Abstract
Background and Purpose
Rilpivirine and etravirine are second-generation non-nucleoside reverse transcriptase inhibitors (NNRTIs) indicated for the treatment of HIV/AIDS. The constitutive androstane receptor (CAR) regulates the expression of genes involved in various biological processes, including the transport and biotransformation of drugs. We investigated the effect of rilpivirine and etravirine on the activity of the wild-type human CAR (hCAR-WT) and its hCAR-SV23 and hCAR-SV24 splice variants, and compared it with first-generation NNRTIs (efavirenz, nevirapine, and delavirdine).
Experimental Approach
Receptor activation, ligand-binding domain (LBD) transactivation, and co-activator recruitment were investigated in transiently transfected, NNRTI-treated HepG2 cells. Nuclear translocation of green fluorescent protein-tagged hCAR-WT and CYP2B6 gene expression were assessed in NNRTI-treated human hepatocytes.
Key Results
Rilpivirine and etravirine activated hCAR-WT, but not hCAR-SV23 or hCAR-SV24, and without transactivating the LBD or recruiting steroid receptor coactivators SRC-1, SRC-2, or SRC-3. Among the first-generation NNRTIs investigated, only efavirenz activated hCAR-WT, hCAR-SV23, and hCAR-SV24, but none of them transactivated the LBD of these receptors or substantively recruited SRC-1, SRC-2, or SRC-3. Rilpivirine, etravirine, and efavirenz triggered nuclear translocation of hCAR-WT and increased hCAR target gene (CYP2B6) expression.
Conclusion and Implications
NNRTIs activate hCAR-WT, hCAR-SV23, and hCAR-SV24 in a drug-specific and isoform-selective manner. The activation occurs by a mechanism that does not appear to involve binding to the LBD or recruitment of SRC-1, SRC-2, or SRC-3.
Tables of Links
| LIGANDS | |
|---|---|
| CITCO |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c).
Introduction
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are allosteric inhibitors of HIV reverse transcriptase (De Clercq, 2009). When used as part of the highly active antiretroviral therapy regimen with other anti-HIV drugs, NNRTIs have been reported to decrease HIV load and increase cluster of differentiation 4 (CD4) counts in patients with HIV/AIDS (Camacho and Teofilo, 2011). Rilpivirine and etravirine (Figure 1) belong to the second-generation NNRTIs that are clinically approved for the treatment of HIV/AIDS infection (Usach et al., 2013). In various clinical studies, rilpivirine and etravirine have been reported to be efficacious against both NNRTI-naive and NNRTI-resistant strains of HIV-1 (Goebel et al., 2006; Arasteh et al., 2009; Gazzard et al., 2011; Santos et al., 2011). Furthermore, the second-generation NNRTIs are reported to have a superior genetic barrier to resistance development (Fofana et al., 2013). Because of its greater potency, smaller dosage, and less frequent dosing, two fixed-dose combinations have been developed and approved for rilpivirine (De Clercq, 2012).
Figure 1.
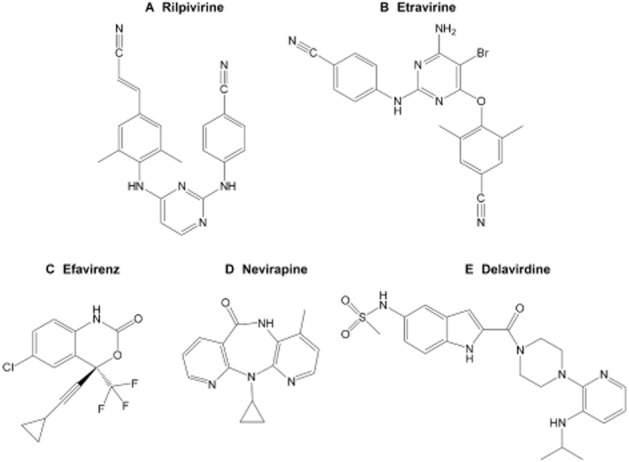
Chemical structures of the US Food and Drug Administration approved NNRTIs: (A) rilpivirine, (B) etravirine, (C) efavirenz, (D) nevirapine, and (E) delavirdine.
The constitutive androstane receptor (CAR) is a member of the superfamily of nuclear receptors (subfamily 1, group I, member 3; NR1I3). It plays an important role in the absorption, distribution, metabolism, and excretion of xenobiotics and of endogenous chemicals by regulating the expression of genes involved in transport and biotransformation (Yang and Wang, 2014). CAR has also been implicated in energy metabolism, endocrine regulation, liver diseases, and other physiological processes and pathophysiological conditions (Jiang and Xie, 2013). Human CAR (hCAR) target genes include the prototypical cytochrome P450 2B6 (CYP2B6) (Sueyoshi et al., 1999). Multiple isoforms of hCAR have been identified, including the wild-type (hCAR-WT) and naturally occurring splice variants (Lamba et al., 2005; Choi et al., 2013), such as hCAR-SV23 (insertion of amino acids SPTV) (Auerbach et al., 2003) and hCAR-SV24 (insertion of amino acids APYLT) (Arnold et al., 2004; Jinno et al., 2004).
hCAR is known to be activated by a diverse range of chemicals, including endogenous chemicals, prescription drugs, and herbal medicines (Chang and Waxman, 2006). Like hCAR, the human pregnane X receptor (hPXR) is another important nuclear receptor that is involved in diverse biological roles and is activated by various endobiotics and xenobiotics (Kodama and Negishi, 2013). Whereas some modulators, such as phenobarbital, activate both hCAR (Sueyoshi et al., 1999) and hPXR (Moore et al., 2000), others exhibit receptor selectivity. For example, carbamazepine preferentially activates hCAR (Faucette et al., 2007), whereas meclizine activates hPXR, but not hCAR (Lau et al., 2011b). Previous studies showed that rilpivirine (Sharma et al., 2013; Weiss and Haefeli, 2013) and etravirine (Sharma et al., 2013) are activators of hPXR. These drugs appear to activate hPXR by a mechanism that involves binding to the ligand-binding domain (LBD) of the receptor and recruitment of steroid receptor coactivator (SRC)-1, SRC-2, and SRC-3 (Sharma et al., 2013). However, it remains to be investigated whether and how rilpivirine and etravirine activate hCAR.
The present study systematically investigated the effects of rilpivirine and etravirine on the activity of hCAR-WT and its hCAR-SV23 and hCAR-SV24 splice variants. As a comparison, we also determined the effects of the first-generation NNRTIs (efavirenz, nevirapine, and delavirdine; Figure 1). Our experimental approaches involved the use of cell-based luciferase reporter gene assays to assess receptor activation, mammalian two-hybrid assay to examine steroid receptor co-activator recruitment, and primary cultures of human hepatocytes to characterize ligand-triggered nuclear translocation of hCAR-WT and hCAR target gene (CYP2B6) expression. The results are discussed from the perspective of drug-dependent and isoform-selective activation of hCAR by NNRTIs.
Methods
Culture and treatment of human hepatocytes
The demographics of the donors (GC4008, HUM4021, HUM4034, and HUM4038) are listed in Table 2013a. Cryopreserved human hepatocytes (Triangle Research Labs, LLC) were thawed and plated according to protocols listed at http://triangleresearchlabs.net/products-hepatocytes/cryopreserved-hepatocytes/. Cell viability, was 88, 90, 94, and 93% for hepatocyte samples GC4008, HUM4021, HUM4034, and HUM4038, respectively, as assessed by trypan blue exclusion (Jauregui et al., 1981). Hepatocytes were plated and cultured as described previously (Sharma et al., 2013). Cultured hepatocytes were treated with DMSO (vehicle control), rilpivirine, etravirine, efavirenz, nevirapine, delavirdine, or a known CYP2B6 inducer such as rifampin (Chang et al., 1997), 6-(4-chlorophenyl)imidazo[2,1-b][1,3] thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (CITCO) (Maglich et al., 2003) or phenobarbital (Chang et al., 1997), as described in the appropriate figure legends. Drug-containing culture medium was replaced every 24 h.
Table 1.
Demographics of human hepatocyte donors
| Donor identification | Race | Gender | Age (years) | Reported BMI | Smoker | Alcohol use | Medicine use | Serological data |
|---|---|---|---|---|---|---|---|---|
| GC4008 | Caucasian | Male | 69 | 24.7 | Stopped 24 years ago | No | No | All negative |
| HUM4021 | Caucasian | Male | 13 | 18.0 | No | No | No | All negative |
| HUM4034 | Caucasian | Male | 21 | 23.6 | Yes | Yes | Marijuana | All negative |
| HUM4038 | Caucasian | Female | 33 | 26.0 | No | Rare | No | CMV+ |
BMI, body mass index; CMV+, cytomegalovirus positive.
