Abstract
Background and Purpose
This study aimed to address the questions of whether Δ9-tetrahydrocannabivarin (THCV) can (i) enhance activation of 5-HT1A receptors in vitro and (ii) induce any apparent 5-HT1A receptor-mediated antipsychotic effects in vivo.
Experimental Approach
In vitro studies investigated the effect of THCV on targeting by 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) of 5-HT1A receptors in membranes obtained from rat brainstem or human 5-HT1A CHO cells, using [35S]-GTPγS and 8-[3H]-OH-DPAT binding assays. In vivo studies investigated whether THCV induces signs of 5-HT1A receptor-mediated antipsychotic effects in rats.
Key Results
THCV (i) potently, albeit partially, displaced 8-[3H]-OH-DPAT from specific binding sites in rat brainstem membranes; (ii) at 100 nM, significantly enhanced 8-OH-DPAT-induced activation of receptors in these membranes; (iii) produced concentration-related increases in 8-[3H]-OH-DPAT binding to specific sites in membranes of human 5-HT1A receptor-transfected CHO cells; and (iv) at 100 nM, significantly enhanced 8-OH-DPAT-induced activation of these human 5-HT1A receptors. In phencyclidine-treated rats, THCV, like clozapine (i) reduced stereotyped behaviour; (ii) decreased time spent immobile in the forced swim test; and (iii) normalized hyperlocomotor activity, social behaviour and cognitive performance. Some of these effects were counteracted by the 5-HT1A receptor antagonist, WAY100635, or could be reproduced by the CB1 antagonist, AM251.
Conclusions and Implications
Our findings suggest that THCV can enhance 5-HT1A receptor activation, and that some of its apparent antipsychotic effects may depend on this enhancement. We conclude that THCV has therapeutic potential for ameliorating some of the negative, cognitive and positive symptoms of schizophrenia.
Tables of Links
| LIGANDS | |
|---|---|
| 8-OH-DPAT | GTPγS |
| Adenosine | MK-801 |
| AM251 | Phencyclidine (PCP) |
| Cannabidiol (CBD) | Rimonabant |
| Clozapine (CLZ) | THCV |
| Glutamine |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,dAlexander et al., 2013a,b,c,d).
Introduction
In 2005, Russo et al. (2005) showed that one of the main components of Cannabis sativa, cannabidiol (CBD), in the micromolar range binds to and functionally activates 5-HT1A receptors. More recently, we have reported that, at concentrations in the nanomolar range, CBD as well as its immediate precursor cannabidiolic acid can enhance the ability of the selective 5-HT1A receptor agonist, 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), to stimulate [35S]-GTPγS binding to rat brainstem membranes (Rock et al., 2012; Bolognini et al., 2013). Cannabigerol, another phytocannabinoid, has been reported by our group to behave as a potent apparent competitive antagonist of the 5-HT1A receptor (Cascio et al., 2010; Rock et al., 2011).
The research described in this paper focused on the phytocannabinoid, Δ9-tetrahydrocannabivarin (THCV) (Figure 1), a propyl-analogue of Δ9-tetrahydrocannabinol, and on the 5-HT1A receptor. So far, it has been shown that this constituent of Cannabis can behave in both in vitro and in vivo experiments as a CB1 receptor antagonist (Thomas et al., 2005; Pertwee et al., 2007; Dennis et al., 2008; Ma et al., 2008) and a CB2 receptor partial agonist (Bolognini et al., 2010). In addition, THCV has been reported to activate or block certain transient receptor potential (TRP) cation channels and to target GPR55 receptors (De Petrocellis et al., 2011; 2012; Anavi-Goffer et al., 2012). However, the ability of THCV to interact with 5-HT1A receptors has not yet been investigated.
Figure 1.
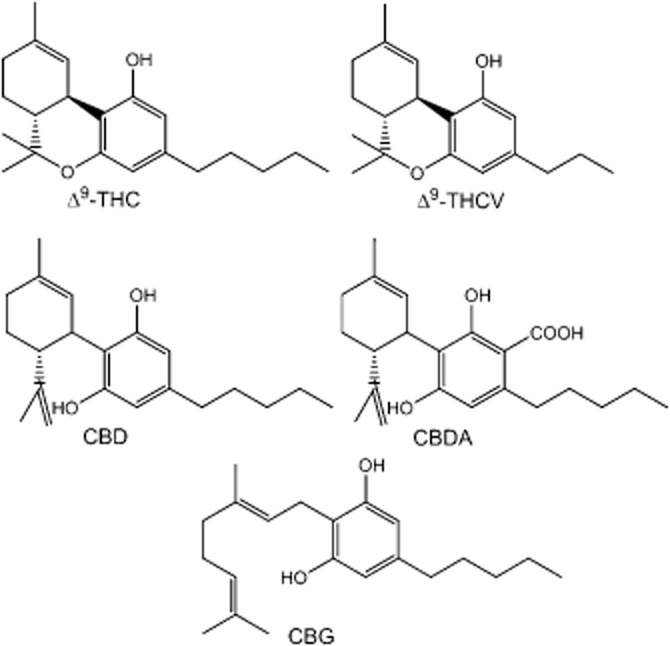
Chemical structures of the phytocannabinoids Δ9-tetrahydrocannabinol (THC), THCV, CBD, cannabidiolic acid (CBDA) and CBG.
Here, for the first time, we present evidence that THCV (i) shares the ability of CBD to enhance 8-OH-DPAT-induced activation of 5-HT1A receptors in vitro in pharmacological assays performed with membranes obtained from rat brainstem or from CHO cells stably transfected with the human 5-HT1A receptor and (ii) produces, in rat models of schizophrenia-like symptoms, apparent antipsychotic effects that are, at least in part, 5-HT1A receptor-mediated.
Methods
Receptor nomenclature
The nomenclature of all the receptors mentioned in this paper conforms to BJP's Concise Guide to Pharmacology (Alexander et al., ,b2013a).
Animals
For in vitro experiments, brainstem tissues were obtained from six adult male Sprague–Dawley rats maintained on a 12/12 h light/dark cycle with free access to food and water. These animals were purchased from Harlan UK Ltd. (Blackthorn, UK). Before the removal of the brainstem, rats were killed by exposure to CO2 followed by cervical dislocation. All animal care and experimental procedures complied with the UK Animals (Scientific Procedures) Act of 1986 and associated guidelines for the use of experimental animals. For in vivo experiments, male Sprague–Dawley rats (280–300 g at the time of arrival) were purchased from Charles River (Calco, Italy) and randomly housed in groups of four, on a 12/12 h light–dark cycle (lights on 08:00 h) and in a temperature (24 ± 2°C) and humidity-controlled environment (50 ± 10%), with a plastic tube for environmental enrichment. All animals had free access to food and water. We used a total of 204 rats that were randomly allocated to the experimental groups as follows: 18 control and 84 treated animals (six rats for each experimental group) were tested for acute phencyclidine (PCP) experiments and 18 control and 84 treated animals (six rats for each experimental group) were subjected to sub-chronic PCP experiments. All in vivo experiments were carried out during the light phase and performed in accordance with the guidelines released by the Italian Ministry of Health (D.L.116/92) and (D.L.111/94-B) and the European Community directives regulating animal research (86/609/EEC). All efforts were made to minimize the number of animals used and their suffering. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
In vitro procedures
CHO cells
CHO cells stably transfected with cDNA encoding human 5-HT1A receptors (a generous gift from Dr Keith Parker) were maintained at 37°C and 5% CO2 in DMEM nutrient mixture F-12 HAM supplemented with 2 mM L-glutamine, 10% FBS, 0.6% penicillin streptomycin and G418 (600 mg·mL−1).
