Abstract
Infection and inflammation can be antecedents of neonatal encephalopathy (NE) and increase the risk of neurological sequelae. Activated protein C (APC) has anti-coagulant and anti-inflammatory effects and provides neuroprotection in brain and spinal cord injury. We examined neutrophil and monocyte responses to lipopolysaccharide (LPS) in infants with NE compared with healthy adult and neonatal controls, and also studied the effect of APC. Whole blood was incubated with LPS and APC and Toll-like receptor (TLR)-4 (LPS recognition), CD11b expression (activation) and intracellular reactive oxygen intermediate (ROI; function) release from neutrophils and monocytes was examined by flow cytometry serially from days 1 to 7. We found a significant increase in neutrophil ROI in infants with NE on day 3 following LPS compared to neonatal controls and this augmented response was reduced significantly by APC. Neutrophil and monocyte CD11b expression was increased significantly on day 1 in infants with NE compared to neonatal controls. LPS-induced neutrophil TLR-4 expression was increased significantly in infants with NE on days 3 and 7 and was reduced by APC. LPS-induced monocyte TLR-4 was increased significantly in infants with NE on day 7. Neutrophil and monocyte activation and production of ROIs may mediate tissue damage in infants with NE. APC modified LPS responses in infants with NE. APC may reduce the inflammatory responses in NE and may ameliorate multi-organ dysfunction. Further study of the immunomodulatory effects of protein C may be warranted using mutant forms with decreased bleeding potential.
Keywords: CD11b, neonatal encephalopathy, protein C, reactive oxygen intermediates, Toll-like receptor-4
Introduction
Neonatal encephalopathy (NE) is one of the leading causes of morbidity and mortality in neonates, with a prevalence of 1·8 to 7·7/1000 live births 1. Epidemiological and experimental evidence suggests that pre-existing intrauterine infection and inflammation are implicated in the evolution of brain white matter injury and subsequent cerebral palsy 2–5. Inflammatory mediators play a major role in the pathogenesis of experimental neonatal hypoxic–ischaemic brain injury 6. In neonatal rats, systemic injection of lipopolysaccharide (LPS) induces cerebral infarction and augments tissue damage after short periods of hypoxia–ischaemia that by themselves caused no or little injury 7. Adult stroke patients with systemic inflammation exhibit clinically poorer outcomes 8,9, and inhibiting the inflammatory response decreases infarct size and improves neurological deficit in experimental stroke 10,11.
Systemic inflammation and leucocyte mobilization is associated with NE and adult stroke. High leucocyte and neutrophil counts in first 96 h of life in term neonates with hypoxia–ischaemia are associated with abnormal neurodevelopmental outcome 12 and neutropenia is neuroprotective in a neonatal rat model with induced hypoxia–ischaemia 13. CD11b is a marker of neutrophil transmigration and activation. In hypoxic–ischaemic injury, neutrophil CD11b/CD18 expression is increased significantly in adults compared to neonates 14,15. Although anti-inflammatory approaches (blocking CD11b) have improved outcomes in animal models, attempts to translate this into clinical application have been unsuccessful 16,17. Toll-like receptor (TLR)-4 is the receptor for lipopolysaccharide (LPS) or endotoxin on innate immune cells and is expressed on both monocytes and neutrophils. The size of cerebral infarction and the inflammatory response following experimental stroke is decreased in TLR-4-deficient mice 18.
Neutrophils damage healthy tissues in many inflammatory diseases, such as acute respiratory distress syndrome, inflammatory bowel disease and rheumatoid arthritis. Intracellular generation of reactive oxygen intermediates (ROIs) by neutrophils and monocytes is a vital defence against bacterial infection. The respiratory burst activity of phagocytes is the essential metabolic event to the killing of ingested microbes by ROIs generated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 19. ROIs can lead to tissue damage by oxidizing membrane phospholipids, proteins, nucleic acids and nucleotides, leading to neuronal injury secondary to cerebral inflammation 20 and ischaemia–reperfusion injury 21.
Activation of the immune system in NE and treatment with hypothermia is beneficial, but additional adjuvant therapies are required and systemic inflammation may be a suitable target. Activated protein C (APC) has potent anti-coagulant activity by inactivating Factor Va and FVIIIa 22. The clinical use of APC remains controversial, as it did not fulfil the initial promise as a panacea for sepsis in adults and children 23. However, subsequent studies did not show similar efficacy. In rat pups after hypoxic ischaemic injury, APC reduced significantly apoptotic cell death and nitric oxide (NO) production in brain, indicating a neuroprotective effect in hypoxic–ischaemic injury in the developing brain 24. APC reduces cell death in white matter and hypomyelination in neonatal rats treated with LPS and APC compared to controls 25. We were therefore interested in examining the effect of APC on systemic immune activation in NE, as newer mutant versions of APC are available with decreased bleeding and increased anti-inflammatory properties.