Isolation of total RNA, reverse transcription and real-time PCR analysis
Isolation of total RNA and reverse transcription were conducted as described previously (Lau et al., 2011b). The sequences of the primers used to amplify human CYP2B6 cDNA, HPRT1 cDNA, 18s rRNA cDNA, and cyclophilin cDNA are listed in Table 2013b. The primers were synthesized and their specificity was verified by sequencing the purified amplicons (Integrated DNA Technologies, Inc., Coralville, IA, USA). CYP2B6 cDNA, HPRT1 cDNA, 18s rRNA cDNA, and cyclophilin cDNA were amplified by real-time PCR in a LightCycler (Roche Diagnostics, Laval, QC, Canada), according to conditions described in Table 2013b. Each PCR reaction contained 1 ng total cDNA, 1 U Platinum Taq DNA polymerase in 1× PCR buffer (20 mM Tris-HCl, pH 8.4 and 50 mM KCl), 4 mM MgCl2 (3 mM MgCl2 for 18s rRNA), 0.2 mM deoxynucleoside triphosphate, 0.25 mg·mL−1 BSA, 0.2 μM forward and reverse primers (0.5 μM for HPRT1), and 1:30 000 SYBR Green I. To construct the calibration curves (cross point vs. log cDNA copies) for CYP2B6, HPRT1, 18s rRNA, and cyclophilin, amplicons were generated using human liver QUICK-Clone cDNA (Clontech, Mountain View, CA, USA), and then purified and quantified as described previously (Lau et al., 2011b). CYP2B6 mRNA expression was normalized to that of a housekeeping gene (HPRT1, 18s rRNA, and cyclophilin).
Table 2.
Primer sequences and RT-PCR cycling conditions
| Gene | Primer sequence (5′ to 3′) | Denaturation | Annealing | Extension | Reference |
|---|---|---|---|---|---|
| CYP2B6 | GCG-TGT-GGT-TCA-TTC-ACA-AA (forward) | 95°C, 5 s | 65°C, 5 s | 72°C, 15 s | (Chang et al., 2003) |
| AAT-TTA-GCC-AGG-CGT-GGT-G (reverse) | |||||
| HPRT1 | GAA-GAG-CTA-TTG-TAA-TGA-CC (forward) | 95°C, 5 s | 60°C, 10 s | 72°C, 15 s | (Qiu et al., 2007) |
| GCG-ACC-TTG-ACC-ATC-TTT-G (reverse) | |||||
| 18s rRNA | CTT-TGG-TCG-CTC-GCT-CCT-C (forward) | 95°C, 1 s | 62°C, 6 s | 72°C, 10 s | (Sharma et al., 2013) |
| CTG-ACC-GGG-TTG-GTT-TTG-AT (reverse) | |||||
| Cyclophilin | CTC-CTT-TGA-GCT-GTT-TGC-AG (forward) | 95°C, 1 s | 55°C, 5 s | 72°C, 15 s | (Simpson et al., 2000) |
| CAC-CAC-ATG-CTT-GCC-ATC-C (reverse) |
Bupropion hydroxylation assay
The CYP2B6-catalysed bupropion hydroxylation assay was performed as described previously (Lau et al., 2011b). Formation of hydroxybupropion was quantified using an optimized ultra-HPLC-MS/MS method (Lau and Chang, 2009).
Cell culture
HepG2 human hepatocellular carcinoma cells (American Type Culture Collection, Manassas, VA, USA) were cultured in T-75 culture flasks in minimum essential medium/Earle's balanced salt solution (MEM/EBSS) supplemented with 10% v v−1 heat-inactivated fetal bovine serum (FBS) (Life Technologies, Inc., Burlington, ON, Canada), penicillin G (100 U·mL−1), streptomycin (100 μg·mL−1), MEM/non-essential amino acid (1×), and L-glutamine (2 mM). Cells were maintained at 37°C in a humidified incubator with 95% air and 5% CO2. Culture medium was changed every 3 days, and cells were subcultured weekly.
Plasmids
pCMV6-XL4-hCAR-WT, pCMV6-neo-hCAR-SV23, pCMV6-XL4-hCAR-SV24, pCMV6-XL4-human retinoid X receptor α (hRXRα), pCMV6-AC-GFP-hCAR-WT, pCMV6-neo, and pCMV6-XL4 were purchased from OriGene Technologies, Inc. (Rockville, MD, USA). The pFR-luc reporter was purchased from Agilent Technologies (Santa Clara, CA, USA). The internal control Renilla reniformis luciferase pGL4.74 [hRluc/TK] plasmid was procured from Promega (Madison, WI, USA). The pVP16 and pM empty vectors were purchased from Clontech. pM-hCAR-WT-LBD, pVP16-hCAR-WT-LBD, pM-hCAR-SV23-LBD, pVP16-hCAR-SV23-LBD, pM-hCAR-SV24-LBD, pVP16-hCAR-SV24-LBD, pGL3-basic-CYP2B6-PBREM/XREM-luc, pM-hSRC1-RID, pM-hSRC2-RID, pM-hSRC3-RID, pVP16-hSRC1-RID, pVP16-hSRC2-RID, and pVP16-hSRC3-RID were constructed as mentioned previously (Lau et al., 2011a; Lau and Chang, 2013). The constructs were sequenced (Integrated DNA Technologies, Inc.) and their sequence identity was confirmed by comparing with published sequence.
Transient transfection and reporter gene assays
HepG2 cells were cultured in minimum essential medium-reduced serum (MEM-RS) supplemented with penicillin G (100 U·mL−1), streptomycin (100 μg·mL−1), L-glutamine (2 mM), and 1% v v−1 charcoal-stripped FBS. This smaller concentration of serum was used to decrease the normally high basal activity associated with hCAR-WT as a result of receptor activation by contaminants (Lau and Chang, 2014), such as di-(2-ethylhexyl)phthalate (DEHP), which is present in FBS (DeKeyser et al., 2009). Cells were seeded at a density of 100 000 cells per well in a volume of 0.5 mL of supplemented culture medium. At 24 h post-plating, transfection of cultured HepG2 cells was carried out as described previously (Lau and Chang, 2014), except that MEM-RS was used instead of serum-free Opti-MEM. The transfection master mix contained FuGENE 6 transfection reagent (3 μL·μg−1 of DNA), MEM-RS (20 μL per well), and various plasmids. In the hCAR-WT-dependent reporter gene assays, the transfection master mix contained pGL4.74 [hRluc/TK] internal control vector (5 ng per well), pGL3-basic-CYP2B6-PBREM/XREM-luc reporter construct (50 ng per well), and either a receptor expression plasmid or the corresponding empty vector (50 ng per well) for 24 h, as detailed in the appropriate figure legend. In the hCAR-SV23- and hCAR-SV24-dependent reporter gene assays, the transfection master mix also contained pCMV6-XL4-hRXRα (10 ng per well) (Auerbach et al., 2005). Transfected cells were treated with 0.5 mL of supplemented culture medium (without charcoal-stripped FBS) containing DMSO (vehicle control), a NNRTI (rilpivirine, etravirine, efavirenz, nevirapine, or delavirdine), 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP; negative control) (Lau et al., 2011a), CITCO (positive control for hCAR-WT and hCAR-SV24) (Maglich et al., 2003; Auerbach et al., 2005), or DEHP (positive control for hCAR-SV23) (DeKeyser et al., 2009), at the concentrations indicated in appropriate figure legends. In hCAR-WT-dependent reporter gene assays, androstanol (10 μM), which is an inverse agonist of the receptor (Moore et al., 2000), was added to decrease the constitutive activity (Burk et al., 2005). At 24 h post-treatment, HepG2 cells were lysed. Firefly luciferase and R. reniformis (internal control) luciferase activities were quantified and normalized as described previously (Sharma et al., 2013). Results are expressed as fold increase over the vehicle-treated control group. Each experiment was performed in triplicate and a total of four or five independent experiments were conducted.
LBD-transactivation assay
The hCAR-WT-LBD-transactivation assays were performed as described previously (Lau and Chang, 2014), except that MEM-RS was used instead of serum-free Opti-MEM. At 24 h post-plating, cultured HepG2 cells were transfected with a master mix containing FuGENE 6 transfection reagent (3 μL·μg−1 of DNA), MEM-RS (20 μL per well), pFR-luc reporter plasmid (100 ng per well), pGL4.74 [hRluc/TK] internal control plasmid (1 ng per well), and either the pM-hCAR-WT-LBD (Gln-105 to Ser-348) receptor expression plasmid or the pM empty vector (40 ng per well) for 24 h. The hCAR-SV23-LBD- and hCAR-SV24-LBD-dependent transactivation assays were carried out in HepG2 cells transfected with a master mix containing FuGENE 6 transfection reagent (3 μL·μg−1 of DNA), MEM-RS (20 μL per well), pCMV6-XL4-hRXRα (10 ng per well), pFR-luc reporter plasmid (100 ng per well), pGL4.74 [hRluc/TK] internal control plasmid (1 ng per well), and either a receptor expression plasmid or the pM empty vector (40 ng per well) for 24 h. The receptor expression plasmids were pM-hCAR-SV23-LBD (Gln-105 to Ser-352) and pM-hCAR-SV24-LBD (Gln-105 to Ser-353). Transfected cells were treated with 0.5 mL of supplemented culture medium (without charcoal-stripped FBS) containing DMSO (vehicle control), an NNRTI (rilpivirine, etravirine, efavirenz, nevirapine, or delavirdine), TCPOBOP (negative control) (Lau et al., 2011a), CITCO (positive control for hCAR-WT and hCAR-SV24) (Maglich et al., 2003; Auerbach et al., 2005), or DEHP (positive control for hCAR-SV23) (DeKeyser et al., 2009) for 24 h, at the concentrations detailed in appropriate figure legends. In the hCAR-WT-dependent LBD-transactivation assay, cells were co-treated with an hCAR-WT inverse agonist, androstanol (10 μM) (Moore et al., 2000). Luciferase activity was measured and normalized as described under Transient transfection and reporter gene assays. Each experiment was performed in triplicate and a total of three independent experiments were conducted.