Radioligand displacement assay
Membranes from Sprague–Dawley rat brainstem were prepared as described by Bolognini et al. (2013). Each assay was carried out with 0.7 nM [3H]-8-OH-DPAT, rat brainstem membranes (50 μg per well) or human 5-HT1A CHO cell membranes (50 μg per well) using Tris-binding buffer (50 mM Tris-HCl, 50 mM Tris-base, 0.1% BSA, pH 7.4), total assay volume 500 μL. All assays were performed at 37°C for 60 min before termination by the addition of ice-cold Tris-binding buffer and vacuum filtration described previously by Ross et al. (1999b). Specific binding was defined by the presence and absence of 1 μM unlabelled 8-OH-DPAT.
[35S]-GTPγS binding assay
Each assay was carried out with rat brainstem membranes (10 μg protein per well) or human 5-HT1A CHO cell membranes (50 μg protein per well), GTPγS-binding buffer (50 mM Tris-HCl; 50 mM Tris-Base; 5 mM MgCl2; 1 mM EDTA; 100 mM NaCl; 1 mM dithiothreitol; and 0.1% BSA), 0.1 nM [35S]-GTPγS and 30 μM GDP, in a final volume of 500 μL (Cascio et al., 2010). Membranes from rat brainstem were pre-incubated for 30 min at 30°C with 0.5 U·mL−1 adenosine deaminase (200 U·mL−1) to remove any endogenous adenosine. Non-specific binding was measured in the presence of 30 μM GTPγS. Assays were performed at 30°C for 60 min (Cascio et al., 2010).
Dissociation kinetics
Dissociation kinetic assays were performed with the 5-HT1A receptor agonist 8-[3H]-OH-DPAT (0.7 nM), human 5HT1A CHO cells (50 μg protein per well) and Tris-binding buffer, total assay volume 500 μL (Price et al., 2005). 8-[3H]-OH-DPAT was incubated with human 5HT1A CHO cells for 60 min at 25°C. Dissociation was initiated by the addition of 1 μM unlabelled ligand in the presence or absence of test compounds. Dissociation times of 0.5–120 min at 25°C were used. Non-specific binding was determined in the presence of a 1 μM concentration of the unlabelled ligand. Binding was terminated by addition of ice-cold wash buffer (50 mM Tris-HCl, 50 mM Tris-base and 0.1% BSA) followed by vacuum filtration.
In vitro data analysis
Values have been expressed as means and variability as SEM or as 95% confidence limits. Values for IC50, EC50, maximal effect (Emax) and SEM or 95% confidence limits of these values have been calculated by non-linear regression analysis using the equation for a sigmoid concentration–response curve (GraphPad Prism, GraphPad Software, San Diego, CA, USA). The dissociation rate constant for 8-[3H]-OH-DPAT was calculated using a one-phase exponential decay equation (GraphPad Prism). P-values < 0.05 were considered significant.
In vivo procedures
Drug administration
PCP was dissolved in saline and administered at a dose of 5 mg·kg−1, i.p. (volume of injection 1 mL·kg−1). THCV was dissolved in ethanol, cremophor and saline (1:1:18) and administered at a dose of 2 mg·kg−1, i.p. (volume of injection 5 mL·kg−1), 30 min before the test. CLZ was dissolved in 0.2% acetic acid and saline, and pH was adjusted to 6.5 using 10 M NaOH. It was administered at a dose of 2.5 mg·kg−1, i.p. (volume of injection 5 mL·kg−1), 30 min before testing. WAY100635 was dissolved in saline and administered at a dose of 1 mg·kg−1, i.p. (volume of injection 1 mL·kg−1), 45 min before the test sessions. AM251 was dissolved in DMSO, Tween-80 and saline (1:1:8) and administered at a dose of 0.5 mg·kg−1, i.p. (volume of injection 5 mL·kg−1), 45 min before testing.
Acute phencyclidine (PCP) administration
Acute inhibition of the NMDA receptor induces positive-like symptoms of schizophrenia in rodents, such as hyperlocomotion and stereotypies, and this is a model often used to predict the effect of substances with potential antipsychotic properties (Large, 2007; Bubenikova-Valesova et al., 2008a).
At post-natal day 75, the effects of drug treatments on the stereotyped behaviour and increases in locomotor activity induced by acute PCP administration (5 mg·kg−1, i.p.) were assessed according to the treatment schedule shown in ‘Results’.
Sub-chronic PCP schedule
A sub-chronic treatment regime with PCP followed by a washout period, with animals tested in the drug-free state, gives lasting cognitive deficits and negative-like signs with reasonable similarity to the neuropathological and behavioural disturbances of the disorder and is currently considered a useful model for testing the efficacy of novel antipsychotics against affective components and cognitive impairments of psychotic disorders (Neill et al., 2010; 2014,). Animals were treated with either saline or PCP once a day for 7 days, according to a slightly modified version of the treatment schedule described by Seillier et al. (2010) and shown in ‘Results’. After 7 days of withdrawal, rats were tested in the novel object recognition (NOR) test, social interaction test and forced swim test (FST).
Behavioural tests
Spontaneous locomotor activity
Rats were placed in a computer-controlled infra-red activity monitor arena. The arena consisted of a clear acrylic box, 43 × 43 × 32 cm (Ugo Basile, Varese, Italy) placed in a sound-attenuating room. The cage was fitted with two parallel infrared beams, located 2 and 6 cm from the floor and cumulative horizontal and vertical movement counts were recorded for 50 min. During this period, stereotyped behaviours were scored by two observers blind to the treatment groups according to the rating scale described by Sams-Dodd (1998). Horizontal locomotor activity and stereotypies were calculated in 10 min blocks. The total scores of the whole 50 min test session (i.e. the sum of the scores recorded for each 10 min block) were calculated and converted to AUC values using GraphPad Prism 5.0 software.
Novel object recognition (NOR) test (classic and spatial)
The experimental apparatus used for the object recognition test was an open-field box (43 × 43 × 32 cm) made of Plexiglas, placed in a dimly illuminated room. Animals performed each test individually. The experiment was performed and analysed as previously described by Zamberletti et al. (2012). Briefly, each animal was placed in the arena and allowed to explore two identical previously unseen objects for 5 min (familiarization phase). After an inter-trial interval of 3 min, one of the two familiar objects was replaced by a novel, previously unseen object and rats were returned to the arena for the 5 min test phase. During the test phase, the time spent exploring the familiar object (Ef) and the new object (En) was videotaped and recorded separately by two observers blind to the treatment groups and the discrimination index was calculated as follows: [(En − Ef)/(En + Ef)] × 100.
