 diabetes (T1D) is an autoimmune disease resulting in part from the CD8 T cell-mediated killing of insulin-producing pancreatic β cells. The non-obese diabetic (NOD) mouse has been a widely used model of this disease for many years 1. Many autoantigens found to be targeted by T cells in NOD mice have also been found to be targets of T cells in the human disease 2. Thus, the NOD mouse has potential for testing therapies that eliminate antigen-specific T cells or induce tolerance. However, while many aspects of the pathogenesis of diabetes in NOD mice have been elucidated and numerous treatments based on these insights have prevented diabetes in mice 2,3, the disease process in humans is more complex, and much remains unknown 4. Clinical trials based on rodent data have shown only temporary and partial efficacy in a subset of those treated 5,6. An improved understanding of human diabetogenic T cells would help to ascertain more clearly which antigen-specific therapies are likely to be successful.
The study of human diabetogenic T cells has been limited by their low frequency in peripheral blood 7, the difficulty in obtaining islets from T1D patients and the challenges inherent in propagating islet-autoreactive T cell clones. However, it is possible to ‘reprogram’ human T cells to express a defined, new T cell receptor (TCR) using retroviral or lentiviral transduction 8–12. This strategy is being used clinically to generate autologous antigen-specific T cells against tumour or viral antigens in order to confer a protective T cell response to patients 13. Conversely, we reasoned that human T cells redirected to recognize β cell antigens could be transferred to an appropriate murine host and used as targets for the development of antigen-specific therapies for T1D.
The NOD-severe combined immunodeficiency (SCID) IL2rγnull (NSG) mouse strain is a highly effective model for the engraftment of both human haematopoietic stem cells 14 and peripheral blood mononuclear cells (PBMC) 15. The interleukin (IL)-2Rγ-chain deficiency eliminates the residual natural killer (NK) cell activity present in NOD-SCID mice that reduces engraftment efficiency 14. As these mice lack a competent immune system of their own, particularly CD4 and CD8 T cells essential for disease development, they cannot develop autoimmune diabetes 16. However, they provide a potential system for the in-vivo study of human autoreactive T cells. Transgenic NSG mice have been developed to express the human class I major histocompatibility complex (MHC) molecule HLA-A2 17,18, which is a T1D susceptibility allele in humans 19–21. These NSG-A2 mice develop islet inflammation (insulitis) when engrafted with PBMC from HLA-A2+ T1D patients 22, demonstrating the potential use of this mouse model for studying human β cell-specific T cells.
Islet-specific glucose-6-phosphatase catalytic-subunit related protein (IGRP) is an antigen recognized by autoreactive T cells in both NOD mice 23–25 and humans 7,26–30. The epitope IGRP265–273 (VLFGLGFAI), identical in mice and humans, was first found to be recognized by islet-infiltrating CD8 T cells in NOD mice transgenic for HLA-A2 31, and also shown later to be a target of CD8 T cells in the peripheral blood 7,27,29 and islets 26 of HLA-A2+ human T1D patients. We have generated lentiviral vectors encoding three distinct human TCRs specific for IGRP265–273/HLA-A2, two isolated from T1D patients and one from a healthy donor. The TCRs were compared in vitro by transduction of a TCR-deficient Jurkat cell line and were found to vary in their avidity for peptide/MHC (pMHC) multimers and to support antigen-specific responses to varying degrees. Lentiviral transduction of primary human CD8 T cells redirected them to be specific for the β cell antigen IGRP, and to exhibit antigen-dependent cytokine secretion and cytotoxic activity. After transfer into NSG-A2 mice, the transduced human CD8 T cells could be detected in the blood, spleen and pancreas of recipient mice up to 5 weeks post-transfer. We propose NSG-A2 mice engrafted with human β cell-specific T cells, generated by lentiviral TCR transduction, as a new system for the study of human autoreactive T cells and the development and testing of antigen-specific therapies for T1D.
Materials and methods
Cells and cell culture
Human C1R 32 and T2 cells 33 were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). C1R cells stably expressing HLA-A2 (C1R-A2) 34 were obtained from V. Engelhard. Human Jurkat cells expressing a chimeric class I MHC molecule consisting of the α1 and α2 domains of HLA-A2 and the α3, transmembrane and cytoplasmic portions of H-2Kb (Jurkat-A2/Kb) 35 were provided by L. Sherman. Jurkat/MA cells, a TCR-β chain-deficient Jurkat derivative modified to express human CD8α and to contain a luciferase reporter gene controlled by nuclear factor of activated T cells (NFAT) 36, were obtained from E. Hooijberg and then modified further by lentiviral transduction to increase human CD8α expression. All cell lines were maintained in Iscove's modified Dulbecco's medium (IMDM) (Invitrogen, Grand Island, NY, USA) containing 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT, USA) and penicillin/streptomycin (Invitrogen). For lentiviral production, the 293T cell line 37 was used at no more than 15 passages of a stock obtained from the ATCC.
Cloning of the TCR-α and -β chains from human CD8 T cell clones specific for IGRP265–273/HLA-A2
CD8 T cells specific for IGRP265–273/HLA-A2 were cloned from the peripheral blood of HLA-A*0201-positive T1D patients (clones 7 and 32) or an HLA-A*0201-positive healthy blood donor (clone FSB) and characterized as described 29. Following nomenclature of the international ImMunoGeneTics information system (IMGT; http://www.imgt.org), the TCR-α and -β chain gene usage of clones 7, FSB and 32 was, respectively, TRAV41/TRAJ48/TRBV6-2 (or -3)/TRBJ2-7; TRAV29/TRAJ29/TRBV28/TRBJ2-7; and TRAV12-1/TRAJ48/TRBV20-1/TRBJ2-1. The TCR-α and -β chains of each of the three T cell clones were linked with the self-cleaving 2A peptide derived from porcine teschovirus-1 by polymerase chain reaction (PCR), as described previously 9, to allow equimolar expression of both TCR chains 38. The PCR product was then cloned into a lentiviral transfer construct regulated by the spleen focus-forming virus promoter 39 and followed by the 2A peptide derived from Thoseassigna virus and the coding sequence for green fluorescent protein (GFP). The transfer constructs encoding the control HLA-A2-restricted TCRs 1803 and 1·9 A2B, specific for HIV-1 p17gag77–85 (SLYNTVATL; SL9), have been described 9,40. With the exception of 1803, all TCR transfer constructs were codon-optimized for expression in human cells 41.
293T cell transfection and lentiviral vector production
Lentiviral vectors were produced by calcium phosphate transfection of 293T cells, as described previously 39. Briefly, the transfer construct encoding the TCR-α–2A–TCR-β sequence was co-transfected into 293T cells with three additional plasmids: a packaging construct expressing the gag and pol genes, a construct expressing rev and a construct expressing the VSV-G envelope. Culture supernatant was replaced 16 h after transfection and lentiviral supernatant was collected 24 and 48 h later and passed through a 0·22 μm filter. Lentivirus was concentrated by ultracentrifugation, resuspended in sterile PBS and frozen in aliquots at −80°C until use. Viral titres ranged from 3 to 11 × 109 transducing units/ml.
Jurkat/MA cell transduction and lentiviral titring
Jurkat/MA cells 36 were transduced in complete IMDM containing 4 μg/ml polybrene in 24-well plates (1 × 105 cells/well in 500 μl). After the addition of an infectious dose of lentivirus sufficient to obtain greater than 95% transduction, the plates were centrifuged at 1350 g for 30 min. Cells were incubated for 16 h at 37°C and 500 μl fresh medium without polybrene was added. Transduced cells were cultured an additional 3–5 days before checking transduction efficiency by flow cytometry. Additionally, lentivirus was quantified by titring in Jurkat/MA cells. For this, cells were plated into six-well plates (1 × 105 cells/well) and transduced with 10-fold serial dilutions of lentivirus. Transduction efficiency was determined by flow cytometric analysis of GFP expression. Titre was determined from the viral dilution that gave GFP expression in 1–10% of cells 39.
Primary human T cell transduction
PBMC were isolated from HLA-A2+ leucopacks from anonymous donors (New York Blood Center) by Ficoll density gradient centrifugation. HLA-A2 expression was determined by flow cytometry using a fluorescein isothiocyanate (FITC)-conjugated anti-HLA-A2-specific antibody (BB7·2; BD Biosciences, San Jose, CA, USA). For some experiments, cryopreserved HLA-A2+ PBMC were obtained from AllCells (Alameda, CA, USA). CD8 T cells were isolated from PBMCs by positive selection with Miltenyi magnetic beads and cultured in RPMI-1640 (Invitrogen) containing 10% heat-inactivated FBS (Hyclone), penicillin/streptomycin (Invitrogen), non-essential amino acids (Invitrogen) and sodium pyruvate (Invitrogen). Prior to transduction, CD8 T cells were activated with 30 ng/ml anti-CD3 (OKT3; eBioscience, San Diego, CA, USA) and 1 μg/ml anti-CD28 (CD28·2; BD Biosciences) for 2 days and 50 U/ml of recombinant human (rh)IL-2 (Peprotech, Rocky Hill, NJ, USA) for 1 day. Cells were transduced with lentivirus at a multiplicity of infection of ≥ 50 in 24-well plates (5 × 105 cells/well in 500 μl) in the presence of 8 μg/ml polybrene while being centrifuged at 1350 g for 1 h. The next day an equal volume of complete medium with 50 U/ml IL-2 was added, and cells were cultured for 2–5 days before monitoring transduction efficiency by flow cytometry.