We hypothesized that APC diminished neutrophil and monocyte activation and survival, and therefore may reduce tissue damage in neonates at risk of brain injury. In this study we aimed to examine the neutrophil and monocyte CD11b and TLR-4 expression and ROI production in response to LPS in infants with risk of brain injury and investigate the effect of APC as a possible immunomodulator.
Materials and methods
Reagents
LPS Escherichia coli serotype 0111:B4, phorbol 12-myristate 13-acetate (PMA), dihydrorhodamine 123 (DHR), fetal bovine serum (heat-inactivated) and phosphate-buffered saline (PBS) were purchased from Sigma Aldrich (St Louis, MO, USA) (http://www.sigmaaldrich.com). Dulbecco's modified Eagle's medium (DMEM) was purchased from Bioscience (Dublin, Ireland) (http://www.biosciences.ie). Penicillin streptomycin solution and l-glutamine serum [fetal calf serum (FCS)] were purchased from Gibco Life Technologies (Carlsbad, CA, USA) (http://www.invitrogen.com). Drotrecogin alpha (Xigris: APC) was purchased from Eli Lilly (Dublin, Ireland) (http://www.lilly.ie). CD11b was purchased from eBioscience (San Jose, CA, USA) (http://www.ebioscience.com). Fluorescence activated cell sorter (FACS) lysing solution and TLR-4 were purchased from BD Bioscience (San Jose, CA, USA) (http://www.bdbiosciences.com) and all remaining chemicals were purchased from Sigma Aldrich (Dorset UK) unless stated otherwise.
Patient population
Ethical committee approval was received from the National Maternity Hospital, a tertiary referral university maternity hospital with ∼10 000 deliveries per annum for the study period from July 2011 to June 2012. Fully informed written consent was obtained from parents of all participants.
The following study groups were enrolled: adult controls, healthy male and female volunteers; neonatal controls, umbilical cord blood from term infants with uncomplicated normal vaginal delivery and postnatal course; and neonatal encephalopathy, term neonates at risk of brain injury with at least two of the inclusion criteria of Huang et al. 26 – (i) evidence or suspicion of hypoxic–ischaemic injury based on a history of fetal distress, i.e. type II dips, loss of beat-to-beat variability on cardiotocograph and/or abnormal scalp pH; (ii) the need for resuscitation after birth, i.e. bag and mask ventilation; and (iii) base deficit >15 mmol/l or pH < 7·2 in cord blood or admission arterial sample 26. The exclusion criteria were major congenital anomalies or maternal substance abuse. Adult controls were used as internal controls to ensure the validity of the experiments. They were used as regular positive (LPS-induced responses) and negative controls (spontaneous CD11b and TLR-4 expression) and acted as internal controls to ensure that the laboratory model was functioning consistently.
Infants were subdivided into mild or no neonatal encephalopathy (NE) grades 0/I and moderate/severe encephalopathy grades II/III for analysis 27. All neonates at risk of brain injury had serial cranial ultrasounds on days 1, 3 and 7 of life and a magnetic resonance imaging (MRI) brain scan on days 5−7 of life. A single paediatric radiologist (V.D.) who was blinded to the laboratory and clinical findings reported and scored the MRI results using the Barkovich criteria 28. This employs a combination five-point score including components of both basal ganglia and watershed patterns of injury. Patients were divided into ‘normal’ (score = 0) and ‘abnormal’ (score = 1–4) neuroimaging groups for the purpose of data analysis (Table 1).
Table 1.
Demographics data NE
| Normal MRI | Abnormal MRI | P value | |
|---|---|---|---|
| n = 17 | n = 6 | ||
| Gestational age (weeks) | 40·4 ± 1·4 | 40·0 ± 1·1 | 0·616 |
| Birth weight (kg) | 3·7 ± 0·9 | 3·2 ± 0·4 | 0·24 |
| Cord pH | 7·1 ± 0·2 | 7·1 ± 0·1 | 0·67 |
| Lactate | 8·4 ± 4·3 | 11·6 ± 5·5 | 0·23 |
| Apgar score @ 1 min | 2·7 ± 2·7 | 1·5 ± 0·8 | 0·32 |
| Apgar score @ 5 min | 3·8 ± 2·6 | 4·0 ± 2·5 | 0·88 |
| Apgar score @ 10 min | 5·1 ± 1·9 | 5·0 ± 1·8 | 0·88 |
| Blood glucose @ admission | 6·4 ± 3·2 | 5·7 ± 4·9 | 0·72 |
MRI = magnetic resonance imaging.