Social interaction test
This test was carried out in a room illuminated with a dim overhead light. On the day of testing, each animal was habituated for 10 min in the test arena (60 × 60 × 60 cm), an open-field box made of Plexiglas. During the test session, each animal was allowed to explore freely an unfamiliar congener in the arena for 10 min. The arena was cleaned with 0.1% acetic acid and dried after each trial. Social behaviours were defined as sniffing, following, grooming, mounting and nosing. Aggressive behaviours were defined as attacking, biting, tail rattling and aggressive grooming. The whole testing phase was videotaped, analysed by two observers blind to the treatment groups; we also recorded the time spent in social behaviours and the number of aggressive behaviours.
Forced swim test (FST)
Animals were tested in a modified version of the FST that included only a single session of swimming (Realini et al., 2011; Zamberletti et al., 2012) as our objective was to measure any changes in a pre-existing behavioural deficit induced by PCP. Briefly, rats were forced to swim for 15 min inside a clear 50 cm tall, 20 cm diameter glass cylinder filled to 30 cm with 25°C water. The session was videotaped for later analysis of the following parameters: immobility (time spent by the animal floating in the water making only those movements necessary to keep its head above the water), swimming (active swimming movements to the centre of the cylinder) and climbing (forceful thrashing movements with forelimbs against the walls of the cylinder). The time spent in each of these behaviours was measured by an experimenter blind to the treatment groups.
In vivo data analysis
Behavioural data were expressed as mean values ± SEM of six animals per group and analysed by three-way anova with PCP, THCV and WAY100635 as independent variables, or by two-way anova with PCP and THCV/CLZ/AM251 as independent variables followed by Bonferroni's post hoc test to examine group differences. The level of statistical significance was set at P < 0.05.
Drugs and materials
THCV was provided by GW Pharmaceuticals (Salisbury, UK). For in vitro experiments we used a Cannabis sativa extract containing THCV at a concentration of 99.4% (w/w), whereas for in vivo experiments we used a Cannabis sativa extract that contained THCV (71.0% w/w), Δ9-tetrahydrocannabinol (0.4%, w/w), cannabigerovarin (0.2%, w/w), cannabinol (0.4% w/w), propyl cannabinol (1.4% w/w) and small amounts of unidentified compounds each at a concentration of less than 1% w/w. 8-OH-DPAT was supplied by Tocris Bioscience (Bristol, UK). [35S]=GTPγS (1250 Ci·mmol−1) and 8-[3H]-OH-DPAT (135.2 Ci·mmol−1) were purchased from PerkinElmer Life Sciences, Inc. (Boston, MA, USA), GTPγS and adenosine deaminase from Roche Diagnostic (Indianapolis, IN, USA), and GDP, DMSO and PCP from Sigma-Aldrich UK (Dorset, UK). Clozapine (CLZ), WAY100635 and N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM251) were obtained from Tocris Bioscience (Italy).
Results
In vitro experiments
First, we investigated whether THCV shares the ability of CBD to enhance the activation of 5-HT1A receptors in rat brainstem membranes. Interestingly, we found that, unlike CBD, THCV (100 nM) induced a statistically significant increase (240.9-fold) in the potency (EC50), but not in the efficacy (Emax), with which 8-OH-DPAT stimulates [35S]-GTPγS binding to these membranes (Figure 2A and Table 2001). When tested alone, THCV (1 nM to 10 μM) did not affect [35S]-GTPγS binding to the same membranes (Figure 2B). Furthermore, we found that in these membranes, THCV potently, but only partially, displaced 8-[3H]-OH-DPAT from specific binding sites (Figure 2C and Table 2013a).
Figure 2.
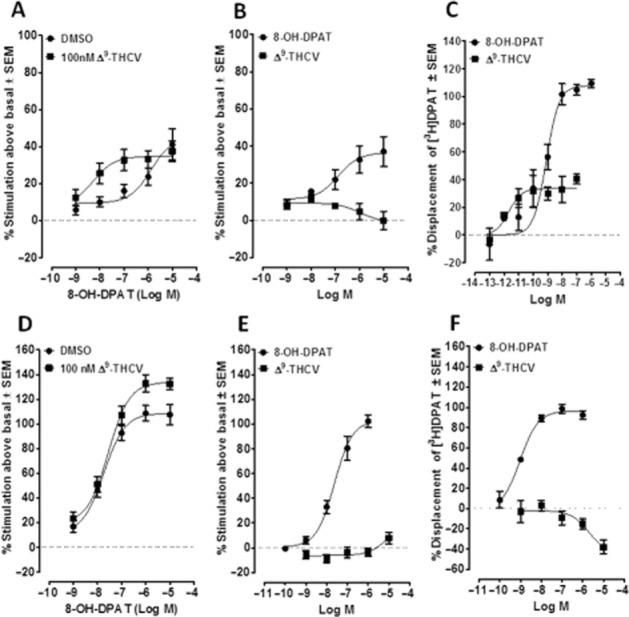
(A) Effect of 8-OH-DPAT on [35S]-GTPγS binding to Sprague–Dawley rat brainstem membranes in the presence of DMSO or 100 nM THCV (n = 11). (B) Effect of 8-OH-DPAT (n = 4) and THCV (n = 6) on [35S]-GTPγS binding to Sprague–Dawley rat brainstem membranes. (C) Displacement of 8-[3H]-OH-DPAT from specific binding sites in Sprague–Dawley rat brainstem membranes by 8-OH-DPAT (n = 4–9) or THCV (n = 4–9). (D) Effect of 8-OH-DPAT on [35S]GTPγS binding to human 5-HT1A CHO cell membranes in the presence of DMSO (vehicle) or 100 nM THCV (n = 8). (E) Effect of 8-OH-DPAT (n = 9) and THCV (n = 10) on [35S]GTPγS binding to human 5-HT1A CHO cell membranes. (F) Displacement of 8-[3H]-OH-DPAT from specific binding sites in human 5-HT1A CHO cell membranes by 8-OH-DPAT (n = 6) and THCV (n = 10). Symbols represent mean values ± SEM.
Table 1.
Effect of 100 nM Δ9-THCV on the mean EC50 and Emax values of 8-OH-DPAT for its stimulation of [35S]-GTPγS binding to membranes obtained from Sprague–Dawley rat brainstem or human 5-HT1A CHO cells
| Pretreatment | Tissue | EC50, nM (95% CI) | Emax, % (95% CI) | n |
|---|---|---|---|---|
| Vehicle (DMSO) | Rat brainstem | 1301 (234, 7236) | 45.0 (30.0, 60.0) | 11 |
| 100 nM THCV | Rat brainstem | 5.4* (0.4, 67.4) | 34.7 (27.8, 41.5) | 11 |
| Vehicle (DMSO) | Human 5-HT1A CHO cells | 18.4 (8.8, 38.5) | 108.5 (99.7, 117.4) | 8 |
| 100 nM THCV | Human 5-HT1A CHO cells | 28.3 (14.9, 53.7) | 133.8* (124.5, 143.1) | 8 |
The 95% confidence intervals (CI) are shown in parentheses. *The 95% confidence intervals of this mean value do not overlap with those of the mean value in the previous row, indicating it to be significantly lower than the mean value obtained from experiments with vehicle-treated membranes (P < 0.05). See also Figure 2A and D.