Analysis of transduced cells by flow cytometry
Transduced Jurkat/MA and primary human CD8 T cells were stained for 30 min on ice with an antibody to human TCR-αβ (T10B9·1A-31; BD Biosciences) as well as with antibodies to human TCR-β chain variable regions: anti-Vβ2-phycoerythrin (PE) (MPB2D5; Beckman Coulter, Brea, CA, USA), anti-Vβ3-RPE (JOVI-3; Ancell, Bayport, MN, USA) and anti-Vβ13·2-PE (H132; eBioscience). TCR-Vα2 was detected using anti-Vα2 (F1; Pierce, Rockford, IL, USA) and rat anti-mouse IgG2a-allophycocyanin (344701; R&D Systems, Minneapolis, MN, USA). Transduced Jurkat/MA cells were stained with HLA-A2/IGRP265–273 or HLA-A2/SL9 tetramer-PE at 34 nM for 1 h at room temperature. To evaluate tetramer staining, gates were set based on unstained controls. Transduced primary human CD8 T cells were stained with HLA-A2/IGRP265–273 or HLA-A2/SL9 dextramer-allophycocyanin (Immudex, Copenhagen, Denmark) for 10 min at room temperature according to the manufacturer's recommendations. To evaluate dextramer staining, gates were set based on the irrelevant dextramer controls. In certain experiments, cells were pretreated with 50 nM dasatinib (Axon Medchem, Groningen, the Netherlands) for 1 h at 37°C, followed by tetramer or dextramer staining in the presence of dasatinib. For detection of granzyme B, cells were treated with 1 × fixation and permeabilization buffer (eBioscience) and anti-granzyme B-AlexaFluor 700 (GB11; BD Biosciences). 4’,6-Diamidino-2-phenylindole (DAPI; Sigma-Aldrich, St Louis, MO, USA) was added just prior to acquisition on a BD LSRII flow cytometer, allowing for the exclusion of dead cells from further analysis. Data were analysed with FlowJo software (Tree Star Inc., Ashland, OR, USA).
Jurkat/MA cell luciferase assay
T2 33, C1R 32, C1R-A2 34, Jurkat-A2/Kb 35 or splenic dendritic cells (DCs) from HLA-A2-transgenic NOD.β2mnull.HHD mice 31 were loaded with the HIV gag peptide SL9 or IGRP265–273 for 1 h at 26°C. An equal number of transduced Jurkat/MA cells (generally greater than 95% GFP+, as determined by flow cytometry) were added and cells were co-cultured for 16 h at 37°C. In certain experiments, T2 cells were loaded with peptide for 30 min at 26°C, and then treated with 5, 10 or 20 μg/ml HLA-A2 blocking antibody (BB7·2; BD Biosciences) for 30 min at 26°C before the addition of Jurkat/MA cells. The cells were washed with PBS, then lysed with Reporter Lysis Buffer (Promega, Madison, WI, USA) and frozen at −80°C and then thawed to complete lysis. Luciferase activity was measured using the Promega Luciferase Assay System. Luminescence was measured using a Victor plate reader.
Determination of tetramer avidity
Jurkat/MA cells were transduced with TCRs 1803, 7, FSB and 32 and sorted by fluorescence-activated cell sorting (FACS) as needed to generate cells with comparable levels of TCR expression with a FACSAria. Equivalent numbers of GFP+ cells were stained with HLA-A2/IGRP265–273 tetramer-PE serial dilutions of 34, 17, 8·5, 4·25 and 2·125 nM in 25 μl FACS buffer (1% FBS, 0·1% sodium azide in PBS) for 1 h at room temperature. Cells were washed once with 200 μl FACS buffer and then resuspended in 200 μl 1% paraformaldehyde. Data were collected using a BD LSRII flow cytometer and analysed with FlowJo software (Tree Star). GFP+ cells were gated on for analysis of tetramer staining. To determine the avidity of tetramer binding to each TCR, non-linear regression analysis of tetramer concentration versus tetramer-PE mean fluorescence intensity (MFI) was performed using GraphPad Prism software. The MFI of unstained cells was subtracted from all values when assessing tetramer avidity.
Human interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay
T2 cells were plated at 5 × 104 cells/well in 50 μl complete RPMI in a 96-well multi-screen filter plate (Millipore, Billerica, MA, USA) precoated with anti-IFN-γ antibody (MAB285; R&D Systems) and blocked with 1% bovine serum albumin (BSA), and loaded with 10 μM peptide SL9 or IGRP265–273 for 1 h at 26°C. Transduced human CD8 T cells and untransduced control cells were added at 5 × 104 cells/well in 50 μl complete RPMI and incubated for 40 h. IFN-γ was detected with a second, biotinylated anti-IFN-γ antibody (BAF285; R&D Systems), and spots were developed using streptavidin–alkaline phosphatase (Zymed Laboratories, Carlsbad, CA, USA) and 5-bromo-4-chloro-3-indolyl-phosphate/nitro-blue tetrazolium (NBT) substrate (Sigma-Aldrich). Spots were counted by an automated ELISPOT reader system (Autoimmun Diagnostika, Strasberg, Germany).
Lactate dehydrogenase (LDH) cytotoxicity assay
T2 cells were plated in 96-well round-bottomed plates at 2 × 104 cells/well in 50 μl RPMI with 5% FBS and loaded with 10 μM SL9 or IGRP265–273 peptide for 1 h at 26°C. Transduced human CD8 T cells and untransduced controls were added at effector : target ratios of 10 : 1 and 2·5 : 1 in 50 μl RPMI with 5% FBS. Cells were co-cultured for 4 h at 37°C, centrifuged, and lactate dehydrogenase (LDH) was detected in the supernatant using an LDH Cytotoxicity Assay Kit (Pierce). Percentage of cytotoxicity was calculated as 100 × (measured reading – effector only – target only)/(target maximum lysis – target only).
Engraftment of transduced human CD8 T cells in NSG-A2 mice
NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(HLA-A2·1)1Enge/SzJ (NSG-A2) mice 18 were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Human HLA-A2+ CD8 T cells were transduced with lentiviral vectors as described above. Four days later, 4 × 106 cells were combined with 8 × 106 CD8 T cell-depleted PBMC from the same donor and transferred via tail vein into NSG-A2 mice. The CD8 T cell-depleted PBMC had been activated with anti-CD3, anti-CD28 and IL-2 and maintained in culture until use, as described above for the transduced CD8 T cells. Blood was taken weekly from the tail of the mice starting at week 1 and analysed for engraftment by flow cytometry. At week 5 mice were euthanized, and blood, spleen and pancreas were analysed for engraftment by flow cytometry. Cells were stained with anti-human CD45-V450 (HI30; BD Biosciences), anti-human CD8-allophycocyanin-cyanine 7 (Cy7) (RPA-T8; BD Biosciences), anti-human CD4-peridinin chlorophyll (PerCP)-Cy5·5 (L200; BD Biosciences), allphycocyanin-labelled HLA-A2/IGRP265–273 or HLA-A2/SL9 dextramers (Immudex), anti-mouse CD45-PE-Cy7 (30-F11; BD Biosciences) and LIVE/DEAD Fixable Yellow (Invitrogen) prior to fixing with 1% paraformaldehyde. NSG-A2 mice that did not receive human cells were used as negative controls for identifying the human CD45+ population, and human PBMC were used as single-stained controls for the anti-human antibodies. All animal studies were approved by Albert Einstein College of Medicine's Institutional Animal Care and Use Committee.
Results
Lentiviral transduction of TCR-deficient Jurkat/MA cells
The TCR-α and -β chains from three distinct human T cell receptors specific for HLA-A2/IGRP265–273 were linked with a self-cleaving viral 2A sequence to achieve equimolar expression of both TCR chains 38 and inserted into a lentiviral vector regulated by the spleen focus-forming virus promoter. Despite their common specificity, the α and β chains of the TCRs utilized different Vα and Vβ gene segments (Table 1). However, sequence analysis of the complementarity determining regions (CDRs) revealed several common residues, particularly in the CDR3α sequences of TCRs 7 and 32, and the CDR3β sequences of TCRs 7 and FSB.
Table 1.
Amino acid comparison of human islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)265–273-specific T cell receptor (TCR)-α and -β chains
| (a) TCR-α chain CDRs* | ||||
|---|---|---|---|---|
| TCR | Vα† | CDR1α | CDR2α | CDR3α |
| 7 | Vα19 | VGISA | LSSGK | AVTSNFGNEKLT† |
| FSB | Vα21 | NSMFDY | ISSIKDK | AASAGSGNTPLV |
| 32 | Vα2 | NSASQS | VYSSGN | VVNILSNFGNEKLT |
| (b) TCR-β chain CDRs* | ||||
| TCR | Vβ† | CDR1β | CDR2β | CDR3β |
| 7 | Vβ13·2 | MNHEY | SVGEGT | ASSSRFVGEGLFRYGYEQY |
| FSB | Vβ3 | MDHEN | SYDVKM | ASSSISGYEQY |
| 32 | Vβ2 | DFQATT | SNEGSKA | SASRQGWVNEQF |
Underlined letters indicate amino acid identity among the TCRs.