Blood sampling
Serial blood samples were taken from infants at risk of brain injury on days 1, 3 and 7 of life during routine phlebotomy. The blood samples were collected in sodium citrate anti-coagulant blood bottles. Blood samples were kept on ice and processed within 90 min of collection. Whole blood was incubated for 1 h at 37°C with the proinflammatory agent LPS 1 μg/ml to mimic an inflammatory response in vitro. In addition, APC 200 ng/ml was added ± LPS following a dose–response study 29.
Protein C activity
The protein C level in patient plasma is measured in two stages: incubation of the plasma with protein C activator and quantification of activated protein C with a synthetic chromogenic substrate. The paranitroaniline released is monitored kinetically at 405 nm and is directly proportional to the protein C level in the test sample. The HemosIL Protein C Kit used contains lyophilized substrate, protein C activator and a saline diluent. Blood was collected (9 vol.) in 0·109 M (i.e. 3·2%) trisodium citrate anti-coagulant bottles (1 vol.) and samples were centrifuged at 2500 g for 10 min. The testing was completed within 4 h of sample collection. The sample was inverted a number of times or vortexed to ensure adequate mixing. Any samples that were clotted or appeared haemolysed were rejected and internal quality control included two different levels of controls at the start of each working day and subsequently every 4 h throughout the day. The controls used were HemosIL normal control and HemosIL low abnormal.
Intracellular respiratory burst activity
Generation of ROIs was evaluated by flow cytometry using the technique of Smith and Wiedemann. Whole blood (50 μl) was incubated with DHR 123 (50 μl) and PBS (450 μl) at 37°C for 10 min. Cells were then stimulated with 1 μl (16 μM) of PMA for 20 min at 37°C. The reaction was then halted by placing samples on ice. Neutrophil and monocyte fluorescence intensity was assessed by flow cytometry and expressed as Ln mean channel fluorescence (LnMCF). DHR 123 has been shown to detect mainly intracellular H2O2 and OH– radical production 30.
Quantification of cell surface antigen expression
The expression of CD11b and TLR-4 antigens on the surface of neutrophils and monocytes was measured by flow cytometry. Whole blood (50 μl) was treated with 5 μl of phycoerythrin (PE)-CD11b and 2·5 μl anti-human TLR-4 antibody and incubated at 4°C for 20 min. FACS added to the whole blood and incubated for 10 min at room temperature. The whole blood was centrifuged at 130g for 5 min at 4°C. The pellet was suspended twice with DMEM 500 μl and stored on ice before flow cytometry analysis. The fluorescence intensity was assessed by flow cytometry and expressed as Ln mean channel fluorescence. Fluorescence intensity is denoted by mean channel fluorescence, which is the average intensity of fluorescence emitted by all cells chosen for measurement, and is comparable to the relative number of receptors present on the surface of each cell. The flow cytometer used was a BD Accuri C6. A minimum of 5000 events was collected and analysed. All measurements were performed under the same instrument settings 31.
Statistical methods
A matched paired t-test was used to compare conditions (control/LPS/LPS + APC) within each group (adults, cord and NE infants). For comparison across groups, the one-way analysis of variance (anova) with Tukey's post-hoc comparison method was used. Statistical analysis was carried out with anova using the PASW statistical package version 18 (http://www.ibm.com/SPSS_Statistics). Significance was assumed for values of P < 0·05. Results are expressed as mean ± standard error of the mean (s.e.m.) unless indicated otherwise.
Results
Demographic data
A total of 63 participants (189 samples) were enrolled into this study: 15 adult controls, eight neonatal controls and 23 infants with NE. The adult controls consisted of nine males and five females with a median (range) age of 26 22–38 years. The neonatal controls were 39·7 ± 1·0 weeks’ gestation, birth weight 3·5 ± 0·4 kg and Apgar score at 5 min of 9 ± 1. The NE group included 17 males and six females (Table 1); 17 infants were eligible for therapeutic hypothermia and treated in accordance with the Total Body Hypothermia for Neonatal Encephalopathy (TOBY) criteria for 72 h duration. Six infants had NE Sarnat grades 0/I and 17 infants were NE grades II/III 27, and no patient died prior to discharge. Three infants in the normal neuroimaging group and five in the abnormal neurimaging group had clinical seizures.
Protein C levels
Adult controls had protein C levels that were within or above the normal range with mean ± standard deviation (s.d.) (1·5 ± 0·5 IU/ml). Protein C levels were available in 17 of 23 infants with NE, as the other samples were unsuitable for analysis. Protein C level was abnormal in five of 17 NE patients. Neonatal controls (0·62 ± 0·19 IU/ml) had significantly elevated levels compared with infants with NE (0·37 ± 0·15 IU/ml) (P < 0·01).