Table 2.
Mean IC50 and maximal percentage displacement values for the displacement of 8-[3H]-OH-DPAT from specific binding sites in membranes obtained from Sprague–Dawley rat brainstem or human 5-HT1A CHO cells
| Compound | Tissue | IC50, nM (95% CI) | Maximal displacement, % (95% CI) | n |
|---|---|---|---|---|
| 8-OH-DPAT | Rat brainstem | 0.8 (0.4, 1.4) | 107.6 (95.4, 119.8) | 4–9 |
| Δ9-THCV | Rat brainstem | 0.002 (0.0002, 0.002) | 33.5* (27.0, 40.0) | 4–9 |
| 8-OH-DPAT | Human 5-HT1A CHO cells | 0.9 (0.5, 1.7) | 96.5 (90.1, 102.9) | 6 |
| Δ9-THCV | Human 5-HT1A CHO cells | 2060 (194.6, 21810) | −45.4*† (−73.5, −17.4) | 10 |
The 95% confidence intervals (CI) are shown in parentheses. *The 95% CIs of this mean value do not overlap with those of the mean value in the previous row, indicating it to be significantly lower than the mean value obtained from experiments with vehicle-treated membranes (P < 0.05). See also Figure 2C and F.
The 95% CIs of this mean maximal displacement value indicate it to be significantly less than zero (P < 0.05). See also Figure 2F.
Next, we performed experiments with membranes obtained from human 5-HT1A-transfected CHO cells that, in contrast to brain tissue, do not express other types of receptor. We found that, in these membranes, THCV (100 nM) induced a significant increase in the efficacy (Emax), but not in the potency (EC50), with which 8-OH-DPAT activates human 5-HT1A receptors (Figure 2D and Table 2001). When THCV was tested alone in the [35S]-GTPγS binding assay, it did not induce any detectable effect at 1 nM to 10 μM (Figure 2E). Also, in the same membranes, we found that THCV significantly increased the binding of 8-[3H]-OH-DPAT to specific binding sites (Figure 2F), while the binding of this tritium-labelled compound was completely prevented by 8-OH-DPAT (Figure 2F and Table 2013a).
Because there is evidence that the 5-HT1A receptor possesses an allosteric binding site (Barrondo and Sallés, 2009), we also investigated the ability of the 8-OH-DPAT-potentiating concentration of THCV (100 nM) to alter the rate at which 8-[3H]-OH-DPAT dissociates from specific binding sites in membranes obtained from human 5-HT1A CHO cells (n = 4). Our experiments showed that this concentration of THCV did not alter this dissociation rate (data not shown).
Effect of THCV administration on PCP-induced behavioural alterations
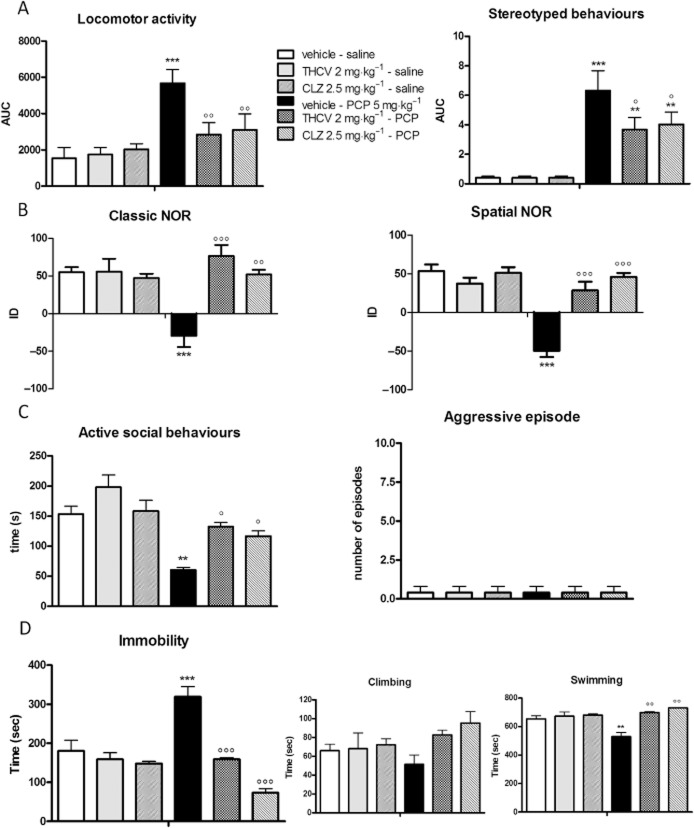
Figure 3 shows the effect of THCV administration (2 mg·kg−1, i.p.) on PCP-induced schizophrenia-like symptoms in rats in comparison with the atypical antipsychotic, CLZ (2.5 mg·kg−1, i.p.). Two different paradigms of PCP administration were chosen: acute PCP to mimic the positive-like signs of schizophrenia (Panel A) and sub-chronic PCP treatment to produce the apparent cognitive deficits (Panel B) and negative-like symptoms (Panels C and D).
Figure 3.
Effect of THCV administration (2 mg·kg−1, i.p.) on PCP-induced hyperlocomotion and stereotyped behaviour (A), cognitive deficits in the classic and spatial NOR test (B), social withdrawal and aggressive behaviours in the social interaction test (C) and immobility in the FST (D). Data are expressed as mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle-saline; °P < 0.05, °°P < 0.01, °°°P < 0.001 versus vehicle-PCP (Bonferroni's post hoc test).
As expected, acute PCP injection (5 mg·kg−1, i.p.) induced marked hyperlocomotion paralleled by the appearance of stereotyped behaviours during the 50-min test session [F(1,20) = 17.56, P = 0.0005]. THCV treatment per se did not affect locomotor activity in control animals but its administration completely normalized PCP-induced hyperlocomotion and significantly reduced stereotyped behaviours [THCV: F(1,20) = 4.452, P = 0.0477; PCP × THCV interaction: F(1,20) = 5.891, P = 0.0248]. Similar results were obtained with the atypical antipsychotic, CLZ (2.5 mg·kg−1, i.p.) [two-way anova for PCP: F(1,20) = 14.85, P = 0.0010; CLZ: F(1,20) = 2.390, P = 0.1378; PCP × CLZ interaction: F(1,20) = 5.133, P = 0.0347] (Figure 3A).
Figure 3B depicts the effect of THCV on the cognitive impairment induced by sub-chronic PCP pretreatment in the NOR test. Sub-chronic PCP significantly impaired recognition memory, as indicated by a significant reduction in the discrimination index of about 50% compared with controls [F(1,20) = 5.266, P = 0.0327]. It induced even greater apparent cognitive impairment in the spatial version of the test, the discrimination index being reduced by about 90% [F(1,20) = 38.64, P < 0.0001]. THCV administration completely restored recognition memory in PCP-pretreated rats both in the classic [THCV: F(1,20) = 14.46, P = 0.0010; PCP × THCV interaction: F(1,20) = 14.46, P = 0.0011] and in the spatial [THCV: F(1,20) = 11.93, P = 0.0025; PCP × THCV interaction: F(1,20) = 27.30, P < 0.0001] variants of the NOR test, without having any effect in control animals. The recovery induced by THCV was very similar to that observed after CLZ administration [classic NOR: two-way anova for PCP: F(1,20) = 19.27, P = 0.0003; CLZ: F(1,20) = 16.47, P = 0.0006; PCP × CLZ interaction: F(1,20) = 24.09, P < 0.0001; spatial NOR: PCP: F(1,20) = 53.18, P < 0.0001; CLZ: F(1,20) = 39.67, P < 0.0001; PCP × CLZ interaction: F(1,20) = 43.37, P < 0.0001]. Neither the time spent exploring the two identical objects during the familiarization phase nor locomotor activity were altered in any of the groups analysed (data not shown).