According to Arden. 42.
Expression of the lentivirus-encoded TCRs was evaluated by transducing the CD8+ TCR-β-deficient cell line Jurkat/MA 36, which does not express endogenous TCR on its surface. Transduction with lentiviral vectors 7, FSB and 32 resulted in the expression of vector-encoded GFP and TCR in greater than 95% of the cells (Fig. 1a). To further characterize the TCRs, transduced cells were stained with TCR Vβ-specific antibodies (Fig. 1a). Vβ staining was comparable to TCR staining, as nearly all GFP+ cells also stained with the Vβ antibody specific for that TCR (i.e. Vβ13·2 for 7, Vβ3 for FSB and Vβ2 for 32). While Vα antibodies specific for TCRs 7 and FSB were not available, we were able to verify co-expression of the lentivirus-encoded TCR-α and -β chains of TCR 32 by co-staining transduced Jurkat/MA cells with antibodies specific for Vα2 and Vβ2 (Fig. 1b). Note that the TCR nomenclature used to designate commercial anti-Vα and -Vβ antibodies is according to Arden 42, and differs from the IMGT nomenclature provided in the Materials and methods.
Fig 1.
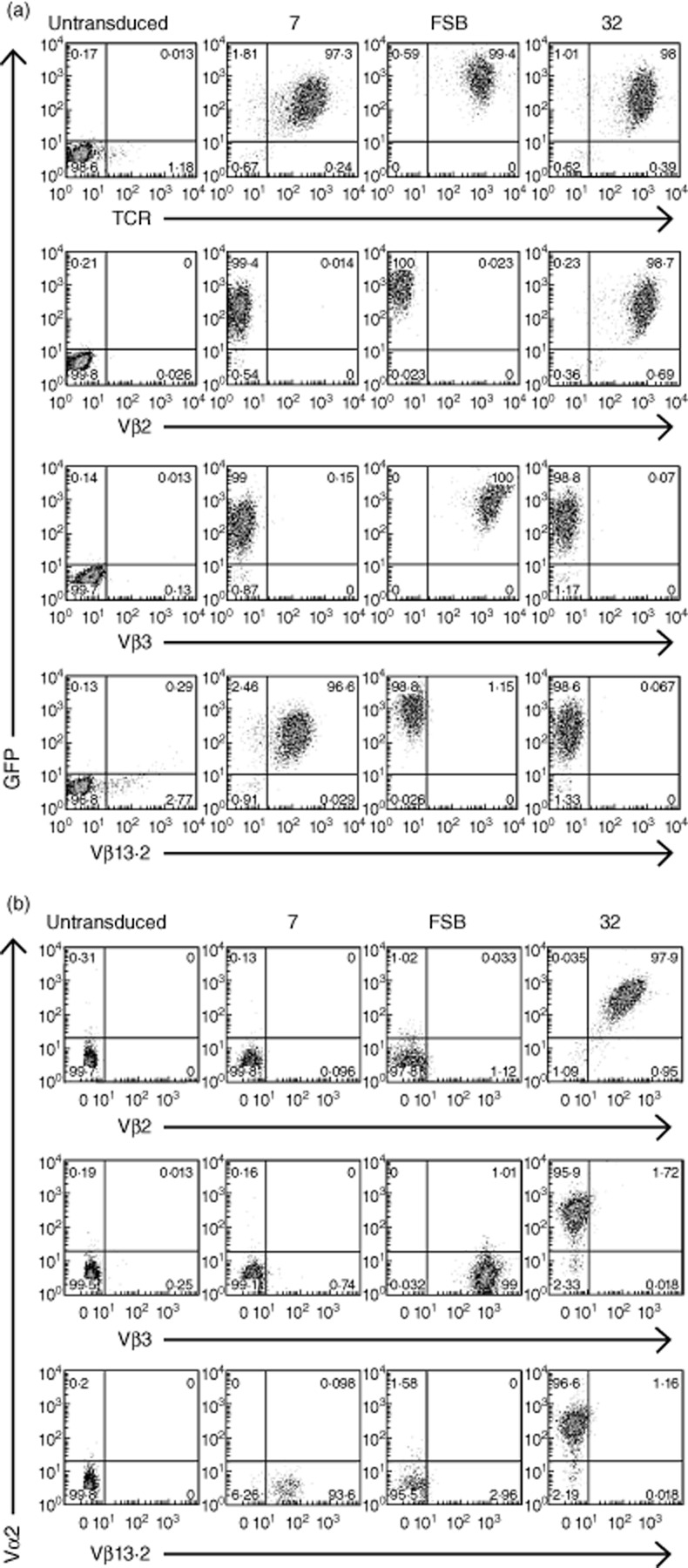
Lentiviral transduction of T cell receptor (TCR)-deficient Jurkat/MA cells. (a) Jurkat/MA cells were transduced with lentiviruses encoding the indicated islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)265–273-specific TCRs and evaluated for TCR expression 4 days after transduction. Cells were stained with anti-human TCR and anti-Vβ antibodies specific for the three TCRs. (b) As in (a), except that an anti-Vα antibody specific for TCR 32 was also included.
Tetramer staining of transduced Jurkat/MA cells reveals varying avidities for pMHC among the three TCRs
To verify the specificity of the transduced TCRs for IGRP265–273, transduced Jurkat/MA cells were stained with PE-labelled HLA-A2/IGRP265–273 tetramers (Fig. 2a). All three TCRs were stained successfully with the tetramers, although with varying intensities. TCR 32 stained very brightly, while FSB stained moderately well and 7 stained weakly, with little separation between positive and negative cells. Tetramer staining has been shown to be correlated with TCR/pMHC monomer affinity 43,44. To verify that the weak tetramer staining of TCRs 7 and FSB was due to low-affinity binding and not the result of the transduced TCR-β chain pairing with the endogenous Jurkat/MA TCR-α chain, transduced cells were pretreated with the protein kinase inhibitor dasatinib prior to tetramer staining. Dasatinib has been shown to improve the tetramer staining of low-affinity TCRs by preventing TCR internalization that occurs during tetramer staining and by reducing tetramer-induced cell death 45. Dasatinib pretreatment of transduced cells resulted in substantial improvements in tetramer staining for all three TCRs (Fig. 2a,b). The most significant effect was seen with TCRs 7 and FSB, for which distinct tetramer-positive populations were now observed. As TCR 32-transduced cells already stained well with tetramer, the effect was less dramatic, resulting in only a slightly higher intensity of staining. These results indicated that the weaker tetramer staining of TCRs 7 and FSB was due probably to a lower-affinity TCR/pMHC interaction. Dasatinib pretreatment did not cause an increase in non-specific binding to a negative control HLA-A2/SL9 tetramer (Fig. 2b).
Fig 2.
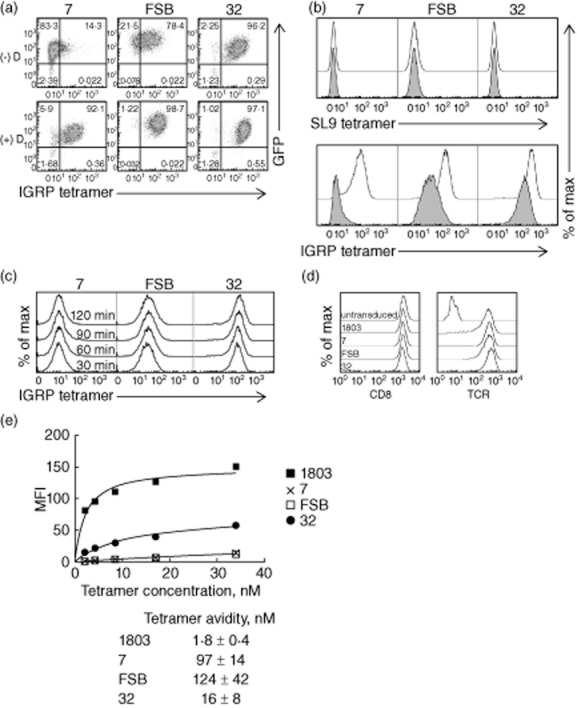
Human T cell receptors (TCRs) specific for islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)265–273 demonstrate varying avidity for peptide/major histocompatibility complex (MHC) tetramers. (a) Jurkat/MA cells were transduced with lentiviruses encoding the indicated TCRs and stained with human leucocyte antigen (HLA)-A2/IGRP265–273 tetramers alone (−D) or following treatment with 50 nM dasatinib (+D). (b) Transduced cells were stained with HLA-A2/SL9 (upper panel) or HLA-A2/IGRP265–273 tetramers (lower panel) with (not shaded) or without (shaded) 50 nM dasatinib pretreatment. Dasatinib pretreatment improves tetramer staining of low-affinity TCRs but does not increase non-specific tetramer staining. (c) To determine the time required for IGRP tetramer staining to reach equilibrium, transduced Jurkat/MA cells were stained with IGRP tetramer for 30, 60, 90 or 120 min at room temperature and analysed by flow cytometry. (d) Transduced Jurkat/MA cells were sorted by fluorescence-activated cell sorting (FACS) for equivalent TCR levels and evaluated for CD8 (left panel) and TCR expression (right panel). (e) The avidity of the TCR/tetramer interaction was determined by staining TCR-equalized Jurkat/MA cells with serial dilutions of HLA-A2/IGRP265–273 tetramers and performing non-linear regression analysis. The mean fluorescence intensity (MFI) of unstained cells was subtracted from all values when assessing tetramer avidity. The graph shown is representative of three separate experiments. Values below the graph are mean ± standard deviation of the three experiments.