In vitro nuclear translocation of GFP-tagged hCAR-WT in primary cultures of human hepatocytes
Human hepatocytes (HUM4038) were cultured on collagen-coated glass cover slips in 24-well plates at a density of 150 000 cells per well. After cell attachment (at 6 h post-plating), the medium was aspirated and 0.5 mL of fresh hepatocyte maintenance medium (Triangle Research Labs, LLC) was added to each well. At 24 h post-plating, human hepatocytes were transfected with a pCMV6-AC-GFP-hCAR-WT (100 ng per well) for 24 h using Effectene® Transfection Reagent (25 μL-μg−1 of DNA) following the manufacturer's protocol. Transfected hepatocytes were treated with 0.5 mL of supplemented hepatocyte maintenance medium containing DMSO (vehicle control), a NNRTI (rilpivirine, etravirine, efavirenz, nevirapine, or delavirdine), or a positive control (CITCO or PB) (Sueyoshi et al., 1999; Maglich et al., 2003) for 24 h, at concentrations shown in the figure legend. At 24 h post-treatment, samples were prepared for confocal imaging as described previously (Sharma et al., 2013). The images were collected using a Carl Zeiss LSM 700 confocal microscope interfaced with the Zen 2011 SP2 software (version 8.0) (Carl Zeiss Microscopy GmbH, Jena, Germany). Post-imaging adjustments in brightness and contrast of the entire image were made in compliance with the Clinical and Laboratory Images in Publications (CLIP) principles (Lang et al., 2012) using Adobe® Photoshop® CS6, version 13.0 ×64 (Adobe Systems Corporation, San Jose, CA, USA).
Mammalian two-hybrid assay
Recruitment of SRC-1, SRC-2, and SRC-3 to hCAR-WT-LBD, hCAR-SV23-LBD, and hCAR-SV24-LBD was assessed by a mammalian two-hybrid assay as described previously (Lau and Chang, 2014), except that MEM-RS was used instead of serum-free Opti-MEM. At 24 h post-plating, cultured HepG2 cells were transfected with a master mix containing FuGENE 6 transfection reagent (3 μL·μg−1 of DNA), MEM-RS (20 μL per well), pFR-luc reporter plasmid (100 ng per well), pGL4.74 [hRluc/TK] internal control plasmid (10 ng per well), a receptor expression plasmid (or the pVP16 empty vector; 40 ng per well), and a coactivator expression plasmid (10 ng per well) for 24 h. The receptor expression plasmids were pVP16-hCAR-WT-LBD, pVP16-hCAR-SV23-LBD, and pVP16-hCAR-SV24-LBD, whereas the coactivator expression plasmids were pM-hSRC1-RID, pM-hSRC2-RID, and pM-hSRC3-RID. In another set of experiments, the mammalian two-hybrid assay was performed as described above, except that a different vector (pM) was used in constructing the receptor expression plasmids (pM-hCAR-WT-LBD, pM-hCAR-SV23-LBD, pM-hCAR-SV24-LBD) and a different vector (pVP16) was used in constructing the coactivator expression plasmids (pVP16-hSRC1-RID, pVP16-hSRC2-RID, pVP16-hSRC3-RID). In the hCAR-SV23-LBD- and hCAR-SV24-LBD-dependent mammalian two-hybrid assay, cells were co-transfected with pCMV6-XL4-hRXRα (10 ng per well) for 24 h. Transfected cells were treated with 0.5 mL of supplemented culture medium (without charcoal-stripped FBS) containing DMSO (vehicle control), a NNRTI (rilpivirine, etravirine, efavirenz, nevirapine, or delavirdine), TCPOBOP (negative control) (Lau et al., 2011a), CITCO (positive control for hCAR-WT and hCAR-SV24) (Maglich et al., 2003; Auerbach et al., 2005), or DEHP (positive control for hCAR-SV23) (DeKeyser et al., 2009) for 24 h, as detailed in the respective figure legends. In the hCAR-WT-dependent co-activator recruitment assay, cells were co-treated with the hCAR-WT inverse agonist, androstanol (10 μM) (Moore et al., 2000). Luciferase activity was measured and normalized as described under Transient transfection and reporter gene assays. Each experiment was performed in triplicate and a total of three independent experiments were conducted.
Data analyses
Data were analysed by two-way anova. Where appropriate, this was followed by the Student Newman–Keuls multiple comparison test (SigmaPlot 11.0; Systat Software, Inc., San Jose, CA, USA). The level of statistical significance was set a priori at P < 0.05.
Chemicals and reagents
Etravirine [Chemical Abstracts Service (CAS) #269055-15-4], efavirenz (CAS #154598-52-4), and nevirapine (CAS #129618-40-2) were obtained from the National Institutes of Health AIDS Reagent Program (Bethesda, MD, USA). Rilpivirine (CAS #500287-72-9), delavirdine (CAS #136817-59-9), and hydroxybupropion (CAS #357399-43-0) were purchased from the Toronto Research Chemicals, Inc. (North York, ON, Canada). FuGENE 6 and Effectene® transfection reagent were purchased from Roche Diagnostics (Laval, QC, Canada), and QIAGEN, Inc. (Mississauga, ON, Canada) respectively. Charcoal-stripped FBS, MEM/EBSS, MEM-RS, and minimum essential medium/non-essential amino acid solution (100×) (HyClone Laboratories, Logan, UT, USA) were purchased from Thermo Fisher Scientific (Nepean, ON, Canada). All other chemicals, reagents and assay kits were purchased from sources listed previously (Lau et al., 2011a; Sharma et al., 2013). Cryopreserved hepatocyte thawing medium, hepatocyte plating medium, hepatocyte maintenance medium, and various medium supplements were provided by Triangle Research Labs, LLC (Research Triangle Park, NC, USA).
Results
Rilpivirine and etravirine induce hepatic expression of a hCAR target gene (CYP2B6)
Sandwich-cultured human hepatocytes were used as an experimental model to compare the effects of rilpivirine and etravirine with first-generation NNRTIs (efavirenz, nevirapine, and delavirdine) on the induction of CYP2B6, a target gene of hCAR (Sueyoshi et al., 1999). As shown in Figure 2A for hepatocyte sample GC4008, rilpivirine, etravirine, and efavirenz (5 μM) increased CYP2B6 mRNA expression by 3.4-, 2.6-, and 3.4-fold, respectively, whereas nevirapine and delavirdine had no effect. Therefore, in subsequent gene expression experiments only the effects of rilpivirine, etravirine, and efavirenz were assessed, not those of nevirapine or delavirdine. Similar effects were observed for sample HUM4021 (Figure 2B), wherein rilpivirine, etravirine, and efavirenz (5 μM) increased the CYP2B6 mRNA level by 3.6-, 3.3-, and 4.3-fold respectively. In human hepatocytes from two other donors (HUM4034 and HUM4038), rilpivirine (0.5 and 5 μM), etravirine (2.5 and 5 μM), and efavirenz (5 and 10 μM) increased CYP2B6 mRNA expression at all the concentrations investigated (Figure 2C and 2D). The positive controls CITCO (1 μM) and rifampicin (10 μM) (Maglich et al., 2003) yielded the expected result (Figure 2A–2D). The same pattern of CYP2B6 induction response was obtained, regardless of whether HPRT1 (Figure 2A–2D), 18s rRNA (data not shown), or cyclophilin (data not shown) was used to normalize the gene expression data. As shown in Figure 2E–2F, the CYP2B6-catalysed bupropion hydroxylation data were consistent with the CYP2B6 mRNA results (Figure 2A–2D).
Figure 2.
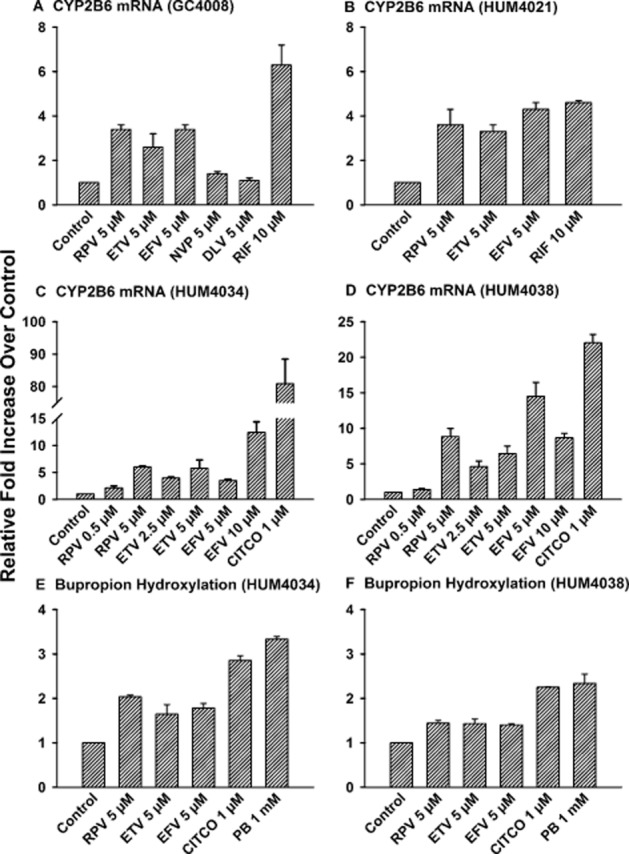
Induction of CYP2B6 in primary cultures of human hepatocytes by rilpivirine and etravirine. Cultured hepatocytes were treated with culture medium (vehicle control for phenobarbital; PB), DMSO (0.1% v v−1; vehicle control for the other drugs), rilpivirine (RPV), etravirine (ETV), efavirenz (EFV), nevirapine (NVP), delavirdine (DLV), rifampicin (RIF; positive control), CITCO (positive control), or PB (positive control) for 48 h (samples GC4008, HUM4034, and HUM4038) or 72 h (sample HUM4021) at the concentrations indicated in (A–D). The hepatocytes were lysed and total RNA was isolated from pooled cell lysates (four wells), and CYP2B6 mRNA and HPRT1 mRNA levels were analysed by real-time PCR, as described under Methods. CYP2B6 mRNA level was normalized to HPRT1 mRNA level. Data are shown as mean ± SD of triplicate PCR analyses for hepatocyte samples GC4008 (A), HUM4021 (B), HUM4034 (C), and HUM4038 (D). CYP2B6 catalytic activity was measured by bupropion hydroxylation using an optimized LC-MS/MS method, as detailed under Methods. Data are expressed as mean ± SD of three wells for hepatocyte samples HUM4034 (E) and HUM4038 (F).