Figure 3C shows the effect of THCV administration in the social interaction test. Sub-chronic PCP pretreatment significantly reduced the amount of time spent in active social behaviours in the 10-min test session by about 60% when compared with vehicle-treated rats [F(1,20) = 154.9, P < 0.0001]. THCV administration restored the normal social behaviour in PCP-pretreated rats [THCV: F(1,20) = 84.40, P < 0.0001; PCP × THCV interaction: F(1,20) = 4.641, P = 0.0436]. Similar results were obtained with CLZ [PCP: F(1,20) = 44.19, P < 0.0001; CLZ: F(1,20) = 9.225, P = 0.0065; PCP × CLZ interaction: F(1,20) = 6.465, P = 0.0194]. Aggressive behaviours were not observed in any of the groups under investigation. In the FST, sub-chronic PCP pretreatment induced a significant increase of about 80% in the time spent in immobility compared with controls [F(1,20) = 11.34, P = 0.0031]. This was paralleled by a simultaneous reduction in swimming activity. THCV administration to PCP-pretreated rats completely normalized the time spent in immobility during the test session [THCV: F(1,20) = 19.62, P = 0.0003; PCP × THCV interaction: F(1,20) = 11.42, P = 0.0030], the effect being very similar to that observed following CLZ injection [PCP: F(1,20) = 17.60, P = 0.0004; CLZ: F(1,20) = 20.89, P = 0.0002; PCP × CLZ interaction: F(1,20) = 8.430, P = 0.0088]. The rescue of this parameter was accompanied by the normalization of the amount of time spent in swimming activity.
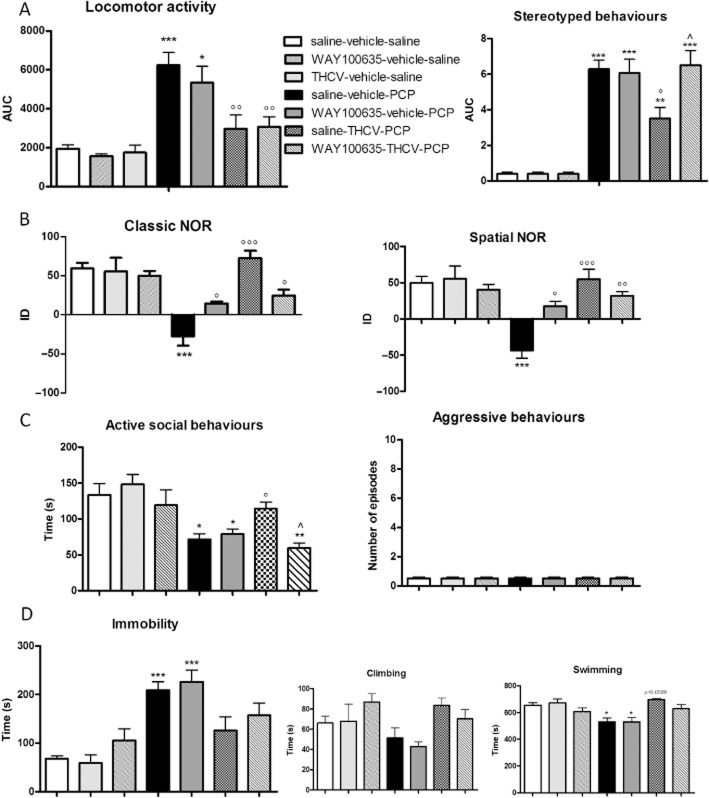
Effect of 5-HT1A receptor blockade on THCV-induced recovery of schizophrenia-like symptoms
Next, we investigated whether the ‘beneficial’ effects exerted by THCV on PCP-induced schizophrenia-like traits were mediated by 5-HT1A receptors. To do this, a selective 5-HT1A antagonist, WAY100635 (1 mg·kg−1, i.p.), was administered prior to THCV according to the treatment protocol shown in Figure 4, and animals were then submitted to behavioural testing. WAY100635 administration per se did not affect any of the behavioural responses under investigation in control animals. Moreover, treatment with WAY100635 had no effect on PCP-induced positive-like (Figure 5A) or negative-like signs (Figure 5C and D). In contrast, WAY100635 administration did partially prevent PCP-induced cognitive deficits in both variants of the NOR test [classic NOR: two-way anova for PCP: F(1,20) = 62.82, P < 0.0001; WAY100635: F(1,20) = 4.617, P = 0.0441; PCP × WAY100635 interaction: F(1,20) = 11.27, P = 0.0031; spatial NOR: PCP: F(1,20) = 44.90, P < 0.0001; WAY100635: F(1,20) = 8.892, P = 0.0074; PCP × WAY100635 interaction: F(1,20) = 16.22, P = 0.0007] (Figure 5B).
Figure 4.
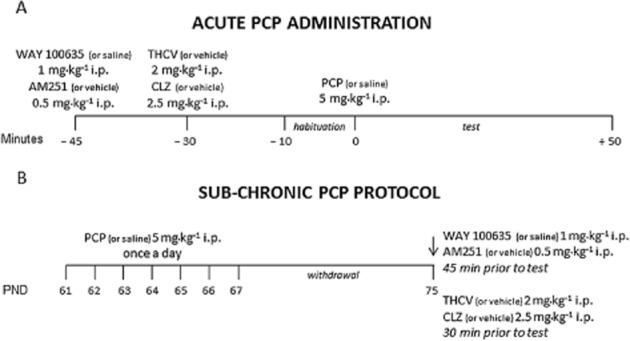
Treatment protocols. (A) PCP (or saline) was injected acutely at the dose of 5 mg·kg−1, i.p. and the effects of drug treatments on the stereotyped behaviour and increases in locomotor activity were monitored in a computer-controlled IR activity cage for 50 min; (B) PCP (or saline) was administered at the dose of 5 mg·kg−1 once a day for 7 days. After 7 days of withdrawal, the effect of drug treatments was tested in the NOR test, social interaction test and FST.
Figure 5.
Effect of WAY100635 pretreatment (1 mg·kg−1, i.p.) on THCV-induced recovery from PCP-induced (A) hyperlocomotion and stereotyped behaviour, (B) cognitive deficits in the classic and spatial NOR test, (C) social withdrawal and aggressive behaviours in the social interaction test and (D) immobility in the FST. Data are expressed as mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01, ***P < 0.001 versus saline-vehicle saline; °P < 0.05, °°P < 0.01, °°°P < 0.001 versus saline-vehicle-PCP; ∧P < 0.05 versus saline-THCV-PCP (Bonferroni's post hoc test).