To investigate further these differences in tetramer staining intensities, transduced cells were stained with serial dilutions of the tetramers, and equilibrium binding results were used to determine the avidity of tetramer binding to each TCR. Binding was carried out for 1 h, as staining intensity was increased only minimally beyond this time-point (Fig. 2c). Because the observed avidity of tetramer binding could be influenced by the expression level of both CD8 and TCR, transduced Jurkat/MA cells were FACS-sorted as needed to generate cells with equivalent TCR expression. These sorted cells expressed comparable levels of both CD8 and TCR (Fig. 2d). As suggested by the initial tetramer staining, of the three IGRP TCRs, TCR 32 bound the most avidly to the HLA-A2/IGRP265–273 tetramer, exhibiting a tetramer avidity of 16 ± 8 nM, compared to 124 ± 42 nM for FSB and 97 ± 14 nM for TCR 7 (Fig. 2e). As expected, the HIV-specific TCR 1803 bound more avidly to its cognate tetramer (1·8 ± 0·4 nM) than did any of the IGRP-specific TCRs (Fig. 2e).
Lentivirus-encoded TCRs support signalling in Jurkat/MA cells to varying degrees
In addition to its TCR deficiency, the Jurkat/MA cell line expresses luciferase under the control of the TCR-induced transcription factor NFAT 36. In order to test the functional activity of the three lentivirus-encoded TCRs, we measured the luciferase activity of the transduced Jurkat/MA cells in response to 1 μM IGRP265–273 presented by the human HLA-A2+ cell line T2 33 (Fig. 3a). Jurkat/MA cells transduced to express the 1803 TCR 46, specific for the HIV gag epitope SL9, were also used in these experiments. The FSB TCR responded to its cognate peptide, although not as strongly as did the HIV TCR 1803, which has a much higher avidity for its pMHC. TCR 7 did not give a detectable response to IGRP265–273. Unexpectedly, TCR 32 responded strongly to T2 cells, regardless of whether or not an exogenous peptide was provided.
Fig 3.
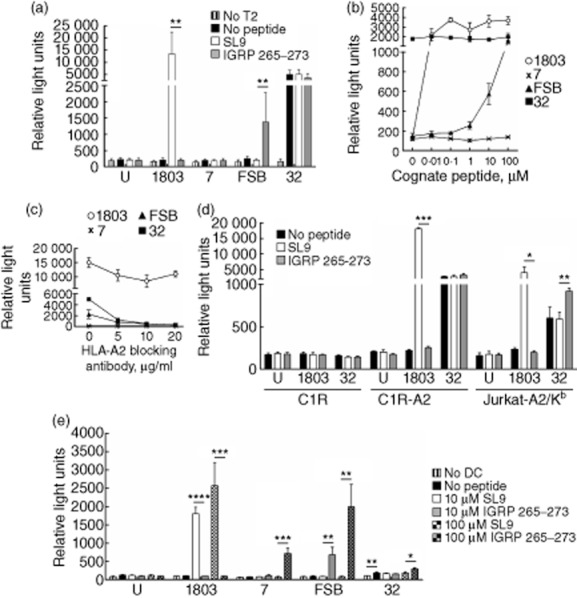
Lentivirus-encoded T cell receptors (TCRs) are functional in Jurkat/MA cells. (a) T2 cells were preincubated with 1 μM SLYNTVATL (SL9) or islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)265–273 for 1 h before addition of Jurkat/MA cells transduced with lentiviruses encoding the indicated TCRs. After overnight co-culture, luciferase activity was measured. Graph depicts mean ± standard deviation (s.d.) of seven independent experiments. U, untransduced Jurkat/MA cells; **P < 0·005. (b) As in (a), except T2 cells were preincubated with 10-fold serial dilutions of SL9 or IGRP265–273 from 0·01 μM to 100 μM. (c) As in (a), except after 30 min incubation with 1 μM SL9 or IGRP265–273, T2 cells were treated with 5, 10 or 20 μg/ml human leucocyte antigen (HLA)-A2 blocking antibody before addition of transduced Jurkat/MA cells. (d) As in (a), except that the indicated cell lines were used as antigen-presenting cells (APC) and 10 μM peptide was used. Graph depicts mean ± s.d. of technical replicates. *P < 0·05; **P < 0·005; ***P < 0·0001. (e) As in (a), except that splenic dendritic cells (DCs) from non-obese diabetic (NOD).β2mnull.HHD mice were used as APC. Graph depicts mean ± s.d. of technical replicates. *P < 0·05; **P < 0·01; ***P < 0·005; ****P < 0·0001.
To correlate further the TCR binding avidity results with functional activity, we performed a dose–response luciferase experiment using the TCR-equalized transduced Jurkat/MA cells (Fig. 3b). Cells transduced with the high-avidity TCR 1803 maintained a strong luciferase response to the SL9 peptide, even at 0·01 μM. The lower-avidity FSB TCR responded to IGRP265–273 at concentrations of 1 μM and above. TCR 7 did not have an observable response, even at 100 μM peptide. TCR 32 continued to demonstrate a non-specific response to the T2 cells, which was unaffected even with high concentrations of IGRP265–273. To verify that these were HLA-A2-restricted responses, T2 cells were loaded with peptide, then treated with an HLA-A2 blocking antibody prior to the addition of Jurkat/MA cells (Fig. 3c). At 20 μg/ml blocking antibody, responses from TCRs FSB and 32 were nearly abolished, while 1803 maintained a reduced but robust response. TCR 32 also responded in varying degrees to other HLA-A2+ human cell lines tested as antigen-presenting cells (APC), including C1R-A2 34 and Jurkat-A2/Kb 35, although it did not respond to the HLA-A2− cell line C1R 32 (Fig. 3d). These results suggest that, in addition to recognizing HLA-A2/IGRP265–273, TCR 32 also cross-reacts with a second peptide presented in the context of HLA-A2 by the three human cell lines examined. This idea is supported by the finding that Jurkat-A2/Kb, which expresses less than half as much HLA-A2 on its surface as T2 and C1R-A2 (data not shown), elicited a weaker response from TCR 32 than did the other two cell lines. Similarly, when DCs from mice transgenic for HLA-A2 (Fig. 3e) were used as the APC, the response of TCR 32 in the absence of exogenous peptide was reduced compared to that observed with the human HLA-A2+ cell lines. These reductions in IGRP peptide-independent signalling were sufficient to allow a modest response of TCR 32 to IGRP265–273 to be observed (Fig. 3d,e). When DCs were used as the APC, TCRs 7 and FSB also responded to IGRP265–273 although, like 32, TCR 7 responded only to the highest concentration of peptide (100 μM) (Fig. 3e). Taken together, these experiments demonstrate the differing functional activities of the lentivector-encoded TCRs 7, FSB and 32 in transduced Jurkat/MA cells and suggest TCR FSB as the best candidate for the in-vivo aspects of our work.
Reprogramming human primary CD8 T cells with IGRP-specific TCRs
We next sought to evaluate the ability of the lentiviral vectors to transduce primary cells and to confirm FSB as the most useful of the three IGRP-specific TCRs. CD8 T cells were purified from peripheral blood of HLA-A2+ human donors, activated to increase transduction efficiency, and transduced separately with the three lentiviral vectors. Transduction efficiency was determined by flow cytometric analysis of GFP expression and staining with the appropriate TCR Vβ-specific antibodies (Fig. 4a). Untransduced cells lacked GFP expression, but had variable levels of endogenous expression of Vβ2, Vβ3 and Vβ13·2 TCRs. Transduction efficiencies varied depending on the donor, but ≥ 90% of GFP+ cells were found consistently to express the lentiviral-encoded TCR-β chain. Additionally, the intensity of Vβ staining was comparable to endogenous levels, indicating that the transduced TCRs were being expressed at normal levels. To verify the proper pairing of the transduced TCR chains, cells were pretreated with dasatinib and stained with HLA-A2/IGRP265–273 dextramers 47, pMHC multimers demonstrated to enable improved detection of low-affinity TCRs compared to standard tetramers 48. Human primary CD8 T cells transduced with TCRs FSB and 32 both stained with the dextramers (Fig. 4b), although no staining was detected in the case of TCR 7. As primary human CD8 T cells have endogenous TCR expression, it is likely that there is some mixed pairing of transduced TCR-α and -β chains with endogenous chains, resulting in reduced expression of the lentivirus-encoded TCR and lower pMHC multimer staining than observed in the case of transduced Jurkat/MA cells (Fig. 2a).
Fig 4.