Rilpivirine and etravirine activate hCAR-WT without involving the LBD
Our previous study indicated that rilpivirine, etravirine, efavirenz, nevirapine, and delavirdine at concentrations ≤10 μM were not cytotoxic to HepG2 cells (Sharma et al., 2013). Therefore, in the present study, the concentration of each of these drugs did not exceed 10 μM in our cell-based reporter gene assays. At an equimolar concentration (5 μM), rilpivirine, etravirine, and efavirenz increased, whereas nevirapine and delavirdine had no effect on the transcriptional activity of hCAR-WT (Figure 3A). By comparison, the positive control (CITCO) (Maglich et al., 2003) and the negative control (TCPOBOP) (Moore et al., 2000) produced the expected results. Dose–response experiments indicated the range of concentrations of rilpivirine (1–10 μM, Figure 3B), etravirine (2.5–10.0 μM, Figure 3C), and efavirenz (2.5–10.0 μM, Figure 3D) that increased hCAR-WT activity.
Figure 3.
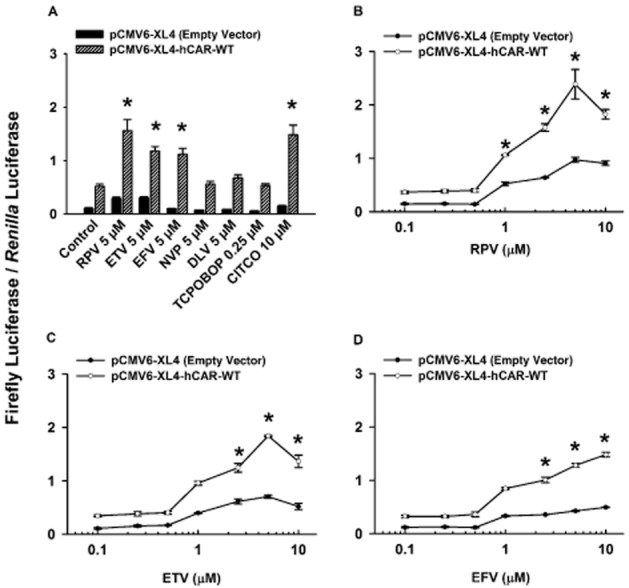
Ligand-specific activation of hCAR-WT by NNRTIs. Cultured HepG2 cells were transfected with pGL3-basic-CYP2B6-PBREM/XREM-luc reporter plasmid, pGL4.74 [hRluc/TK] internal control plasmid, and either the pCMV6-XL4-hCAR-WT receptor expression plasmid or the pCMV6-XL4 empty vector for 24 h. (A) Transfected HepG2 cells were treated with DMSO (0.1% v v−1; vehicle control), rilpivirine (RPV; 5 μM), etravirine (ETV; 5 μM), efavirenz (EFV; 5 μM), nevirapine (NVP; 5 μM), delavirdine (DLV; 5 μM), TCPOBOP (0.25 μM; negative control), or CITCO (10 μM; positive control) for 24 h. (B, C, D) In the concentration–response experiment, transfected HepG2 cells were treated with DMSO (0.1% v v−1; vehicle control), rilpivirine (RPV; 0.1–10.0 μM), etravirine (ETV; 0.1–10.0 μM), or efavirenz (EFV; 0.1–10.0 μM) for 24 h. In all cases, cells were co-treated with androstanol (10 μM; inverse agonist for hCAR-WT) to decrease the constitutive activity of this receptor. Firefly luciferase and Renilla reniformis luciferase activities were measured and normalized as described under Methods. Data are expressed as mean ± SEM for four or five independent experiments performed in triplicate. *Significantly different from the same treatment group transfected with empty vector and the vehicle-treated control group transfected with the receptor expression plasmid (P < 0.05). Androstanol reduced the constitutive activity of hCAR-WT by 32 ± 3%.
To determine whether the NNRTIs transactivate the LBD of hCAR-WT, cultured HepG2 cells were transfected with an hCAR-WT-LBD expression plasmid (i.e. Gln-105 to Ser-348) and treated with 5 μM rilpivirine, etravirine, efavirenz, nevirapine, or delavirdine. As shown in Figure 4, none of the NNRTIs increased the luciferase activity in cells transfected with hCAR-WT-LBD. As expected, CITCO (positive control) (Maglich et al., 2003), but not TCPOBOP (negative control) (Lau et al., 2011a), transactivated hCAR-WT-LBD.
Figure 4.
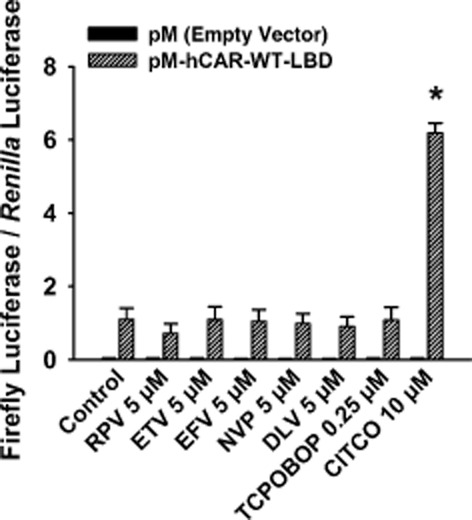
Effect of NNRTIs on transactivation of the LBD of hCAR-WT. Cultured HepG2 cells were transfected with pFR-luc reporter plasmid, pGL4.74 [hRluc/TK] internal control plasmid, and either the pM-hCAR-WT-LBD receptor expression plasmid or the pM empty vector for 24 h. Transfected HepG2 cells were treated with DMSO (0.1% v v−1; vehicle control), rilpivirine (RPV; 5 μM), etravirine (ETV; 5 μM), efavirenz (EFV; 5 μM), nevirapine (NVP; 5 μM), delavirdine (DLV; 5 μM), TCPOBOP (0.25 μM; negative control), or CITCO (10 μM; positive control) for 24 h. In all cases, cells were co-treated with androstanol (10 μM; inverse agonist for hCAR-WT) to decrease the constitutive activity of this receptor. Firefly luciferase and R. reniformis luciferase activities were measured and normalized as described under Methods. Data are expressed as mean ± SEM for three independent experiments performed in triplicate. *Significantly different from the same treatment group transfected with the empty vector and from the vehicle-treated control cells transfected with receptor expression plasmid (P < 0.05). Androstanol reduced the constitutive activity of hCAR-WT by 30 ± 19%.
Rilpivirine and etravirine trigger nuclear translocation of GFP-tagged hCAR-WT
To determine whether the NNRTIs triggered nuclear translocation of hCAR-WT, primary cultures of human hepatocytes were transfected with a GFP-tagged hCAR-WT expression plasmid. As shown in Figure 5, confocal image analyses and comparison with vehicle control (DMSO) group revealed that at a concentration of 5 μM, rilpivirine, etravirine, and efavirenz, but not nevirapine or delavirdine, triggered nuclear translocation of GFP-tagged hCAR-WT in primary cultures of human hepatocytes. Control analysis showed that CITCO and phenobarbital triggered nuclear translocation of hCAR-WT, in agreement with previous findings (Maglich et al., 2003; Li et al., 2009).
Figure 5.