Intracellular reactive oxygen intermediates and NE
Neutrophil ROI baseline was significantly higher in infants with NE on day 7 versus neonatal controls (P = 0·014) (Fig. 1a). LPS-induced neutrophil ROI increased significantly in NE infants on days 3 (P = 0·021) and 7 (P = 0·02) compared to their respective baseline levels. APC reduced the LPS-induced neutrophil ROI significantly in infants with NE on day 3 (P = 0·02) and adult controls (P = 0·014) (Fig. 1a). Monocyte ROI baseline was significantly higher in infants with NE than neonatal controls (P < 0·001). LPS-induced monocyte ROI increased significantly in infants with NE on days 3 and 7 (P = 0·001) and APC did not alter these responses (Fig. 1b).
Fig 1.
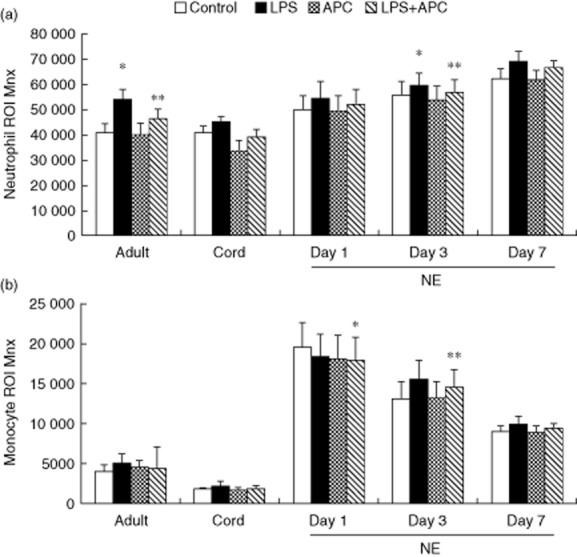
Neutrophil and monocyte reactive oxygen intermediate (ROI) in infants with neonatal encephalopathy (NE) versus controls: whole blood from healthy adult controls (n = 15), neonatal controls (n = 8) and NE patients (n = 23) was incubated with lipopolysaccharide (LPS) ± activated protein C (APC) for 1 h and then stimulated with dihydrorhodamine 12 (DHR) and phorbol 12-myristate 13-acetate (PMA). Results were expressed as the Ln mean channel fluorescence ± standard error of the mean (s.e.m.). (a) Neutrophil reactive oxygen intermediate (ROI): *P < 0·05 versus controls; **P < 0·05 versus LPS response. (b) Monocyte ROI: *P < 0·05 versus adult and neonatal controls; **P < 0·05 versus NE baseline.
LPS-induced neutrophil ROI in NE 0/I on day 1 was higher than severe NE II/III (P = 0·04). However, on day 3, LPS induced similar neutrophil ROI production in NE 0/I and NE II/III. LPS-induced neutrophil ROI production on day 7 was increased significantly in severe NE II/III compared to baseline (P = 0·001).There were similar monocyte ROI baseline and LPS responses in both groups on day 1. However, LPS-induced monocyte ROI was significantly higher in NE II/III than NE 0/I on days 3 (P < 0·001) and 7 (P = 0·037) (Supporting Information Fig. S1–3). APC did not alter monocyte LPS responses.
There was no significant difference in neutrophil and monocyte ROI baseline on days 1, 3 and 7 between infants with normal and abnormal MRIs. There was significantly increased LPS-induced neutrophil ROI on days 3 (P = 0·032) and 7 (P = 0·040) in NE infants with an abnormal MRI, whereas in NE infants with a normal MRI, LPS-induced neutrophil ROI increased on day 7 (P = 0·017).
Neonatal encephalopathy and CD11b expression
Neutrophil CD11b baseline was significantly higher in infants with NE than neonatal controls (P = 0·048 and 0·017, respectively). There was significantly increased LPS-induced neutrophil CD11b expression in infants with NE and control groups compared to baseline levels (P = 0·001). LPS-induced neutrophil CD11b expression was significantly higher in adult controls than infants with NE on day 1 (P = 0·033) and infants with NE on days 3 and 7 and neonatal controls (P < 0·001) (Fig. 2a).
Fig 2.