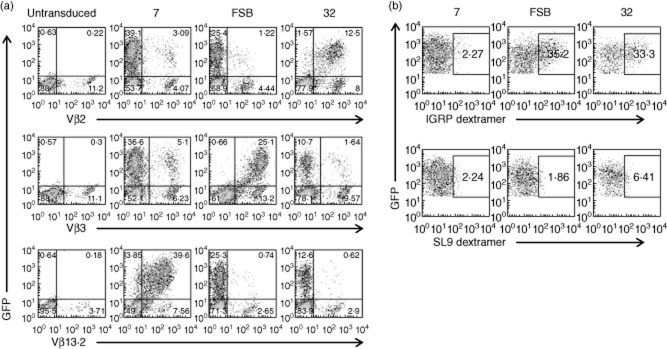
Lentiviral transduction can be used to generate human β cell-specific CD8 T cells. Primary human CD8 T cells were transduced with lentiviruses encoding the indicated T cell receptors (TCRs) and (a) stained with anti-Vβ antibodies specific for each of the three TCRs or (b) pretreated with 50 nM dasatinib and stained with human leucocyte antigen (HLA)-A2/ islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)265–273 or human leucocyte antigen (HLA)-A2/SLYNTVATL (SL9) dextramers. Plots shown in (b) are from the green fluorescent protein (GFP)+ gate.
FSB-transduced human CD8 T cells release IFN-γ in response to their cognate antigen and lyse peptide-pulsed target cells
After demonstrating that human primary CD8 T cells could be engineered to express β cell-specific TCRs, we next examined the effector functions of the transduced cells. To do this, transduced cells were co-cultured with T2 cells pretreated with IGRP265–273, and their response was measured by IFN-γ ELISPOT (Fig. 5a). In agreement with the luciferase results obtained with transduced Jurkat/MA cells, IFN-γ production by FSB-transduced primary human CD8 T cells was the most pronounced. TCR 7 trended towards an increase in IFN-γ-producing cells in the presence of T2 cells loaded with IGRP265–273, but this was not significantly different from the response to an irrelevant peptide or to T2 cells alone. TCR 32-transduced cells did not show a specific response to IGRP265–273-loaded T2 cells.
Fig 5.
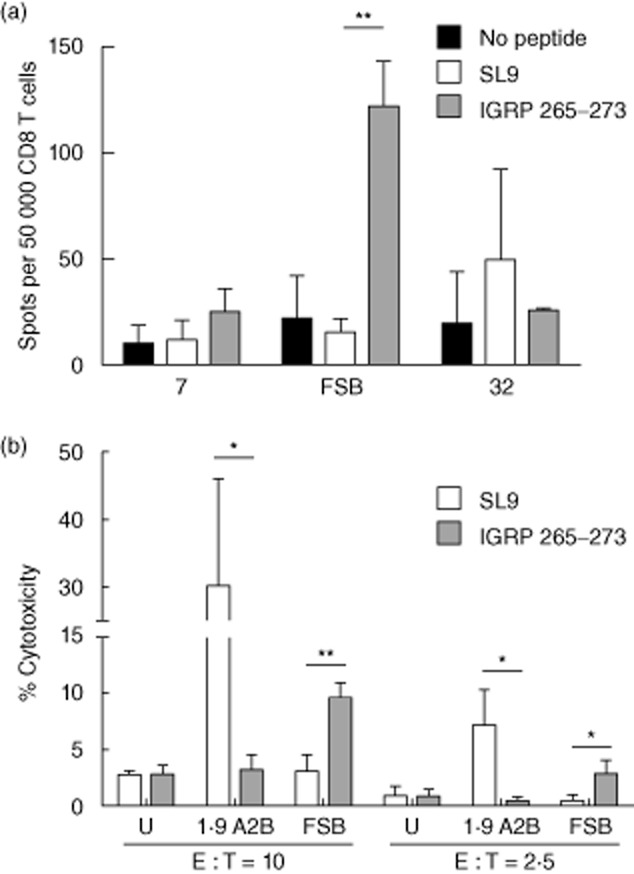
T cell receptor (TCR)-transduced primary human CD8 T cells release interferon (IFN)-γ and are cytotoxic. (a) Primary human CD8 T cells were transduced with lentiviruses encoding the indicated TCRs and incubated with T2 cells loaded with 10 μM SLYNTVATL (SL9) or islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)265–273. IFN-γ production was detected by enzyme-linked immunospot (ELISPOT). Graph depicts mean ± standard deviation (s.d.) of three independent experiments. **P < 0·005. (b) Transduced primary human CD8 T cells were incubated with peptide-loaded T2 cells at an effector : target (E : T) ratio of 10 : 1 or 2·5 : 1. Cytotoxicity was measured by lactate dehydrogenase (LDH) release into the supernatant. Graph depicts mean ± s.d. of technical replicates. U, untransduced primary human CD8 T cells; *P < 0·05; **P < 0·005.
To evaluate further the ability of the transduced CD8 T cells to act as cytotoxic T lymphocytes (CTL), an LDH release assay was performed to measure their cytotoxic activity. T2 cells loaded with IGRP265–273 were specifically lysed by FSB-transduced cells compared to T2 cells loaded with the irrelevant SL9 peptide (Fig. 5b). Cells transduced with the SL9-specific TCR 1·9 A2B lysed SL9-loaded T2 cells, but not IGRP265–273-loaded T2 cells. In contrast to TCR FSB, cells transduced with TCRs 7 or 32 did not exhibit specific lysis of IGRP265–273-pulsed target cells (data not shown). Taken together, these experiments demonstrate successful transduction of primary human CD8 T cells with IGRP-specific TCRs, with FSB yielding cells possessing peptide-specific CTL functions including IFN-γ production and cytotoxic activity. TCR FSB is clearly the preferred TCR for in-vivo studies, given that it responds in a dose-dependent manner to peptide concentrations as low as 1 μM (Fig. 3a,b). Furthermore, primary human T cells transduced to express FSB show cytotoxic activity against peptide-pulsed target cells in vitro even at low effector : target ratios (Fig. 5b).
Long-term survival of engrafted transduced cells in NSG-A2 mice
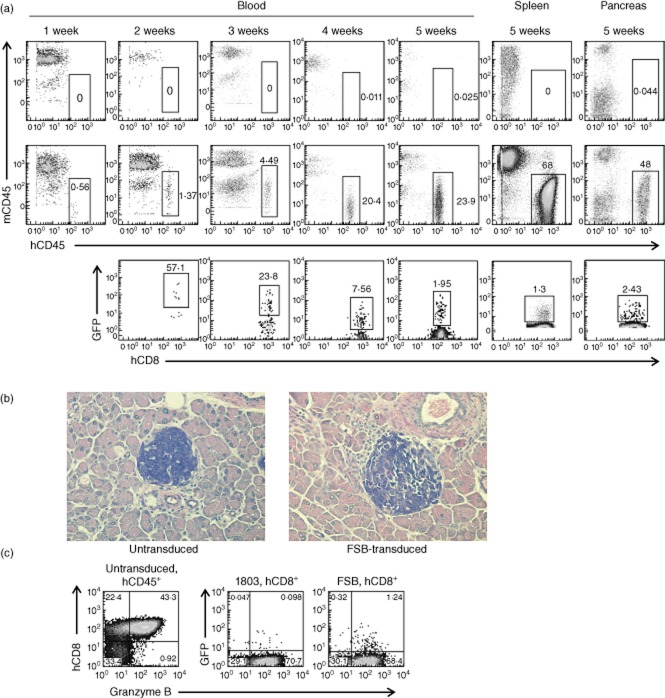
In-vivo studies of the autoimmune activity of the TCR-transduced human CD8 T cells would require long-term engraftment in NSG-A2 hosts. It has been shown that intravenous injection of at least 1 × 107 PBMC yields the best engraftment outcome in NSG mice 15. As CD8 T cells have been found to engraft poorly in NOD-SCID mice when transferred alone 49, we injected NSG-A2 mice intravenously with 4 × 106 untransduced or FSB-transduced human CD8 T cells in combination with 8 × 106 CD8 T cell-depleted PBMC from the same donor. Blood engraftment levels were monitored weekly, and at 5 weeks after transfer mice were euthanized to examine spleen and pancreas engraftment as well. The experiments were terminated at 5 weeks, because xenogeneic graft-versus-host (GvH) disease occurs in NSG mice repopulated with PBMC between 30 and 45 days post-transfer 15,50. The level of engraftment of hCD45+ cells in mice receiving FSB-transduced CD8 T cells was similar to engraftment in mice receiving untransduced cells (Table 2). The percentage of engrafted cells in the blood increased each week, and higher engraftment was seen in the spleen than in the blood. Human CD45+ cells were also observed in the pancreata of both untransduced and FSB-transduced recipient mice. Recipients that received untransduced cells tended to have a higher percentage of engrafted CD8 T cells, but this was only statistically significant at the 3-week time-point. In FSB-transduced recipient mice, GFP+ CD8 cells could be detected as early as 2 weeks after transfer, and remained detectable in blood, spleen and pancreas 5 weeks after transfer (Fig. 6a; Table 2). Histological analysis of pancreas sections revealed mild infiltration around some islets in both untransduced and FSB-transduced recipient mice (Fig. 6b) due probably, at least in part, to a GvH response to murine class I MHC molecules 15,50. Because of this non-specific infiltration, we were unable to discern a difference in histopathology between the two groups, and diabetes was not observed in any of the recipients during the 5-week experimental period. To evaluate whether the transduced cells maintained their CTL capabilities after engraftment, splenocytes from untransduced, FSB-transduced and 1803-transduced recipients were analysed 5 weeks after engraftment for granzyme B expression (Fig. 6c). In all mice tested, granzyme B expression was restricted to the CD8 T cell population. Importantly, granzyme B expression in GFP+ FSB and 1803-transduced cells was comparable to granzyme B expression of GFP− cells in the same host, as well as those from untransduced recipients. This result confirms that the transduced human cells retain CTL function after 5 weeks in the mouse host.