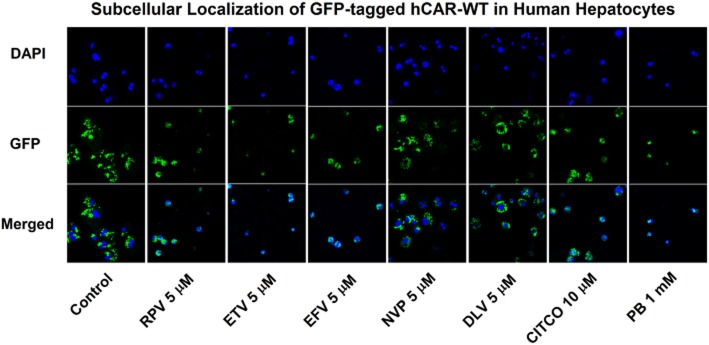
Localization of GFP-tagged hCAR-WT in primary cultures of human hepatocytes (HUM4038) treated with a NNRTI. Cultured hepatocytes were transfected with pCMV6-AC-GFP-hCAR-WT for 24 h and subsequently treated with DMSO (0.1% v v−1; vehicle control), rilpivirine (RPV; 5 μM), etravirine (ETV; 5 μM), efavirenz (EFV; 5 μM), nevirapine (NVP; 5 μM), delavirdine (DLV; 5 μM), CITCO (10 μM; positive control), or phenobarbital (PB; 1 mM; positive control) for 24 h. Cells were fixed with 4% p-formaldehyde and mounted on glass slides using ProLong® Gold Antifade Reagent with DAPI for confocal microscopy. Shown are representative photomicrographs illustrating the localization of GFP-tagged hCAR-WT, DAPI-stained nuclei, and merged images for each treatment group.
In contrast to CITCO, rilpivirine and etravirine do not recruit SRC-1, SRC-2, or SRC-3 to hCAR-WT-LBD
A mammalian two-hybrid assay was performed to determine whether the NNRTIs recruit SRC to the LBD of hCAR-WT. Rilpivirine, etravirine, efavirenz, nevirapine, and delavirdine at 5 μM did not increase the luciferase activity in HepG2 cells co-transfected with a pVP16-hCAR-WT-LBD expression plasmid and a pM-SRC-1 (Figure 6A), pM-SRC-2 (Figure 6B), or pM-SRC-3 coactivator expression plasmid (Figure 6C). The same results were obtained in cells transfected with a pM-hCAR-WT-LBD receptor expression plasmid and a pVP16-SRC-1, pVP16-SRC-2, or pVP16-SRC-3 coactivator expression plasmid (data not shown). By comparison, CITCO (positive control) (Maglich et al., 2003) and TCPOBOP (negative control) (Lau et al., 2011a) produced the expected results.
Figure 6.
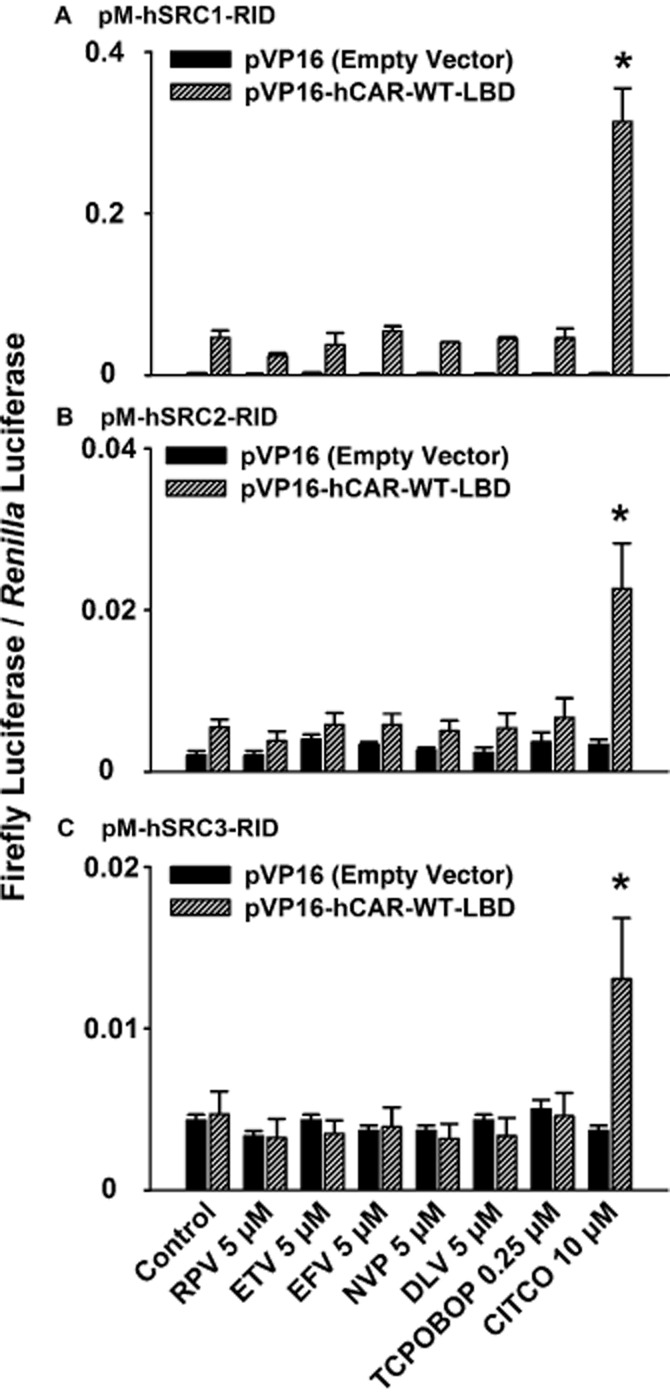
Mammalian two-hybrid assay to evaluate recruitment of SRCs to the LBD of hCAR-WT by specific NNRTIs. Cultured HepG2 cells were co-transfected with a pVP-16-hCAR-WT-LBD receptor expression plasmid (or the pVP-16 empty vector), a coactivator expression plasmid, pFR-luc reporter plasmid, and pGL4.74 [hRluc/TK] internal control plasmid. The coactivator expression plasmids were pM-hSRC1-RID (A), pM-hSRC2-RID (B), and pM-hSRC3-RID (C). Transfected HepG2 cells were treated with DMSO (0.1% v v−1; vehicle control), rilpivirine (RPV; 5 μM), etravirine (ETV; 5 μM), efavirenz (EFV; 5 μM), nevirapine (NVP; 5 μM), delavirdine (DLV; 5 μM), TCPOBOP (0.25 μM; negative control), or CITCO (10 μM; positive control) for 24 h. In all cases, cells were co-treated with androstanol (10 μM; inverse agonist for hCAR-WT) to decrease the constitutive activity of this receptor. Firefly luciferase and Renilla reniformis luciferase activities were measured and normalized as described under Methods. Data are expressed as mean ± SEM for three independent experiments performed in triplicate. *Significantly different from the same treatment group transfected with the empty vector and from the vehicle-treated control cells transfected with receptor expression plasmid (P < 0.05). Androstanol reduced the constitutive activity of hCAR-WT by 49 ± 8%, 36 ± 20%, and 45 ± 9% in cells co-transfected with pVP16-hCAR-WT-LBD and pM-hSRC1-RID, pM-hSRC2-RID, or pM-hSRC3-RID respectively.
Selective activation of hCAR isoforms by rilpivirine and etravirine
As shown in Figure 3A and B, rilpivirine and etravirine activated hCAR-WT. Therefore, the next experiment was to investigate whether NNRTIs influence the activity of other hCAR isoforms. At an equimolar concentration (5 μM), only efavirenz increased, whereas rilpivirine, etravirine, nevirapine, and delavirdine had no effect on the activity of hCAR-SV23 (Figure 7A) or hCAR-SV24 (Figure 7B). Concentration–response data revealed that 0.5–10.0 μM efavirenz increased hCAR-SV23 activity (Figure 7C), whereas ≥5 μM efavirenz increased hCAR-SV24 activity (Figure 7D). The effect of an equimolar concentration (10 μM) of efavirenz on hCAR-SV24 (Figure 7D) was smaller than the response elicited by CITCO (Figure 7B).
Figure 7.
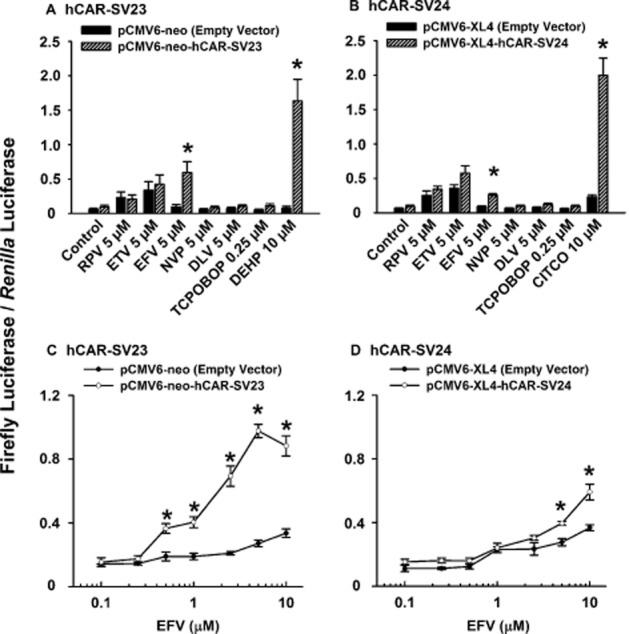
Ligand-specific activation of functional splice variants hCAR-SV23 and hCAR-SV24 by NNRTIs. Cultured HepG2 cells were transfected with pCMV6-XL4-hRXRα, pGL3-basic-CYP2B6-PBREM/XREM-luc reporter plasmid, pGL4.74 [hRluc/TK] internal control plasmid, and either a receptor expression plasmid [pCMV6-neo-hCAR-SV23 (A, C) or pCMV6-XL4-hCAR-SV24 (B, D) ] or an empty vector (pCMV6-neo or pCMV6-XL4) for 24 h. Transfected HepG2 cells were treated with DMSO (0.1% v v−1; vehicle control), rilpivirine (RPV; 5 μM), etravirine (ETV; 5 μM), efavirenz (EFV; 5 μM or 0.1–10.0 μM), nevirapine (NVP; 5 μM), delavirdine (DLV; 5 μM), TCPOBOP (0.25 μM; negative control), DEHP (10 μM; positive control for hCAR-SV23), or CITCO (10 μM; positive control for hCAR-SV24) for 24 h. Firefly luciferase and Renilla reniformis luciferase activities were measured and normalized as described under Methods. Data are expressed as mean ± SEM for four or five independent experiments performed in triplicate. *Significantly different from the same treatment group transfected with empty vector and the vehicle-treated control group transfected with the receptor expression plasmid (P < 0.05).