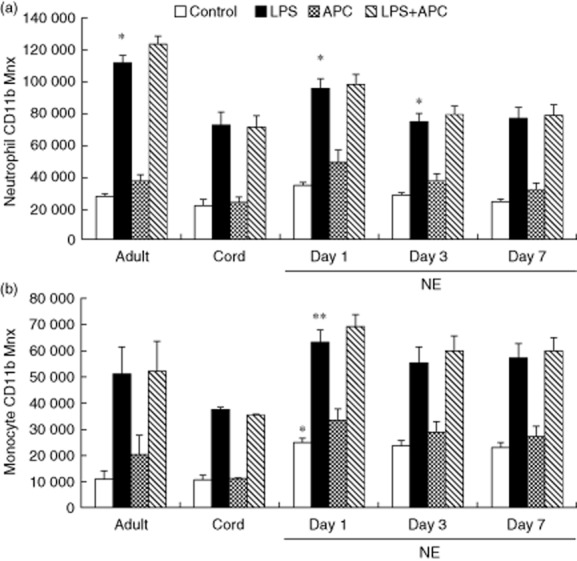
Neutrophil and monocyte CD11b in response to lipopolysaccharide (LPS) and activated protein C (APC) modulation in neonatal encephalopathy (NE): whole blood from healthy adult controls (n = 15), cord controls (n = 8) and NE patients (n = 23) incubated with LPS ± APC for 1 h. Neutrophils were assessed for CD11b using a phycoerythrin (PE)-labelled monoclonal antibody (mAb) and mean channel fluorescence analysed using flow cytometry. The neutrophil and monocyte populations were selected based on their scatter profile (forward- and sideways-scatter): (a) neutrophil CD11b, *P < 0·05 versus control; (b) monocyte CD11b, *P < 0·05 versus adult and cord controls; **P < 0·05 versus controls.
Monocyte CD11b baseline expression was significantly higher in infants with NE than neonatal controls (P < 0·001). There was significantly increased LPS-induced monocyte CD11b expression in infants with NE and controls compared to baseline (P < 0·001). Monocyte CD11b expression in response to LPS was significantly higher in infants with NE than neonatal controls (P < 0·001). APC had no effect on neutrophil or monocyte CD11b expression in response to LPS (Fig. 2b). There was significantly increased LPS-induced neutrophil and monocyte CD11b expression on days 1, 3 and 7 (P < 0·05) compared to baseline in infants with NE 0/I and NE II/III. APC had no effect in reducing this response. There were no significant differences in neutrophil and monocyte CD11b baseline and LPS responses between NE 0/I and NE II/III.
LPS-induced neutrophil and monocyte CD11b on days 1, 3 and 7 (P < 0·001) was increased in infants with NE with both normal and abnormal MRI. LPS-induced neutrophils CD11b was higher in infants with NE with normal MRI on day 1 (P = 0·023) than those with an abnormal MRI. This difference was not seen in neutrophils and monocytes on days 3 and 7. APC had no effect in reducing this response.
TLR-4 and NE
There was no significant difference in neutrophil TLR-4 baseline expression between NE infants and control groups. LPS-induced neutrophil TLR-4 expression was increased significantly in infants with NE on day 3 compared to baseline and APC decreased this LPS response significantly (Fig. 3a). There was no significant difference in monocyte TLR-4 baseline or LPS responses between infants with NE and control groups (Fig. 3b).
Fig 3.
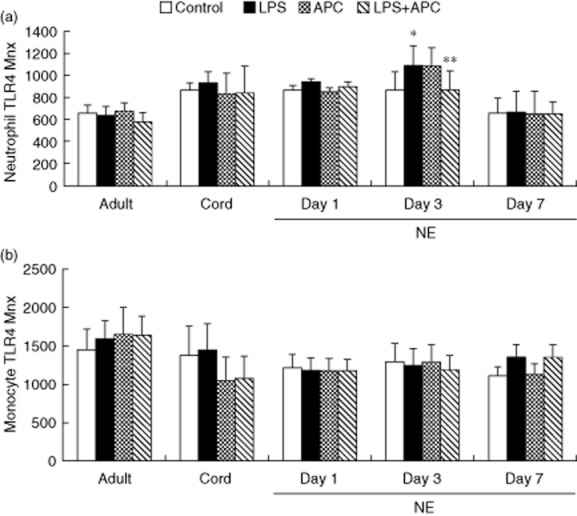
Neutrophil and monocyte Toll-like receptor (TLR)-4 in response to lipopolysaccharide (LPS) and activated protein C (APC) modulation in neonatal encephalopathy (NE): whole blood from healthy adult controls (n = 15), cord controls (n = 8) and NE patients (n = 23) was incubated with LPS ± APC for 1 h. Cells were then labelled with Alexa Fluor 647 TLR-4 monoclonal antibody (mAb) and mean channel fluorescence analysed using flow cytometry. The neutrophil and monoclonal populations were selected via flow cytometry based on their scatter profile. (a) Neutrophil TLR-4: *P < 0·05 versus control; **P < 0·05 versus LPS response. (b) Monocyte TLR-4: no significant differences.