Importantly, WAY100635 did prevent the beneficial effect exerted by THCV on PCP-induced stereotypies and social withdrawal. Thus, three-way anova indicated both a significant PCP × THCV × WAY100635 interaction [F(1,30) = 5.58, P = 0.0053] and a significant THCV × WAY100635 interaction [F(1,30) = 7.23, P = 0.0095] on stereotypies, the data we obtained indicating that WAY100635 pretreatment completely prevented THCV from reducing PCP-induced stereotypies (Figure 5A). A similar statistically significant effect was observed in the social interaction test [PCP × THCV × WAY100635 interaction: F(1,30) = 5.046, P = 0.0129; THCV × WAY100635 interaction: F(1,30) = 8.998, P = 0.0071], in which WAY100635 pretreatment abolished entirely the ability of THCV to prevent PCP-induced social withdrawal (Figure 5C).
In addition, WAY100635 also partially antagonized the recovery induced by THCV on PCP-induced cognitive deficits in both the classic [PCP × THCV × WAY100635 interaction: F(1,30) = 11.80, P = 0.0002; THCV × WAY100635 interaction: F(1,30) = 11.76, P = 0.0027] and the spatial [PCP × THCV × WAY100635 interaction: F(1,30) = 7.690, P = 0.0020; THCV × WAY100635 interaction: F(1,30) = 5.129, P = 0.0348] variants of the NOR test (Figure 5B).
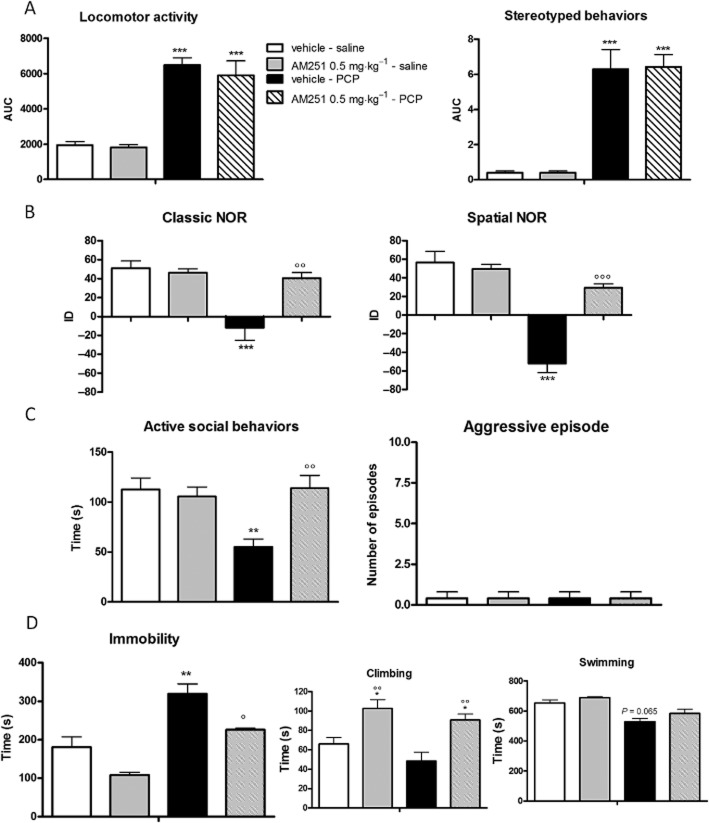
Effect of AM251 administration on PCP-induced behavioural alterations
Acute administration of AM251 (0.5 mg·kg−1, i.p.) did not affect PCP-induced hyperlocomotion or stereotyped behaviours (Figure 6, Panel A). In contrast, its administration completely prevented PCP-induced cognitive impairments in the classic [two-way anova for PCP: F(1,14) = 13.69, P = 0.0024; AM251: F(1,14) = 6.476, P = 0.0234; PCP × AM251 interaction: F(1,14) = 9.297, P = 0.0087] and spatial [two-way anova for PCP: F(1,14) = 46.72, P < 0.0001; AM251: F(1,14) = 15.56, P = 0.0013; PCP × AM251 interaction: F(1,14) = 22.17, P = 0.0003] variants of the NOR test (Figure 6, Panel B). Acute AM251 administration also significantly reduced PCP-induced negative-like symptoms. Thus, AM251 completely abolished PCP-induced social withdrawal in the social interaction test [two-way anova for PCP: F(1,14) = 4.290, P = 0.0560; AM251: F(1,14) = 4.696, P = 0.0467; PCP × AM251 interaction: F(1,14) = 7.676, P = 0.0143] (Figure 6, Panel C) and opposed the increased immobility induced by PCP in the FST [two-way anova for PCP: F(1,14) = 35.59, P < 0.0001; AM251: F(1,14) = 14.93, P = 0.0017; PCP × AM251 interaction: F(1,14) = 5.560, P = 0.0334] by significantly increasing the time spent in climbing activity [F(1,14) = 21,91, P = 0.0001] (Figure 6, Panel D).
Figure 6.
Effect of acute AM251 administration (0.5 mg·kg−1, i.p.) on PCP-induced (A) hyperlocomotion and stereotyped behaviour, (B) cognitive deficits in the classic and spatial NOR test, (C) social withdrawal and aggressive behaviours in the social interaction test and (D) immobility in the FST. Data are expressed as mean ± SEM (n = 6 per group). *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle-saline; °P < 0.05, °°P < 0.01, °°°P < 0.001 versus vehicle-PCP (Bonferroni's post hoc test).
Discussion
The results from our investigation clearly show that THCV possesses an ability to interact with 5-HT1A receptors both in vitro and in vivo. Turning first to our in vitro data, these showed that THCV, at 100 nM, significantly increased the potency (EC50) but not the efficacy (Emax), with which DPAT activates 5-HT1A receptors in rat brainstem membranes, thus behaving as a potential positive allosteric modulator of the 5-HT1A receptor. Also, we found that, like 8-OH-DPAT, THCV potently displaced 8-[3H]-OH-DPAT from specific binding sites in these membranes, although the percentage of the maximum displacement induced by 100 nM THCV was significantly lower than that induced by the same concentration of 8-OH-DPAT. These results raised the possibility that THCV does not bind directly to orthosteric sites on these receptors. Moreover, it remains possible too that THCV enhances DPAT-induced 5-HT1A receptor activation through an indirect mechanism that involves the targeting by THCV of another kind of receptor.
To investigate this hypothesis, we performed experiments with human 5-HT1A-transfected CHO cell membranes that, in contrast to brain membranes, do not express other types of receptors. We found that at 100 nM, THCV did significantly increase the binding of 8-[3H]-OH-DPAT to specific binding sites in these CHO cell membranes. However, in contrast to the results we obtained with rat brainstem membranes, THCV induced a significant increase in the efficacy (Emax), rather than the potency (EC50), with which 8-OH-DPAT activates the human 5-HT1A receptor. In spite of the different manner in which THCV enhanced the effect of 8-OH-DPAT in brain and CHO cell membranes, these results support the hypothesis that THCV might behave as a positive allosteric modulator at 5-HT1A receptors. However, in the same cells, we found that 100 nM THCV did not alter the rate of dissociation of 8-[3H]-OH-DPAT from specific binding sites.