Table 2.
Human cell engraftment in NSG-A2 mice*
| Site | hCD45-positive cells [% of (hCD45 + mCD45)] | hCD8-positive cells (% of hCD45)† | GFP-positive cells (% of hCD8)‡ | ||
|---|---|---|---|---|---|
| Untransduced | FSB-transduced | Untransduced | FSB-transduced | FSB-transduced | |
| Blood | |||||
| Week 1 | 0·2 ± 0·2 (6) | 0·2 ± 0·3 (5)§ | n.d. | n.d. | n.d. |
| Week 2 | 0·5 ± 0·6 (6) | 0·7 ± 0·6 (5) | 61 ± 29 (3) | 33 ± 32 (3) | 26 ± 27 (3) |
| Week 3 | 7·2 ± 14 (6) | 8·0 ± 6·4 (5) | 81 ± 12 (2) | 47 ± 6·0 (4) | 11 ± 10 (4) |
| Week 4 | 14 ± 16 (6) | 22 ± 15 (5) | 64 ± 20 (5) | 39 ± 9·7 (5) | 4·6 ± 3·4 (5) |
| Week 5 | 37 ± 34 (6) | 52 ± 16 (5) | 61 ± 22 (6) | 31 ± 14 (5) | 1·5 ± 1·4 (5) |
| Spleen | 57 ± 29 (6) | 70 ± 14 (5) | 54 ± 20 (6) | 32 ± 16 (5) | 1·1 ± 1·1 (5) |
| Pancreas | 44 ± 35 (6) | 66 ± 16 (5) | 51 ± 22 (6) | 32 ± 12 (5) | 1·9 ± 1·7 (5) |
*Untransduced or T cell receptor (TCR) FSB lentivirus-transduced human leucocyte antigen (HLA)-A2+ human CD8 T cells (4 × 106) were combined with CD8 T cell-depleted peripheral blood monunclear cells (PBMC) from the same donor (8 × 106) and transferred via tail vein into HLA-A2-transgenic non-obese diabetic (NOD)-severe combined immunodeficient (SCID) interleukin (IL)-2rγ null (NSG-A2) mice. Blood was taken weekly from the tail starting at week 1 and analysed for engraftment by flow cytometry. At week 5, mice were killed and blood, spleen and pancreas were analysed for engraftment. Numbers of mice are indicated in parentheses. Sample fluorescence activated cell sorter (FACS) plots are shown in Fig. 6a. n.d., not determined.
Expressed as a % of hCD45-positive cells. Mice having a hCD45-positive cell percentage less than 0·5% for a given time-point were excluded from subsequent analysis for that time-point.
Expressed as a % of hCD8-positive cells.
A sixth mouse had a splenic hCD45-positive cell frequency of less than 1% at week 5 and was excluded from further analysis.
Fig 6.
Transduced human CD8 T cells remain detectable and functional 5 weeks after transfer to human leucocyte antigen (HLA)-A2-transgenic non-obese diabetic (NOD)-severe combined immunodeficient (SCID) interleukin (IL)-2rγ null (NSG-A2) mice. NSG-A2 mice were injected intravenously with 4 × 106 untransduced, FSB-transduced, or 1803-transduced human CD8 T cells in combination with 8 × 106 CD8-depleted peripheral blood mononucleaer cells (PBMC). Blood samples were taken weekly from 1 to 5 weeks after transfer and analysed by flow cytometry. Spleen and pancreas were analysed similarly at 5 weeks post-transfer. (a) Engraftment of a representative FSB recipient mouse (middle panels), with an NSG-A2 mouse that did not receive human cells shown for comparison (top panels). Top and middle panels, total hCD45+mCD45− cell engraftment (% of total cells is shown). Bottom panels, engraftment of green fluorescent protein (GFP)+ FSB-transduced CD8 T cells (% of hCD45+mCD45−hCD8+ cells is shown). Due to the necessity of analysing the time-points on different days, variations in fluorescence intensity were observed. Summary data from all mice are shown in Table 2. (b) Pancreata from untransduced and FSB-transduced recipient mice were fixed 5 weeks after transfer, sectioned, and stained with aldehyde fuchsin. Representative images of islets from untransduced and FSB recipient mice are shown. (c) Splenocytes from untransduced, 1803-transduced and FSB-transduced recipient mice were analysed for intracellular granzyme B expression by flow cytometry 5 weeks after transfer. Left panel, granzyme B expression of untransduced hCD45+mCD45− cells. Middle and right panels, granzyme B expression of hCD45+mCD45−hCD8+ cells from 1803 and FSB recipient mice.
Discussion
The NOD mouse has been the prevailing model for the study of T1D for many years and has greatly improved our understanding of this autoimmune disease 1. However, while the disease can be prevented, and even reversed, in the mice 2,3, a robust immunological therapy for human T1D has not yet been achieved 5,6. We reasoned that the ability to investigate the impact of therapeutic approaches targeting human β cell-specific T cells might support the translation of rodent data to the human disease. To that end, we have used lentiviral transduction to generate human CD8 T cells specific for IGRP265–273/HLA-A2. Cells transduced with TCR FSB exhibited characteristics of CTL, including antigen-dependent IFN-γ secretion and lysis of peptide-pulsed targets, suggesting in-vitro uses for such cells, e.g. investigation of mechanisms of β cell killing by human T cells or antigen identification in the case of β cell-specific TCRs of unknown specificity. Importantly, the transduced cells survived for up to 5 weeks in NSG-A2 hosts and will thus permit the future evaluation of T cell-modulatory interventions in an in-vivo system that incorporates human T cells interacting with human MHC molecules. Although TCR-transduced human T cells have been used to achieve anti-tumour or anti-viral activity in patients 13, their long-term survival and utility in immune-deficient murine models has not been reported previously.
In the course of developing this system, we first characterized three human TCRs specific for IGRP265–273/HLA-A2 in terms of their structural and functional avidities. As determined by measurement of tetramer avidity values, the TCRs exhibited a range of structural avidities, and all were of lower avidity than the HIV-specific TCR 1803 studied for comparison. While the functional avidities of the three IGRP-specific TCRs did not correlate strictly with the measured structural avidities, all were reduced compared to the functional avidity observed for the HIV-specific TCR. This is perhaps not surprising, as it is unlikely that T cells bearing high-affinity autoreactive TCRs would escape negative selection in the thymus 51. It is becoming clear that autoreactive TCRs in both mice and humans often exhibit low avidity, either because of the TCR itself (as in our work) or because its cognate peptide binds poorly to MHC 52–59. It is not unusual for autoreactive T cells to exhibit responses only to relatively high concentrations of peptide in vitro (compared to anti-viral T cells, for example) 52,56,57,59. Some of these T cells are nonetheless pathogenic, presumably because the local peptide concentration in the target organ is high 60, or because the peptide is modified there in a way that improves recognition 61. The relatively high tetramer avidity values for the IGRP-specific TCRs, the poor tetramer staining observed in the absence of dasatinib for TCR 7 (and, to a lesser extent, FSB) and the reduced functional avidities compared to the anti-viral TCR support the autoreactive nature of our TCRs and emphasize some of the challenges inherent in working with autoreactive TCRs. TCR 7 is of particular interest, because its CDR3β loop is unusually long (19 residues). It is tempting to speculate that a loop of this length may impair the formation of intimate contacts between the TCR and the pMHC and, in so doing, may account for the poor tetramer staining and low functional avidity observed for this autoreactive TCR.
Another characteristic that has been reported for autoreactive T cells is antigenic recognition promiscuity (cross-reactivity) 62–69. Our results show that this property, exemplified by TCR 32, can also be studied using lentiviral transduction of T cells. TCR 32 had the highest structural avidity of the three TCRs examined here (as measured by tetramer avidity); however, it was difficult to document a functional peptide-specific response from transduced cells due to its robust cross-reaction to human cell lines expressing HLA-A2 and used as APC. Apparently, in addition to recognizing IGRP265–273/HLA-A2, TCR 32 also responds to one or more endogenous peptides presented by HLA-A2 in these cell lines. When murine HLA-A2-positive DCs were used as APC, the stimulation in the absence of exogenous peptide was considerably reduced, but still present. Surprisingly, however, the peptide-specific response was quite low and required a high peptide concentration for detection. Taken together, these data suggest not only that TCR 32 is promiscuous, but also that one of its peptide ligands may be an antagonist 70 that can dampen its response to IGRP265–273. Alternatively, it is possible that the binding characteristics of TCR 32 to IGRP265–273/HLA-A2 are not suitable to elicit a strong functional response 71. These results illustrate that careful in-vitro analysis of candidate TCRs, as performed here, is necessary before undertaking in-vivo experiments, because tetramer binding does not guarantee a measurable functional response to peptide.