However, LPS-induced neutrophil TLR-4 was increased significantly on day 3 (P = 0·024) and in monocytes on day 7 (P = 0·011) in NE II/III compared to baseline. APC did not alter these responses. There was no significant difference in LPS-induced neutrophil and monocyte TLR-4 between NE 0/1 and NE II/III. No significant difference was found in neutrophil and monocyte TLR-4 baseline between normal and abnormal MRI in infants with NE. LPS-induced neutrophil TLR-4 on day 3 (P = 0·028) and monocytes TLR-4 on day 7 (P = 0·039) were increased significantly in infants with NE with abnormal MRI compared to their baseline. APC had no effect in reducing LPS responses.
Discussion
We have demonstrated that neutrophil ROI in response to LPS was increased significantly in NE infants compared to controls, and this response was exaggerated in infants with severe NE. In many diseases, increased systemic ROIs are associated with multi-organ injury 2. APC reduces this response significantly, which may have clinical relevance as a potential immunomodulator.
Neutrophil and monocyte CD11b expression was increased significantly by LPS in infants with NE and controls. Infants with NE and abnormal MRI had decreased CD11b responses on day 1 compared to those with a normal MRI. This is in keeping with previous research showing decreased neutrophil CD11b in infants with NE and abnormal neurological outcome in cord blood 33. Winerdal et al. demonstrated that local and systemic CD11b is highly expressed on neuronal cells even after a week of ex-vivo-induced cerebral hypoxic–ischaemic injury 34. The damaging potential of systemic neutrophil activation begins as early as 15 min, and remains evident at 24 h after the initiation of reperfusion in a murine stroke model 35.
We have also demonstrated that neutrophil TLR-4 expression in response to LPS was up-regulated significantly in infants with NE compared to unstimulated cells and was reduced significantly by APC. TLR-4 is an essential receptor for LPS binding and LPS does not induce white matter injury in TLR-4-deficient mice 36. The volume of brain injury and neurological deficit secondary to stroke are reduced significantly in TLR-4-deficient mice compared to wild-type mice 37. TLR-4 antagonists reduce inflammatory injury and neurological deficits in a mouse model of intracerebral haemorrhage 38. Umbilical cord blood was used as a neonatal control and can have variable responses to LPS. We used cord blood following labour, which was similar to the infants with NE and is associated with enhanced inflammatory responses, including LPS responses compared with cord blood following elective caesarean section 39. Although APC (Xigris) has been withdrawn following the negative results of the PROWESS-SHOCK (Recombinant Human Protein C Worldwide Evaluation in Severe Sepsis-Shock) trial, it remains controversial, with a recent cohort study showing an overall reduction in hospital mortality in a cohort of 45 000 sepsis patients 40. The Cochrane review of APC in adults and children neonates suggested that APC is not indicated outside clinical trials 41 for sepsis. The anti-coagulant activity of APC involves inactivation of factors Va and VIIIa, whereas the cytoprotective activities involve the endothelial protein C and protease-activated receptor 1. The bleeding side effects associated with APC may be overcome by genetic engineering using the mutated versions of APC that retain the beneficial anti-inflammatory effect of APC, but decrease bleeding risk due to the reduction in APC-dependent profibrinolytic and anti-coagulant activities 42,43. In a murine model, intravenous APC infusion during focal ischaemia induced by ligation of middle cerebral artery decreased significantly brain infarction volume and brain oedema. This protective effect was decreased significantly in endothelial protein C receptor-deficient mice 43,44.
Elevated LPS-induced neutrophil ROI production on day 7 in infants with NE may be evidence of an altered inflammatory response. APC diminished this response and may suggest a potential for immunomodulation as adjunctive therapy in these infants. However, newer forms of APC with increased inflammatory responses and lower bleeding risk require further study. Exploring systemic immunomodulation in neonatal encephalopathy holds promise as a target for future adjunctive therapies.
Disclosure
There are no competing interests.
Acknowledgments
This study was funded by the National Children's Research Centre (NCRC), Crumlin, Dublin 12 and Royal College of Surgeons in Ireland.
Supporting Information
Additional Supportingporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Neutrophil (a) and monocyte (b) reactive oxygen intermediates in infants with neonatal encephalopathy on day 3 divided by severity: NE grade 0/I versus II/III. *P < 0·05 LPS-induced monocyte ROI comparing NE grade 0/I versus II/III.
Fig. S2. Neutrophil (a) and monocyte (b) reactive oxygen intermediates in infants with neonatal encephalopathy (NE) divided by severity on day 7 comparing: NE grade 0/I versus II/III. *P < 0·05 LPS-induced ROI in NE II/III versus controls.