These in vitro results together with evidence already published that activation of 5-HT1A receptors in vivo can ameliorate at least some signs of schizophrenia (Bantick et al., 2001; Ohno, 2011; Shimizu et al., 2013) prompted us to investigate whether THCV can produce any apparent antipsychotic effects in vivo, and if so, whether any of these effects are 5-HT1A receptor-mediated. This we did in a pharmacological model of schizophrenia in which rats are treated acutely or pretreated sub-chronically with PCP. Thus, overall, we found first that on single administration, THCV was as effective as the established atypical antipsychotic, CLZ, in reverting both positive- and negative-like signs of schizophrenia, and cognitive impairments, and second, that many of the effects of THCV that we observed in these in vivo experiments appeared to be mediated, at least in part by the 5-HT1A receptor. In rats pretreated with vehicle instead of PCP, neither THCV nor CLZ produced any effect in any of the behavioural tests that we used.
Our in vivo experiments with THCV showed that it prevented hyperlocomotion and stereotypies induced by acute PCP administration, and that in the sub-chronic PCP model, it restored social behaviours and counteracted the increase in the time spent in immobility in the FST. Moreover, THCV administration was able to normalize cognitive performance in PCP-pretreated rats. Importantly, the ability of THCV to reverse behavioural changes induced by acute PCP injections, such as stereotypies and hyperlocomotion, is suggestive of a beneficial effect on positive-like signs, whereas its efficacy in normalizing the behavioural alterations induced by sub-chronic PCP in the other tests, although not specific to schizophrenia, may predict THCV effectiveness for treating negative and cognitive symptoms of this disorder, such as memory impairment and deficits in social interaction.
We also found that pretreatment with the selective 5-HT1A antagonist, WAY100635, prevented many of the apparent beneficial effects of THCV on PCP-induced behavioural alterations without affecting any of these effects of PCP in the absence of THCV. Thus, the ability of THCV to interact in vivo with these receptors might represent one of the molecular mechanisms responsible for its antipsychotic-like properties, in line with previous reports indicating that increasing the activation of 5-HT1A receptors could be a promising strategy for antipsychotic therapy (Bantick et al., 2001; Kleven et al., 2005; Newman-Tancredi, 2010; Meltzer et al., 2012). It is noteworthy, however, that although WAY100635 completely blocked the reversal by THCV of PCP-evoked stereotypies, it did not reduce the ability of THCV to reverse hyperlocomotion induced by acute administration of PCP, indicating that this latter effect of THCV was probably not 5-HT1A receptor-mediated. These results suggest that 5-HT1A receptors are not mediating the effect of THCV on hyperlocomotion, whereas their modulation may be involved in its action on stereotyped behaviours. Our results are in line with previous findings obtained with wild-type and 5-HT1A receptor knockout mice that demonstrated that 5-HT1A receptors are implicated in the control of stereotyped movements, but not hyperlocomotion, induced by the non-competitive NMDA receptor antagonist, MK-801 (Scorza et al., 2010).
Furthermore, our findings that pretreatment with WAY100635 prevented THCV from producing any recovery from PCP-induced social withdrawal are in line with evidence obtained from other preclinical studies that 5-HT1A receptor agonism can improve PCP-induced social behaviour deficits in rodents (Depoortère et al., 2007; Bubenikova-Valesova et al., 2008b; Snigdha and Neill, 2008), and with the concept that optimized stimulation of 5-HT1A receptors is required to maximize treatment benefits with regard to some aspects of social abilities (Bruins Slot et al., 2005; Depoortère et al., 2007; Bubenikova-Valesova et al., 2008b). Despite the evidence that 5-HT1A receptor agonists can produce apparent antidepressant effects both in traditional and in modified versions of the FST (Lucki et al., 1994; De Vry, 1995; Cryan et al., 1997), the ability of THCV to reverse PCP-induced immobility in the FST was not dependent on its action at 5-HT1A receptors, as pretreatment with WAY10063 did not prevent THCV from producing this effect.
Finally, in the NOR test, WAY100635 by itself partially restored recognition memory in PCP-pretreated rats, possibly indicating that 5-HT1A receptors may be involved in the impairment of recognition memory triggered by sub-chronic PCP administration in rats. Because sub-chronic treatment with PCP has been reported to increase cortical 5-HT1A receptor binding (Choi et al., 2009) and 5-HT release (Etou et al., 1998; Martin et al., 1998; Adams and Moghaddam, 2001; Amargós-Bosch et al., 2006), it is possible that 5-HT1A antagonism could counteract PCP-induced enhancement of serotonergic stimulation, thus resulting in the observed improvement of cognitive performance. However, the fact that the reversal by THCV of PCP-induced cognitive impairment in the NOR test was not completely prevented by WAY100635 suggests that other molecular mechanisms may have contributed to the ameliorating effect of THCV in this test.
One such mechanism may be antagonism of the cannabinoid CB1 receptor by THCV. Thus, at the dose used in the present study, THCV has been reported to produce such antagonism (Pertwee, 2008) and we hypothesize that this action could contribute to the observed recovery of recognition memory induced by THCV as CB1 receptor antagonism has been extensively proven to have pro-cognitive effects (de Bruin et al., 2010; Seillier et al., 2010; Black et al., 2011; Guidali et al., 2011; Vaseghi et al., 2012). In line with previously published data, here we demonstrated that the established CB1 receptor antagonist, AM251, was effective in reversing the negative-like symptoms and cognitive impairment induced by sub-chronic PCP. Interestingly, unlike THCV, AM251 did not counteract acute PCP-evoked hyperlocomotion and stereotypies. This is in line with previous reports that after acute administration, CB1 receptor antagonists fail to show any activity in models of positive symptoms of schizophrenia (Martin et al., 2003; Thiemann et al., 2008; Black et al., 2011), suggesting that antagonism of the CB1 receptor may not reduce such symptoms. In this context, its ability to enhance activation of 5-HT1A receptors and block activation of CB1 receptors simultaneously could make THCV particularly effective as a therapeutic agent for the treatment of schizophrenia, a possibility that merits further exploration, for example, by investigating its efficacy in other animal models of schizophrenia.
It is well-established that drugs, like rimonabant, that are able to antagonize cannabinoid CB1 receptors may also show depressive-like effects, including suicidality (Beyer et al., 2010). The mechanism(s) by which rimonabant shows these side effects are not yet known, one possibility being that at high doses rimonabant behaves as an inverse agonist, rather than as a ‘neutral’ antagonist at the CB1 receptors (Pertwee, 2005). On the contrary, THCV, at the dose used in this study (2 mg·kg−1) lacks an inverse effect, thus behaving as a CB1 receptor ‘neutral’ antagonist (Pertwee, 2008). The lack of inverse effect might make THCV a safer drug than rimonabant. Moreover, it has been reported that a major limitation in the use of neuroleptics is the risk of short- and long-term side effects, such as significant weight gain and alterations in glucose metabolism (Shams and Müller, 2014). In contrast, THCV has been reported to exert anti-obesity effects in mouse models (Wargent et al., 2013), suggesting that, unlike current antipsychotics, its administration would not produce unwanted increases in body weight.