Interestingly, we observed that FSB, isolated from a healthy donor, was the most functionally active of the three TCRs. This finding suggests that useful islet-specific human TCRs need not be derived from diabetic patients, while also once again highlighting the need for rigorous in-vitro analysis of TCRs.
Multiple antigen-specific therapies have demonstrated potential for preventing diabetes development in NOD mice by inducing deletion of β cell-specific CD8 T cells 72–74. We have found that delivery of β cell antigens to DCs via the endocytic receptor DEC-205 can lead to the deletion of both transferred 75 and endogenous CD8 T cells 76 specific for the delivered antigen. Our ability to generate human CD8 T cells specific for β cell antigens and their survival in NSG-A2 mice will now allow such deletional strategies to be explored in a system incorporating human T cells. This line of investigation does not require that the recipient mice develop diabetes, as T cell deletion in response to treatment can be monitored even in the absence of overt disease by evaluating GFP expression. However, our goal is to optimize our system further so that diabetes is observed. The T cell clones from which the TCRs 7, FSB and 32 were obtained originally were able to lyse human β cells (data not shown), supporting their potential diabetogenic nature. It is possible that we did not observe diabetes upon transfer of transduced IGRP-specific CD8 T cells to NSG-A2 mice because this single specificity may be insufficient to cause disease. However, our experimental design was based in part on our finding that transfer to NOD-SCID recipients of cultured islet infiltrates from 8·3 TCR (specific for H-2Kd/IGRP206–214)-transgenic NOD mice induced diabetes in all recipients (data not shown). These cultured infiltrates contained 98% CD8 T cells and, of these, 80% were specific for IGRP206–214, suggesting at least the possibility that a T cell population highly enriched for CD8 T cells having a single specificity can indeed transfer disease to an immunodeficient host. However, our lentiviral transduction approach will allow human CD8 T cells having multiple defined antigenic specificities to be engineered and if necessary transferred in the future. Furthermore, we have found that human CD4 T cells can also be engineered by lentiviral transduction to be specific for β cell antigens (data not shown), suggesting the possibility of simultaneously transferring β cell-specific CD8 and CD4 T cells to NSG mice transgenic for human class I and class II MHC molecules. The contribution of CD4 T cells to islet pathology in such a model is suggested by the recent report that insulitis was observed when immortalized human CD4 T cells specific for HLA-DR4-binding β cell peptides were transferred to NSG mice transgenic for HLA-DR4 77.
In addition to the possible requirements for multiple antigenic specificities or both CD8 and CD4 T cells, the duration of our experiments may have been insufficient for diabetes development to take place. We followed the recipient mice for only 5 weeks after transfer of the engineered IGRP-specific T cells, as a xenogeneic GvH disease develops in NSG mice engrafted with human PBMC within 4–5 weeks after transfer 15. This GvH disease is due largely to a T cell response to murine class I MHC molecules, as NSG mice that are murine class I MHC-deficient are relatively resistant to this disease 50. Murine class I-deficient NSG-A2 mice will probably be an improved recipient for the engraftment of engineered β cell-specific human CD8 T cells, as they will allow the experimental duration to be extended. They should also allow increased numbers of transduced CD8 T cells to be transferred.
We demonstrate here for the first time, to our knowledge, the ability to generate human islet-specific cytotoxic T cells at will by TCR lentiviral transduction and their survival for up to 5 weeks in NSG-A2 mice. We believe that this strategy has the potential to allow for a better understanding of T1D pathogenesis and the evaluation of antigen-specific therapies. Our work highlights the difficulties in working with autoreactive TCRs, including their low affinity for pMHC and their propensity for promiscuity. However, our proof-of-concept study none the less suggests broad applicability of our approach to investigations concerning autoreactive T cells involved in other human T cell-mediated autoimmune diseases.
Acknowledgments
The authors thank Wendy Unger for T cell cloning and Arno van der Slik for TCR sequencing. This work was supported by National Institutes of Health grants R01 DK094327 (T.P.D.), R01 DK064315 (T.P.D.), P60 DK020541 (Albert Einstein College of Medicine's Diabetes Research Center), P30 AI051519 (Einstein-Montefiore Center for AIDS Research), P01 AI046629 (D.L.G., L.D.S), U01 DK089572 (D.L.G., L.D.S.), R01 DA033788 (H.G.) and R01 AI043203 (O.O.Y.), and by grants from the Canadian Institutes of Health Research (P.S.) and The Leona M and Harry B. Helmsley Charitable Trust (2012PG-T1D018; University of Massachusetts Medical School Diabetes Center of Excellence). The flow cytometry facility at Albert Einstein College of Medicine is supported by National Institutes of Health Cancer Center grant P30 CA013330. The Julia McFarlane Diabetes Research Centre is supported by the Canadian Diabetes Association. T. P. D. is the Diane Belfer, Cypres and Endelson Families Faculty Scholar in Diabetes Research. H. G. holds the Charles Michael Chair in Autoimmune Diseases. P. S. is a Scientist of the Alberta Innovates – Health Solutions and a scholar of the Instituto de Investigaciones Sanitarias Carlos III. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosure
There are no commercial or financial interests to disclose.
References
- Driver JP, Serreze DV, Chen YG. Mouse models for the study of autoimmune type 1 diabetes: a NOD to similarities and differences to human disease. Semin Immunopathol. 2011;33:67–87. doi: 10.1007/s00281-010-0204-1. [DOI] [PubMed] [Google Scholar]
- Chaparro RJ, DiLorenzo TP. An update on the use of NOD mice to study autoimmune (Type 1) diabetes. Expert Rev Clin Immunol. 2010;6:939–955. doi: 10.1586/eci.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Herold KC, Vignali DA, Cooke A, Bluestone JA. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat Rev Immunol. 2013;13:243–256. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum CJ, Schatz DA, Haller MJ, Sanda S. Through the fog: recent clinical trials to preserve beta-cell function in type 1 diabetes. Diabetes. 2012;61:1323–1330. doi: 10.2337/db11-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeva TP, Chatenoud L, Insel R, Atkinson MA. Recent lessons learned from prevention and recent-onset type 1 diabetes immunotherapy trials. Diabetes. 2013;62:9–17. doi: 10.2337/db12-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallone R, Martinuzzi E, Blancou P, et al. CD8+ T-cell responses identify β-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–621. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- Hughes MS, Yu YY, Dudley ME, et al. Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther. 2005;16:457–472. doi: 10.1089/hum.2005.16.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph A, Zheng JH, Follenzi A, et al. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol. 2008;82:3078–3089. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Yu YY, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Rohena A, Molloy PE, Dunn SM, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14:1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Morgan RA. Genetic engineering with T cell receptors. Adv Drug Deliv Rev. 2012;64:756–762. doi: 10.1016/j.addr.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2Rγnull mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- King M, Pearson T, Shultz LD, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Saito Y, Najima Y, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2rγnull humanized mice. Proc Natl Acad Sci USA. 2010;107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Gurer C, Ploss A, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206:1423–1434. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennessy M, Metcalfe K, Hitman GA, et al. A gene in the HLA class I region contributes to susceptibility to IDDM in the Finnish population. Childhood Diabetes in Finland (DiMe) Study Group. Diabetologia. 1994;37:937–944. doi: 10.1007/BF00400951. [DOI] [PubMed] [Google Scholar]
- Nejentsev S, Howson JM, Walker NM, et al. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature. 2007;450:887–892. doi: 10.1038/nature06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles DT, Eisenbarth GS, Wang T, et al. Identification of children with early onset and high incidence of anti-islet autoantibodies. Clin Immunol. 2002;102:217–224. doi: 10.1006/clim.2001.5171. [DOI] [PubMed] [Google Scholar]
- Whitfield-Larry F, Young EF, Talmage G, et al. HLA-A2-matched peripheral blood mononuclear cells from type 1 diabetic patients, but not nondiabetic donors, transfer insulitis to NOD-scid/γcnull/HLA-A2 transgenic mice concurrent with the expansion of islet-specific CD8+ T cells. Diabetes. 2011;60:1726–1733. doi: 10.2337/db10-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Serra P, Amrani A, et al. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11:645–652. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- Lieberman SM, Evans AM, Han B, et al. Identification of the β cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R, Wagar D, Stephens TA, Lee-Chan E, Singh B. Identification of CD4+ T cell-specific epitopes of islet-specific glucose-6-phosphatase catalytic subunit-related protein: a novel β cell autoantigen in type 1 diabetes. J Immunol. 2005;174:5306–5315. doi: 10.4049/jimmunol.174.9.5306. [DOI] [PubMed] [Google Scholar]
- Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP. Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol. 2008;127:359–365. doi: 10.1016/j.clim.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standifer NE, Ouyang Q, Panagiotopoulos C, et al. Identification of novel HLA-A*0201-restricted epitopes in recent-onset type 1 diabetic subjects and antibody-positive relatives. Diabetes. 2006;55:3061–3067. doi: 10.2337/db06-0066. [DOI] [PubMed] [Google Scholar]
- Unger WW, Pearson T, Abreu JR, et al. Islet-specific CTL cloned from a type 1 diabetes patient cause beta-cell destruction after engraftment into HLA-A2 transgenic NOD/scid/IL2RG null mice. PLOS ONE. 2012;7:e49213. doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Danke NA, Berger D, et al. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J Immunol. 2006;176:2781–2789. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- Takaki T, Marron MP, Mathews CE, et al. HLA-A*0201-restricted T cells from ‘humanized’ NOD mice recognize autoantigens of potential clinical relevance to type 1 diabetes. J Immunol. 2006;176:3257–3265. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- Storkus WJ, Alexander J, Payne JA, Dawson JR, Cresswell P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc Natl Acad Sci USA. 1989;86:2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter RD, Howell DN, Cresswell P. Genes regulating HLA class I antigen expression in T–B lymphoblast hybrids. Immunogenetics. 1985;21:235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Hogan KT, Clayberger C, Bernhard EJ, et al. Identification by site-directed mutagenesis of amino acid residues contributing to serologic and CTL-defined epitope differences between HLA-A2.1 and HLA-A2.3. J Immunol. 1988;141:2519–2525. [PubMed] [Google Scholar]
- Irwin MJ, Heath WR, Sherman LA. Species-restricted interactions between CD8 and the α3 domain of class I influence the magnitude of the xenogeneic response. J Exp Med. 1989;170:1091–1101. doi: 10.1084/jem.170.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero A, Hospers GA, Kruse KM, et al. Retargeting of a T cell line by anti MAGE-3/HLA-A2 αβ TCR gene transfer. Anticancer Res. 2000;20:1793–1799. [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold PY, Burton AR, Vignali DA. Diabetes incidence is unaltered in glutamate decarboxylase 65-specific TCR retrogenic nonobese diabetic mice: generation by retroviral-mediated stem cell gene transfer. J Immunol. 2004;173:3103–3111. doi: 10.4049/jimmunol.173.5.3103. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- Bennett MS, Joseph A, Ng HL, Goldstein H, Yang OO. Fine-tuning of T-cell receptor avidity to increase HIV epitope variant recognition by cytotoxic T lymphocytes. AIDS. 2010;24:2619–2628. doi: 10.1097/QAD.0b013e32833f7b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten KB, Kramer D, Kueter EW, et al. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clin Immunol. 2006;119:135–145. doi: 10.1016/j.clim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Arden B, Clark SP, Kabelitz D, Mak TW. Human T-cell receptor variable gene segment families. Immunogenetics. 1995;42:455–500. doi: 10.1007/BF00172176. [DOI] [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- Laugel B, van den Berg HA, Gostick E, et al. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem. 2007;282:23799–23810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- Lissina A, Ladell K, Skowera A, et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods. 2009;340:11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Kalams SA, Trocha A, et al. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batard P, Peterson DA, Devevre E, et al. Dextramers: new generation of fluorescent MHC class I/peptide multimers for visualization of antigen-specific CD8+ T cells. J Immunol Methods. 2006;310:136–148. doi: 10.1016/j.jim.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dolton G, Lissina A, Skowera A, et al. Comparison of peptide-major histocompatibility complex tetramers and dextramers for the identification of antigen-specific T cells. Clin Exp Immunol. 2014;177:47–63. doi: 10.1111/cei.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagar EJ, Cromwell MA, Shultz LD, et al. Regulation of human cell engraftment and development of EBV-related lymphoproliferative disorders in Hu-PBL-scid mice. J Immunol. 2000;165:518–527. doi: 10.4049/jimmunol.165.1.518. [DOI] [PubMed] [Google Scholar]
- King MA, Covassin L, Brehm MA, et al. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157:104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Bevan MJ. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 2006;25:261–270. doi: 10.1016/j.immuni.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont D, Mukherjee G, Kumar PR, et al. Compensatory mechanisms allow undersized anchor-deficient class I MHC ligands to mediate pathogenic autoreactive T cell responses. J Immunol. 2014;193:2135–2146. doi: 10.4049/jimmunol.1400997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levisetti MG, Suri A, Petzold SJ, Unanue ER. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol. 2007;178:6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Standifer NE, Qin H, et al. Recognition of HLA class I-restricted β-cell epitopes in type 1 diabetes. Diabetes. 2006;55:3068–3074. doi: 10.2337/db06-0065. [DOI] [PubMed] [Google Scholar]
- Scotto M, Afonso G, Osterbye T, et al. HLA-B7-restricted islet epitopes are differentially recognized in type 1 diabetic children and adults and form weak peptide-HLA complexes. Diabetes. 2012;61:2546–2555. doi: 10.2337/db12-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadinski BD, Delong T, Reisdorph N, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadinski BD, Zhang L, Crawford F, Marrack P, Eisenbarth GS, Kappler JW. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc Natl Acad Sci USA. 2010;107:10978–10983. doi: 10.1073/pnas.1006545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger WW, Velthuis J, Abreu JR, et al. Discovery of low-affinity preproinsulin epitopes and detection of autoreactive CD8 T-cells using combinatorial MHC multimers. J Autoimmun. 2011;37:151–159. doi: 10.1016/j.jaut.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Wong FS, Moustakas AK, Wen L, Papadopoulos GK, Janeway CA., Jr Analysis of structure and function relationships of an autoantigenic peptide of insulin bound to H-2Kd that stimulates CD8 T cells in insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA. 2002;99:5551–5556. doi: 10.1073/pnas.072037299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan JF, Levisetti MG, Calderon B, Herzog JW, Petzold SJ, Unanue ER. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong T, Baker RL, He J, Barbour G, Bradley B, Haskins K. Diabetogenic T-cell clones recognize an altered peptide of chromogranin A. Diabetes. 2012;61:3239–3246. doi: 10.2337/db12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese MA, Jakobsen KB, Friis L, et al. Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis. Nat Med. 2008;14:1227–1235. doi: 10.1038/nm.1881. [DOI] [PubMed] [Google Scholar]
- Li L, Wang B, Frelinger JA, Tisch R. T-cell promiscuity in autoimmune diabetes. Diabetes. 2008;57:2099–2106. doi: 10.2337/db08-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Verdaguer J, Averill N, Santamaria P. A mechanism for the major histocompatibility complex-linked resistance to autoimmunity. J Exp Med. 1997;186:1059–1075. doi: 10.1084/jem.186.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki T, Lieberman SM, Holl TM, et al. Requirement for both H-2Db and H-2Kd for the induction of diabetes by the promiscuous CD8+ T cell clonotype AI4. J Immunol. 2004;173:2530–2541. doi: 10.4049/jimmunol.173.4.2530. [DOI] [PubMed] [Google Scholar]
- Thiessen S, Serra P, Amrani A, Verdaguer J, Santamaria P. T-cell tolerance by dendritic cells and macrophages as a mechanism for the major histocompatibility complex-linked resistance to autoimmune diabetes. Diabetes. 2002;51:325–338. doi: 10.2337/diabetes.51.2.325. [DOI] [PubMed] [Google Scholar]
- Tsai S, Serra P, Clemente-Casares X, et al. Antidiabetogenic MHC class II promotes the differentiation of MHC-promiscuous autoreactive T cells into FOXP3+ regulatory T cells. Proc Natl Acad Sci USA. 2013;110:3471–3476. doi: 10.1073/pnas.1211391110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge L, Ekeruche-Makinde J, van den Berg HA, et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J Biol Chem. 2012;287:1168–1177. doi: 10.1074/jbc.M111.289488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I-restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology. 2009;126:165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niens M, Grier AE, Marron M, Kay TW, Greiner DL, Serreze DV. Prevention of ‘humanized’ diabetogenic CD8 T-cell responses in HLA-transgenic NOD mice by a multipeptide coupled-cell approach. Diabetes. 2011;60:1229–1236. doi: 10.2337/db10-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GS, Fishman S, Khai Siew L, et al. Immunotargeting of insulin reactive CD8 T cells to prevent diabetes. J Autoimmun. 2010;35:390–397. doi: 10.1016/j.jaut.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Vincent BG, Young EF, Buntzman AS, et al. Toxin-coupled MHC class I tetramers can specifically ablate autoreactive CD8+ T cells and delay diabetes in nonobese diabetic mice. J Immunol. 2010;184:4196–4204. doi: 10.4049/jimmunol.0903931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhaya A, Hanafusa T, Jarchum I, et al. Selective delivery of β cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci USA. 2008;105:6374–6379. doi: 10.1073/pnas.0802644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee G, Geliebter A, Babad J, et al. DEC-205-mediated antigen targeting to steady-state dendritic cells induces deletion of diabetogenic CD8+ T cells independently of PD-1 and PD-L1. Int Immunol. 2013;25:651–660. doi: 10.1093/intimm/dxt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viehmann Milam AA, Maher SE, Gibson JA, et al. A humanized mouse model of autoimmune insulitis. Diabetes. 2014;63:1712–1724. doi: 10.2337/db13-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]