Fig. S3. Neutrophil (a) reactive oxygen intermediates (ROI) on day 3 and (b) monocyte ROI day 7 comparing normal versus normal neuroimaging. Neutrophil ROI on day 3 and day 7 infants with abnormal neuroimaging had significantly increased LPS-induced neutrophil ROI production (*P < 0·05).
References
- Badawi N, Keogh JM, Dixon G, Kurinczuk JJ. Developmental outcomes of newborn encephalopathy in the term infant. Indian J Pediatr. 2001;68:527–530. doi: 10.1007/BF02723247. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1β, and tumor necrosis factor-α), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- Hermansen MC, Hermansen MG. Perinatal infections and cerebral palsy. Clin Perinatol. 2006;33:315–333. doi: 10.1016/j.clp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Barks JDE, Liu YQ, Shangguan Y, Li J, Pfau J, Silverstein FS. Impact of indolent inflammationon neonatal hypoxic–ischemic brain injury in mice. Int J Dev Neurosci. 2008;26:57–65. doi: 10.1016/j.ijdevneu.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklind S, Mallard C, Leverin AL, Gilland E, Blomgren K, Mattsby-Baltzer I. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind MS, Cheng J, Rundek T, Boden-Albala B, Sacco RL. Leukocyte count predicts outcome after ischemic stroke: the Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis. 2004;13:220–227. doi: 10.1016/j.jstrokecerebrovasdis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wang X. Investigational anti-inflammatory agents for the treatment of ischemic brain injury. Expert Opin Investig Drugs. 2005;14:393–409. doi: 10.1517/13543784.14.4.393. [DOI] [PubMed] [Google Scholar]
- Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkos AA, Hopper AO, Deming DD, et al. Elevated total peripheral leukocyte count may identify risk for neurological disability in asphyxiated term neonates. J Perinatol. 2007;27:365–370. doi: 10.1038/sj.jp.7211750. [DOI] [PubMed] [Google Scholar]
- Hudome S, Palmer C, Roberts RL, Mauger D, Housman C, Towfighi J. The role of neutrophils in the production of hypoxic–ischemic brain injury in the neonatal rat. Pediatr Res. 1997;41:607–616. doi: 10.1203/00006450-199705000-00002. [DOI] [PubMed] [Google Scholar]
- Liu F, McCullough LD. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol Sin. 2013;34:1121–1130. doi: 10.1038/aps.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy EJ, O'Neill AJ, Doyle BT, et al. Effects of heat shock and hypoxia on neonatal neutrophil lipopolysaccharide responses: altered apoptosis, Toll-like receptor-4 and CD11b expression compared with adults. Biol Neonate. 2006;90:34–39. doi: 10.1159/000091743. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang RL, Lu M, Krams M, Chopp M. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke. 2003;34:1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- Becker KJ. Anti-leukocyte antibodies: LeukArrest (Hu23F2G) and Enlimomab (R6.5) in acute stroke. Curr Med Res Opin. 2002;18:18–22. doi: 10.1185/030079902125000688. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Izasoation I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- Kontos CD, Wei EP, Williams JI, Kontos HA, Povlishock JT. Cytochemical detection of superoxide in cerebral inflammation and ischemia in vivo. Am J Physiol. 1992;263:H1234–1242. doi: 10.1152/ajpheart.1992.263.4.H1234. [DOI] [PubMed] [Google Scholar]
- Defraigne JO, Detry O, Pincemail J, et al. Direct evidence of free radical production after ischaemia and reperfusion and protective effect of desferrioxamine: ESR and vitamin E studies. Eur J Vasc Surg. 1994;8:537–543. doi: 10.1016/s0950-821x(05)80587-0. [DOI] [PubMed] [Google Scholar]
- Esmon CT. The protein C anticoagulant pathway. Arterioscler Thromb. 1992;12:135–145. doi: 10.1161/01.atv.12.2.135. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JLV, Laterre PF, et al. PROWESS study group. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Yesilirmak DC, Kumral A, Tugyan K, Cilaker S, Baskin H, Yilmaz O. Effects of activated protein C on neonatal hypoxic ischemic brain injury. Brain Res. 2008;1210:56–62. doi: 10.1016/j.brainres.2008.02.088. [DOI] [PubMed] [Google Scholar]
- Yesilirmak DC, Kumral A, Baskin H, et al. Activated protein C reduces endotoxin-induced white matter injury in the developing rat brain. Brain Res. 2007;20:14–23. doi: 10.1016/j.brainres.2007.04.083. [DOI] [PubMed] [Google Scholar]