None of the drugs at 5 μM transactivated the LBD of hCAR-SV23 (Gln-105 to Ser-352) (Figure 8A) or hCAR-SV24 (Gln-105 to Ser-353) (Figure 8B). Among the NNRTIs investigated in the mammalian two-hybrid assay for each combination of the LBD of a hCAR splice variant (pVP16-hCAR-SV23-LBD or pVP16-hCAR-SV24-LBD) and an SRC (pM-hSRC1-RID, pM-hSRC2-RID, or pM-hSRC3-LBD) (Figure 8C, 8D, and data not shown), only efavirenz minimally recruited SRC-1 to the LBD of hCAR-SV23 (Figure 8C). No recruitment was obtained in cells transfected with a pM-containing receptor expression plasmid and a pVP16-containing coactivator expression plasmid (data not shown). In each of the assays mentioned earlier, positive control analysis with DEHP and CITCO and negative control analysis with TCPOBOP (Lau et al., 2011a) produced the expected results (Figure 8C, 8D and data not shown).
Figure 8.
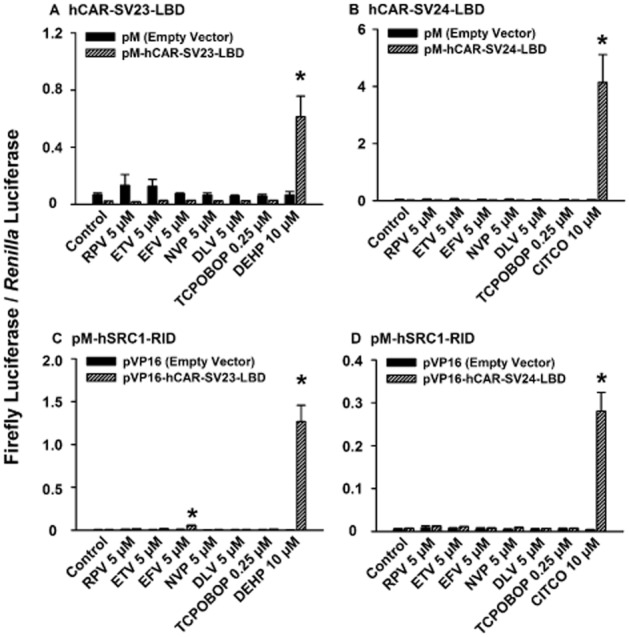
Effect of rilpivirine, etravirine, efavirenz, nevirapine, and delavirdine on transactivation of the LBD of hCAR-SV23 and hCAR-SV24, and recruitment of SRC1 to the ligand-binding domain of hCAR-SV23 and hCAR-SV24. LBD-transactivation assays were performed in HepG2 cells were transfected with pCMV6-XL4-hRXRα, pFR-luc reporter plasmid, pGL4.74 [hRluc/TK] internal control plasmid, and either a receptor expression plasmid [pM-hCAR-SV23-LBD (A) or pM-hCAR-SV24-LBD (B) ] or an empty vector (pM) for 24 h. In the mammalian two-hybrid assays, HepG2 cells were co-transfected with a pM-hSRC1-RID coactivator expression plasmid, pCMV6-XL4-hRXRα, pFR-luc reporter plasmid, pGL4.74 [hRluc/TK] internal control plasmid, and either a receptor expression plasmid [pVP16-hCAR-SV23-LBD (C) or pVP16-hCAR-SV24-LBD (D) ] or an empty vector (pVP16) for 24 h. Transfected cells were treated with DMSO (0.1% v v−1; vehicle control), rilpivirine (RPV; 5 μM), etravirine (ETV; 5 μM), efavirenz (EFV; 5 μM), nevirapine (NVP; 5 μM), or delavirdine (DLV; 5 μM), TCPOBOP (0.25 μM; negative control), DEHP (10 μM; positive control for hCAR-SV23), or CITCO (10 μM; positive control for hCAR-SV24) for 24 h. Firefly luciferase and Renilla reniformis luciferase activities were measured and normalized as described under Methods. Data are expressed as mean ± SEM for three independent experiments performed in triplicate. *Significantly different from the same treatment group transfected with corresponding empty vector and from the vehicle-treated control cells transfected with respective receptor expression plasmid (P < 0.05).
Discussion
CAR is a transcriptional factor of practical importance as changes in its activity influence a variety of physiological functions and pathological conditions (Jiang and Xie, 2013). Moreover, owing to its effect on genes involved in transport and biotransformation of various endogenous substances and xenobiotics, alteration in CAR activity represents a molecular basis for pharmacokinetic drug interactions and chemical toxicity (Yang and Wang, 2014). Our study is the first to demonstrate that rilpivirine and etravirine are activators of hCAR-WT. This conclusion is based on our experimental evidence indicating that rilpivirine and etravirine: (i) activated hCAR-WT, as assessed in a cell-based reporter gene assay in which cultured HepG2 cells were transfected with a plasmid expressing the full-length hCAR-WT; (ii) triggered nuclear translocation of GFP-tagged hCAR-WT in primary cultures of human hepatocytes; and (iii) induced hCAR target gene (CYP2B6) expression in primary cultures of human hepatocytes. The activation of hCAR-WT by rilpivirine and etravirine did not appear to involve a direct interaction between each of these drugs and the LBD of hCAR-WT, as suggested by the lack of an effect in a hCAR-WT-LBD-transactivation assay and the lack of coactivator (SRC-1, SRC-2, and SRC-3) recruitment in a mammalian two-hybrid assay. The molecular mechanism of hCAR-WT activation is still not well-understood. Previous studies have shown orthosteric agonism as a mechanism of hCAR-WT activation by CITCO (Maglich et al., 2003) and 3-hydroxyflavone (Lau and Chang, 2013). However, other ligands, such as phenobarbital (Moore et al., 2000), phenytoin (Wang et al., 2004), and Ginkgo biloba (Lau et al., 2011a), do not activate hCAR-WT by binding directly to the LBD of the receptor and recruiting coactivators to the ligand–receptor complex. A recent study indicated inhibition of EGF receptor (EGFR) signalling as a mechanism of indirect activation of mouse CAR by phenobarbital (Mutoh et al., 2013). Given the pronounced species-dependency in chemical activation of CAR (Chang and Waxman, 2006; Omiecinski et al., 2011), it remains to be investigated whether the EGFR signalling pathway also contributes to the activation of hCAR by rilpivirine, etravirine, and other indirect activators of hCAR. Overall, our data provide the novel identification of rilpivirine and etravirine as activators of hCAR-WT and show that the activation occurs in a manner distinct from the orthosteric agonism of hCAR-WT by CITCO (Maglich et al., 2003) and 3-hydroxyflavone (Lau and Chang, 2013).
Another novel finding of the present study is that rilpivirine and etravirine activate hCAR in an isoform-dependent manner. Rilpivirine and etravirine activated hCAR-WT, but not hCAR-SV23 or hCAR-SV24. This finding corroborates with the notion that these hCAR isoforms do not have identical ligand activation profiles (Lau et al., 2011a). This may be explained by the proposed alteration in receptor conformation of hCAR-SV23 as a result of the amino acid (SPTV) insertion between helices 6 and 7 (DeKeyser et al., 2011) and the proposed compromise in receptor heterodimerization of hCAR-SV24 as a result of the amino acid (APYLT) insertion between helices 8 and 9 (Omiecinski et al., 2011). As reported previously, phenobarbital (Lau et al., 2011a) and various flavonol analogues (galangin, datiscetin, kaempferol, quercetin, isorhamnetin, and tamarixetin) (Lau and Chang, 2013) activate hCAR-WT, but not hCAR-SV23 or hCAR-SV24. In contrast, di-isononyl phthalate (DeKeyser et al., 2009) and pheniramine (Dring et al., 2010) activate hCAR-SV23 and hCAR-SV24, respectively, whereas neither affects the activity of hCAR-WT. The finding that rilpivirine and etravirine activate hCAR-WT, but not hCAR-SV23 or hCAR-SV24 leads to the possibility of inter-individual differences in hCAR-mediated effects, given the inter-individual differences reported for hepatic gene expression of hCAR-WT (Chang et al., 2003).
Among the first-generation NNRTIs investigated in the present study, efavirenz at concentrations of 2.5–10.0 μM, 0.5–10.0 μM and 5–10 μM activated hCAR-WT, hCAR-SV23, and hCAR-SV24 respectively. This occurred by a mechanism that did not appear to involve binding to the LBD of the respective receptor or recruitment of the SRC-1, SRC-2, and SRC-3 coactivators. In a previous report (Svard et al., 2010), 10 μM efavirenz was shown not to activate hCAR-WT in a cell-based reporter gene assay. However, any effect of efavirenz on hCAR-WT activity may have been masked in that study (Svard et al., 2010) because their HepG2 cells were not treated with an inverse agonist (e.g. androstanol) to attenuate the high basal activity normally associated with hCAR-WT in cultured HepG2 cells (Burk et al., 2005). Our results on activation of hCAR-SV24 by efavirenz are consistent with those reported in a previous study (Faucette et al., 2007). Among the other first-generation NNRTIs investigated in the present study, nevirapine and delavirdine at concentrations of up to 10 μM did not influence the activity of hCAR-WT, hCAR-SV23 or hCAR-SV24. In the case of nevirapine, it appears that a greater concentration is needed to activate hCAR-SV24, as shown in a previous finding that suprapharmacological concentrations (50 and 100 μM) of this drug activate hCAR-SV24 (Faucette et al., 2007; Chen et al., 2010). Overall, the first-generation NNRTIs activated hCAR in an isoform-selective and drug-specific manner. Furthermore, efavirenz activated hCAR-WT, hCAR-SV23, and hCAR-SV24 by a mechanism that is distinct from that of hCAR-WT and hCAR-SV24 activation by CITCO and of hCAR-SV23 activation by DEHP (Lau and Chang, 2013).
hCAR regulates the expression of CYP2B6, which is its prototypical target gene (Sueyoshi et al., 1999). The CYP2B6 enzyme metabolizes a diverse group of drugs, including bupropion (Hesse et al., 2000), efavirenz (Ward et al., 2003), and nevirapine (Erickson et al., 1999). The CYP2B6-inducing concentrations of rilpivirine (0.5–5.0 μM), etravirine (2.5–5.0 μM), and efavirenz (5–10 μM) evident in our sandwich-cultured human hepatocyte experiment are comparable with the steady-state maximum plasma concentrations reported for rilpivirine (0.30 ± 0.08 μM; mean ± SD) (Goebel et al., 2006), etravirine (up to 5 μM) (Gagliardini et al., 2014), and efavirenz (12.98 μM; 95% confidence interval, 7.95–18.27 μM) (Nanzigu et al., 2012). Whether rilpivirine and etravirine influence the elimination pharmacokinetics of CYP2B6-metabolized drugs is not known, but efavirenz has been reported to decrease by approximately one-half the systemic exposure to bupropion (i.e. the area under the plasma bupropion concentration–time curve) in a study of healthy human subjects (Robertson et al., 2008). Interestingly, in vitro experiments have identified rilpivirine (IC50 = 4.2 ± 1.6 μM) (Weiss and Haefeli, 2013) and efavirenz (at 10 and 100 μM concentrations) (Hesse et al., 2001) as inhibitors of CYP2B6 catalytic activity. Therefore, in our human hepatocyte samples treated with one of these drugs, the magnitude of bupropion hydroxylation reflects perhaps not only an inductive effect on CYP2B6 gene expression, but also an inhibitory effect on CYP2B6 catalytic activity by rilpivirine and efavirenz. Studies are planned to investigate in detail the effect of these NNRTIs on CYP2B6 catalytic activity.
In conclusion, NNRTIs activate hCAR in an isoform-selective and drug-specific manner. Rilpivirine and etravirine activate hCAR-WT, whereas efavirenz activates hCAR-WT and its SV23 and SV24 splice variants. The activation of these hCAR isoforms occurs by a mechanism that does not appear to involve binding to the LBD of the respective receptor or recruitment of SRC-1, SRC-2, or SRC-3 coactivators. This indicates that rilpivirine, etravirine, and efavirenz are not a CITCO-type of hCAR-WT and hCAR-SV24 activator nor are they DEHP-type of hCAR-SV23 activator.
Acknowledgments
This research was supported by the Natural Sciences and Engineering Research Council of Canada (Grant RGPIN-2014-03734). T. K. H. C. received a Senior Scholar Award from the Michael Smith Foundation for Health Research. The authors thank Dr Guixiang Yang and Catherine Hu for their assistance, Triangle Research Labs, LLC for their generous provision of cryopreserved human hepatocytes, specialty culture media, and the various culture medium supplements, and the National Institutes of Health AIDS Reagent Program (Bethesda, MD, USA) for supplying etravirine, efavirenz, and nevirapine.
Glossary
Abbreviations
- CAR
constitutive androstane receptor
- CAS
Chemical Abstracts Service
- CITCO
6-(4-chlorophenyl)imidazo[2,1-b][1,3] thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime
- CYP2B6
cytochrome P450 2B6
- DEHP
di-(2-ethylhexyl)phthalate
- hCAR
human CAR
- hRXRα
human retinoid X receptor α
- LBD
ligand-binding domain
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- SRC
steroid receptor coactivator
- TCPOBOP
1,4-bis-[2-(3,5-dichloropyridyloxy) ]benzene
Author contributions
All authors participated in the research design. D. S. and A. J. L. conducted the experiments and performed the data analysis. D. S. and T. K. H. C. wrote the paper with input and discussion from all of the co-authors.
Conflict of interest
None.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear hormone receptors. Br J Pharmacol. 2013a;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013b;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasteh K, Rieger A, Yeni P, Pozniak A, Boogaerts G, van Heeswijk R, et al. Short-term randomized proof-of-principle trial of TMC278 in patients with HIV type-1 who have previously failed antiretroviral therapy. Antivir Ther. 2009;14:713–722. [PubMed] [Google Scholar]
- Arnold KA, Eichelbaum M, Burk O. Alternative splicing affects the function and tissue-specific expression of the human constitutive androstane receptor. Nucl Recept. 2004;2:1–16. doi: 10.1186/1478-1336-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SS, Ramsden R, Stoner MA, Verlinde C, Hassett C, Omiecinski CJ. Alternatively spliced isoforms of the human constitutive androstane receptor. Nucleic Acids Res. 2003;31:3194–3207. doi: 10.1093/nar/gkg419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach SS, Stoner MA, Su S, Omiecinski CJ. Retinoid X receptor-alpha-dependent transactivation by a naturally occurring structural variant of human constitutive androstane receptor (NR1I3) Mol Pharmacol. 2005;68:1239–1253. doi: 10.1124/mol.105.013417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk O, Arnold KA, Nussler AK, Schaeffeler E, Efimova E, Avery BA, et al. Antimalarial artemisinin drugs induce cytochrome P450 and MDR1 expression by activation of xenosensors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol. 2005;67:1954–1965. doi: 10.1124/mol.104.009019. [DOI] [PubMed] [Google Scholar]
- Camacho R, Teofilo E. Antiretroviral therapy in treatment-naive patients with HIV infection. Curr Opin HIV AIDS. 2011;6(Suppl. 1):S3–S11. doi: 10.1097/01.COH.0000410239.88517.00. [DOI] [PubMed] [Google Scholar]
- Chang TKH, Waxman DJ. Synthetic drugs and natural products as modulators of constitutive androstane receptor (CAR) and pregnane X receptor (PXR) Drug Metab Rev. 2006;38:51–73. doi: 10.1080/03602530600569828. [DOI] [PubMed] [Google Scholar]
- Chang TKH, Yu L, Maurel P, Waxman DJ. Enhanced cyclophosphamide and ifosfamide activation in primary human hepatocyte cultures: response to cytochrome P-450 inducers and autoinduction by oxazaphosphorines. Cancer Res. 1997;57:1946–1954. [PubMed] [Google Scholar]
- Chang TKH, Bandiera SM, Chen J. Constitutive androstane receptor and pregnane X receptor gene expression in human liver: interindividual variability and correlation with CYP2B6 mRNA levels. Drug Metab Dispos. 2003;31:7–10. doi: 10.1124/dmd.31.1.7. [DOI] [PubMed] [Google Scholar]
- Chen T, Tompkins LM, Li L, Li H, Kim G, Zheng Y, et al. A single amino acid controls the functional switch of human constitutive androstane receptor (CAR) 1 to the xenobiotic-sensitive splicing variant CAR3. J Pharmacol Exp Ther. 2010;332:106–115. doi: 10.1124/jpet.109.159210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EJ, Jang YJ, Cha EY, Shin JG, Lee SS. Identification and characterization of novel alternative splice variants of human constitutive androstane receptor in liver samples of Koreans and Caucasians. Drug Metab Dispos. 2013;41:888–896. doi: 10.1124/dmd.112.049791. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Anti-HIV drugs: 25 compounds approved within 25 years after the discovery of HIV. Int J Antimicrob Agents. 2009;33:307–320. doi: 10.1016/j.ijantimicag.2008.10.010. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Where rilpivirine meets with tenofovir, the start of a new anti-HIV drug combination era. Biochem Pharmacol. 2012;84:241–248. doi: 10.1016/j.bcp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- DeKeyser JG, Stagliano MC, Auerbach SS, Prabhu KS, Jones AD, Omiecinski CJ. Di(2-ethylhexyl) phthalate is a highly potent agonist for the human constitutive androstane receptor splice variant CAR2. Mol Pharmacol. 2009;75:1005–1013. doi: 10.1124/mol.108.053702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKeyser JG, Laurenzana EM, Peterson EC, Chen T, Omiecinski CJ. Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor. Toxicol Sci. 2011;120:381–391. doi: 10.1093/toxsci/kfq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dring AM, Anderson LE, Qamar S, Stoner MA. Rational quantitative structure-activity relationship (RQSAR) screen for PXR and CAR isoform-specific nuclear receptor ligands. Chem Biol Interact. 2010;188:512–525. doi: 10.1016/j.cbi.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999;27:1488–1495. [PubMed] [Google Scholar]
- Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, et al. Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther. 2007;320:72–80. doi: 10.1124/jpet.106.112136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fofana DB, Soulie C, Maiga AI, Fourati S, Malet I, Wirden M, et al. Genetic barrier to the development of resistance to rilpivirine and etravirine between HIV-1 subtypes CRF02_AG and B. J Antimicrob Chemother. 2013;68:2515–2520. doi: 10.1093/jac/dkt251. [DOI] [PubMed] [Google Scholar]
- Gagliardini R, Fabbiani M, Fortuna S, Visconti E, Navarra P, Cauda R, et al. Pharmacokinetics of etravirine in HIV-infected patients concomitantly treated with rifampin for tuberculosis. Infection. 2014;42:775–778. doi: 10.1007/s15010-014-0599-z. [DOI] [PubMed] [Google Scholar]
- Gazzard B, Duvivier C, Zagler C, Castagna A, Hill A, van Delft Y, et al. Phase 2 double-blind, randomized trial of etravirine versus efavirenz in treatment-naive patients: 48-week results. AIDS. 2011;25:2249–2258. doi: 10.1097/QAD.0b013e32834c4c06. [DOI] [PubMed] [Google Scholar]
- Goebel F, Yakovlev A, Pozniak AL, Vinogradova E, Boogaerts G, Hoetelmans R, et al. Short-term antiviral activity of TMC278 – a novel NNRTI – in treatment-naive HIV-1-infected subjects. AIDS. 2006;20:1721–1726. doi: 10.1097/01.aids.0000242818.65215.bd. [DOI] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, et al. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos. 2000;28:1176–1183. [PubMed] [Google Scholar]
- Hesse LM, von Moltke LL, Shader RI, Greenblatt DJ. Ritonavir, efavirenz, and nelfinavir inhibit CYP2B6 activity in vitro: potential drug interactions with bupropion. Drug Metab Dispos. 2001;29:100–102. [PubMed] [Google Scholar]
- Jauregui HO, Hayner NT, Driscoll JL, Williams-Holland R, Lipsky MH, Galletti PM. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro. 1981;17:1100–1110. doi: 10.1007/BF02618612. [DOI] [PubMed] [Google Scholar]
- Jiang M, Xie W. Role of the constitutive androstane receptor in obesity and type 2 diabetes: a case study of the endobiotic function of a xenobiotic receptor. Drug Metab Rev. 2013;45:156–163. doi: 10.3109/03602532.2012.743561. [DOI] [PubMed] [Google Scholar]
- Jinno H, Tanaka-Kagawa T, Hanioka N, Ishida S, Saeki M, Soyama A, et al. Identification of novel alternative splice variants of human constitutive androstane receptor and characterization of their expression in the liver. Mol Pharmacol. 2004;65:496–502. doi: 10.1124/mol.65.3.496. [DOI] [PubMed] [Google Scholar]
- Kodama S, Negishi M. PXR cross-talks with internal and external signals in physiological and pathophysiological responses. Drug Metab Rev. 2013;45:300–310. doi: 10.3109/03602532.2013.795585. [DOI] [PubMed] [Google Scholar]
- Lamba J, Lamba V, Schuetz E. Genetic variants of PXR (NR1I2) and CAR (NR1I3) and their implications in drug metabolism and pharmacogenetics. Curr Drug Metab. 2005;6:369–383. doi: 10.2174/1389200054633880. [DOI] [PubMed] [Google Scholar]
- Lang TA, Talerico C, Siontis GC. Documenting clinical and laboratory images in publications: the CLIP principles. Chest. 2012;141:1626–1632. doi: 10.1378/chest.11-1800. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Chang TKH. Inhibition of human CYP2B6-catalyzed bupropion hydroxylation by Ginkgo biloba extract: effect of terpene trilactones and flavonols. Drug Metab Dispos. 2009;37:1931–1937. doi: 10.1124/dmd.109.028118. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Chang TKH. Indirect activation of the SV23 and SV24 splice variants of human constitutive androstane receptor: analysis with 3-hydroxyflavone and its analogues. Br J Pharmacol. 2013;170:403–414. doi: 10.1111/bph.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AJ, Chang TKH. Fetal bovine serum and human constitutive androstane receptor: evidence for activation of the SV23 splice variant by artemisinin, artemether, and arteether in a serum-free cell culture system. Toxicol Appl Pharmacol. 2014;277:221–230. doi: 10.1016/j.taap.2014.03.023. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Yang G, Chang TKH. Isoform-selective activation of human constitutive androstane receptor by Ginkgo biloba extract: functional analysis of the SV23, SV24, and SV25 splice variants. J Pharmacol Exp Ther. 2011a;339:704–715. doi: 10.1124/jpet.111.186130. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Yang G, Rajaraman G, Baucom CC, Chang TKH. Differential effect of meclizine on the activity of human pregnane X receptor and constitutive androstane receptor. J Pharmacol Exp Ther. 2011b;336:816–826. doi: 10.1124/jpet.110.175927. [DOI] [PubMed] [Google Scholar]
- Li H, Chen T, Cottrell J, Wang H. Nuclear translocation of adenoviral-enhanced yellow fluorescent protein-tagged-human constitutive androstane receptor (hCAR): a novel tool for screening hCAR activators in human primary hepatocytes. Drug Metab Dispos. 2009;37:1098–1106. doi: 10.1124/dmd.108.026005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- Mutoh S, Sobhany M, Moore R, Perera L, Pedersen L, Sueyoshi T, et al. Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal. 2013;6:ra31. doi: 10.1126/scisignal.2003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanzigu S, Eriksen J, Makumbi F, Lanke S, Mahindi M, Kiguba R, et al. Pharmacokinetics of the nonnucleoside reverse transcriptase inhibitor efavirenz among HIV-infected Ugandans. HIV Med. 2012;13:193–201. doi: 10.1111/j.1468-1293.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- Omiecinski CJ, Coslo DM, Chen T, Laurenzana EM, Peffer RC. Multi-species analyses of direct activators of the constitutive androstane receptor. Toxicol Sci. 2011;123:550–562. doi: 10.1093/toxsci/kfr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098-106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Durand K, Rabinovitch-Chable H, Rigaud M, Gazaille V, Clavere P, et al. Gene expression of HIF-1alpha and XRCC4 measured in human samples by real-time RT-PCR using the sigmoidal curve-fitting method. Biotechniques. 2007;42:355–362. doi: 10.2144/000112331. [DOI] [PubMed] [Google Scholar]
- Robertson SM, Maldarelli F, Natarajan V, Formentini E, Alfaro RM, Penzak SR. Efavirenz induces CYP2B6-mediated hydroxylation of bupropion in healthy subjects. J Acquir Immune Defic Syndr. 2008;49:513–519. doi: 10.1097/QAI.0b013e318183a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JR, Llibre JM, Domingo P, Imaz A, Ferrer E, Podzamczer D, et al. Short communication: high effectiveness of etravirine in routine clinical practice in treatment-experienced HIV type 1-infected patients. AIDS Res Hum Retroviruses. 2011;27:713–717. doi: 10.1089/AID.2010.0283. [DOI] [PubMed] [Google Scholar]
- Sharma D, Lau AJ, Sherman MA, Chang TKH. Agonism of human pregnane X receptor by rilpivirine and etravirine: comparison with first generation non-nucleoside reverse transcriptase inhibitors. Biochem Pharmacol. 2013;85:1700–1711. doi: 10.1016/j.bcp.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Simpson DA, Feeney S, Boyle C, Stitt AW. Retinal VEGF mRNA measured by SYBR green I fluorescence: A versatile approach to quantitative PCR. Mol Vis. 2000;6:178–183. [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem. 1999;274:6043–6046. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- Svard J, Spiers JP, Mulcahy F, Hennessy M. Nuclear receptor-mediated induction of CYP450 by antiretrovirals: functional consequences of NR1I2 (PXR) polymorphisms and differential prevalence in whites and sub-Saharan Africans. J Acquir Immune Defic Syndr. 2010;55:536–549. doi: 10.1097/QAI.0b013e3181f52f0c. [DOI] [PubMed] [Google Scholar]
- Usach I, Melis V, Peris JE. Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability. J Int AIDS Soc. 2013;16:1–14. doi: 10.7448/IAS.16.1.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Faucette S, Moore R, Sueyoshi T, Negishi M, LeCluyse E. Human constitutive androstane receptor mediates induction of CYP2B6 gene expression by phenytoin. J Biol Chem. 2004;279:29295–29301. doi: 10.1074/jbc.M400580200. [DOI] [PubMed] [Google Scholar]
- Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- Weiss J, Haefeli WE. Potential of the novel antiretroviral drug rilpivirine to modulate the expression and function of drug transporters and drug-metabolising enzymes in vitro. Int J Antimicrob Agents. 2013;41:484–487. doi: 10.1016/j.ijantimicag.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H. Signaling control of the constitutive androstane receptor (CAR) Protein Cell. 2014;5:113–123. doi: 10.1007/s13238-013-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