- Huang CC, Wang ST, Chang YC, Lin KP, Wu PL. Measurement of the urinary lactate: creatinine ratio for the early identification of newborn infants at risk for hypoxic-ischemic encephalopathy. N Engl J Med. 1999;341:328–335. doi: 10.1056/NEJM199907293410504. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, Hajnal BL, Vigneron D, et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. Am J Neuroradiol. 1998;19:143–149. [PMC free article] [PubMed] [Google Scholar]
- Galley HF, El Sakka NE, Webster NR, et al. Activated protein C inhibits chemotaxis and interleukin-6 release by human neutrophils without affecting other neutrophil functions. Br J Anaesth. 2008;100:815–819. doi: 10.1093/bja/aen079. [DOI] [PubMed] [Google Scholar]
- Smith JA, Wiedemann MJ. Further characterization of the neutrophil oxidative burst by flow cytometry. J Immunol Methods. 1993;3:261–268. doi: 10.1016/0022-1759(93)90391-j. [DOI] [PubMed] [Google Scholar]
- Molloy EJ, O'Neill AJ, Grantham JJ, et al. Labor induces a maternal inflammatory response syndrome. Am J Obstet Gynecol. 2004;190:448–455. doi: 10.1016/j.ajog.2003.08.027. [DOI] [PubMed] [Google Scholar]
- Molloy EJ, O'Neill AJ, Grantham-Sloan JJ, Webb DW, Watson RW. Maternal and neonatal lipopolysaccharide and Fas responses are altered by antenatal risk factors for sepsis. Clin Exp Immunol. 2008;151:244–250. doi: 10.1111/j.1365-2249.2007.03540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy EJ, O'Neill AJ, Grantham-Sloan JJ, et al. Neonatal encephalopathy is associated with altered perinatal systemic neutrophil apoptosis. Am J Perinatol. 2007;24:525–530. doi: 10.1055/s-2007-986678. [DOI] [PubMed] [Google Scholar]
- Winerdal M, Winerdal ME, Kinn J, Urmaliya V, Winqvist O, Ådén U. Long lasting local and systemic inflammation after cerebral hypoxic ischemia in newborn mice. PLOS ONE. 2012;7:e36422. doi: 10.1371/journal.pone.0036422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, McKee D, Ritter L. Systemic neutrophil activation in a mouse model of ischemic stroke and reperfusion. Biol Res Nurs. 2011;13:154–163. doi: 10.1177/1099800410384500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnard S, Lachance C, Patrizi S, et al. The Toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Wang PF, Fang H, Chen J, Xiong XY, Yang QW. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke. 2013;44:2545–2552. doi: 10.1161/STROKEAHA.113.001038. [DOI] [PubMed] [Google Scholar]
- Molloy EJ, O'Neill AJ, Grantham JJ, et al. Labor promotes neonatal neutrophil survival and lipopolysaccharide responsiveness. Pediatr Res. 2004;56:99–103. doi: 10.1203/01.PDR.0000130473.30874.B6. [DOI] [PubMed] [Google Scholar]
- Christiaans SC, Wagener BM, Esmon CT, Pittet JF. Protein C and acute inflammation: a clinical and biological perspective. Am J Physiol Lung Cell Mol Physiol. 2013;305:455–466. doi: 10.1152/ajplung.00093.2013. [DOI] [PubMed] [Google Scholar]
- Marti-Carvaial AJ, Sola I, Lathyris D, Cardona AF. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst Rev. 2011;((4)) doi: 10.1002/14651858.CD004388.pub4. : CD004388. doi: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- Mosnier LO, Yanq XV, Griffin JH. Activated protein C mutant with minimal anticoagulant activity, normal cytoprotective activity, and preservation of thrombin activable fibrinolysis inhibitor-dependent cytoprotective functions. J Biol Chem. 2007;282:33022–33033. doi: 10.1074/jbc.M705824200. [DOI] [PubMed] [Google Scholar]
- Kerschen EJ, Fernandez JA, Cooley BC, et al. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34:198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Neutrophil (a) and monocyte (b) reactive oxygen intermediates in infants with neonatal encephalopathy on day 3 divided by severity: NE grade 0/I versus II/III. *P < 0·05 LPS-induced monocyte ROI comparing NE grade 0/I versus II/III.
Fig. S2. Neutrophil (a) and monocyte (b) reactive oxygen intermediates in infants with neonatal encephalopathy (NE) divided by severity on day 7 comparing: NE grade 0/I versus II/III. *P < 0·05 LPS-induced ROI in NE II/III versus controls.
Fig. S3. Neutrophil (a) reactive oxygen intermediates (ROI) on day 3 and (b) monocyte ROI day 7 comparing normal versus normal neuroimaging. Neutrophil ROI on day 3 and day 7 infants with abnormal neuroimaging had significantly increased LPS-induced neutrophil ROI production (*P < 0·05).