In conclusion, this investigation has shown for the first time that THCV can affect the activation of 5-HT1A receptors both in vitro and in vivo. Our in vitro results, obtained from experiments performed with both rat brainstem and human 5-HT1A CHO cell membranes, strongly support the hypothesis that THCV might modulate the activation of these receptors indirectly, rather than by binding directly to their orthosteric sites. Our in vivo experiments with rats yielded data showing that, like the established antipsychotic drug, CLZ, THCV can potently antagonize stereotyped behaviour, reduce the amount of time spent immobile in the FST and normalize hyperlocomotor activity, social behaviour and cognitive performance in PCP models of schizophrenia-like symptoms. The 5-HT1A receptor antagonist, WAY100635, abolished the ability of THCV to modify PCP-induced stereotyped and social behaviour, but it had no effect in the FST and only partially reduced the suppressant effect of THCV on PCP-induced cognitive deficiency in the NOR test, thus suggesting that these apparent beneficial effects of THCV were not mediated only by 5-HT1A receptors. We speculate that one additional action that may underlie these apparent beneficial effects is the antagonism by THCV of the cannabinoid CB1 receptor.
Acknowledgments
The authors wish to thank Mrs Lesley Stevenson for technical support and Dr John Raymond, Dr Keith Parker and Dr Ethan Russo for providing human 5-HT1A CHO cells. This research was supported by a grant from GW Pharmaceuticals to M. G. C. and R. G. P.
Glossary
Abbreviations
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CBD
cannabidiol
- CLZ
clozapine
- FST
forced swim test
- NOR
novel object recognition test
- PCP
phencyclidine
- THCV
Δ9-tetrahydrocannabivarin
Author contributions
M. G. C. designed the research study, performed the in vitro research and wrote the in vitro part of this paper. E. Z. performed the in vivo research and wrote the in vivo part of this paper. P. M. performed some of the in vitro research. D. P. helped with data analysis, in vivo data interpretation and paper writing. R. G. P. helped with data analysis, in vitro data interpretation and paper writing.
Conflict of interest
None.
References
- Adams BW, Moghaddam B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol Psychiatry. 2001;50:750–757. doi: 10.1016/s0006-3223(01)01195-7. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: Ligand-gated ion channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: Ion channels. Br J Pharmacol. 2013c;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: Enzymes. Br J Pharmacol. 2013d;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargós-Bosch M, López-Gil X, Artigas F, Adell A. Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int J Neuropsychopharmacol. 2006;9:565–573. doi: 10.1017/S1461145705005900. [DOI] [PubMed] [Google Scholar]
- Anavi-Goffer S, Baillie G, Irving AJ, Gertsch J, Greig IR, Pertwee RG, et al. Modulation of L-alpha-lysophosphatidylinositol/GPR55 mitogen-activated protein kinase (MAPK) signaling by cannabinoids. J Biol Chem. 2012;287:91–104. doi: 10.1074/jbc.M111.296020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantick RA, Deakin JF, Grasby PM. The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics? J Psychopharmacol. 2001;15:37–46. doi: 10.1177/026988110101500108. [DOI] [PubMed] [Google Scholar]
- Barrondo S, Sallés J. Allosteric modulation of 5-HT(1A) receptors by zinc: binding studies. Neuropharmacology. 2009;56:455–462. doi: 10.1016/j.neuropharm.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Dwyer JM, Piesla MJ, Platt BJ, Shen R, Rahman Z, et al. Depression-like phenotype following chronic CB1 receptor antagonism. Neurobiol Dis. 2010;39:148–155. doi: 10.1016/j.nbd.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Black MD, Stevens RJ, Rogacki N, Featherstone RE, Senyah Y, Giardino O, et al. AVE1625, a cannabinoid CB1 receptor antagonist, as a co-treatment with antipsychotics for schizophrenia: improvement in cognitive function and reduction of antipsychotic-side effects in rodents. Psychopharmacology (Berl) 2011;215:149–163. doi: 10.1007/s00213-010-2124-0. [DOI] [PubMed] [Google Scholar]
- Bolognini D, Costa B, Maione S, Comelli F, Marini P, Di Marzo V, et al. The plant cannabinoid D9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol. 2010;160:677–687. doi: 10.1111/j.1476-5381.2010.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognini D, Rock EM, Cluny NL, Cascio MG, Limebeer CL, Duncan M, et al. Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br J Pharmacol. 2013;168:1456–1470. doi: 10.1111/bph.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin NM, Prickaerts J, Lange JH, Akkerman S, Andriambeloson E, de Haan M, et al. SLV330, a cannabinoid CB1 receptor antagonist, ameliorates deficits in the T-maze, object recognition and Social Recognition Tasks in rodents. Neurobiol Learn Mem. 2010;93:522–531. doi: 10.1016/j.nlm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Bruins Slot LA, Kleven MS, Newman-Tancredi A. Effects of novel antipsychotics with mixed D(2) antagonist/5-HT(1A) agonist properties on PCP-induced social interaction deficits in the rat. Neuropharmacology. 2005;49:996–1006. doi: 10.1016/j.neuropharm.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008a;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Stuchlik A, Svoboda J, Bures J, Vales K. Risperidone and ritanserin but not haloperidol block effect of dizocilpine on the active allothetic place avoidance task. Proc Natl Acad Sci U S A. 2008b;105:1061–1066. doi: 10.1073/pnas.0711273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent alpha(2)-adrenoceptor agonist and moderately potent 5HT(1A) receptor antagonist. Br J Pharmacol. 2010;159:129–141. doi: 10.1111/j.1476-5381.2009.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YK, Snigdha S, Shahid M, Neill JC, Tarazi FI. Subchronic effects of phencyclidine on dopamine and serotonin receptors: implications for schizophrenia. J Mol Neurosci. 2009;38:227–235. doi: 10.1007/s12031-009-9204-9. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Redmond AM, Kelly JP, Leonard BE. The effects of the 5-HT1A agonist flesinoxan, in three paradigms for assessing antidepressant potential in the rat. Eur Neuropsychopharmacol. 1997;7:109–114. doi: 10.1016/s0924-977x(96)00391-4. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Orlando P, Moriello AS, Aviello G, Stott C, Izzo AA, et al. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol (Oxf) 2012;204:255–266. doi: 10.1111/j.1748-1716.2011.02338.x. [DOI] [PubMed] [Google Scholar]
- De Vry J. 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacology (Berl) 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- Dennis I, Whalley BJ, Stephens GJ. Effects of D9-tetrahydrocannabivarin on S-35 GTP gamma S binding in mouse brain cerebellum and piriform cortex membranes. Br J Pharmacol. 2008;154:1349–1358. doi: 10.1038/bjp.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortère R, Auclair AL, Bardin L, Bruins Slot L, Kleven MS, Colpaert F, et al. , a compound with D2/D3 antagonist, 5-HT 1A agonist and D4 partial agonist properties. III. Activity in models of cognition and negative symptoms. Br J Pharmacol. 2007;151:266–277. doi: 10.1038/sj.bjp.0707160. F15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etou K, Kuroki T, Kawahara T, Yonezawa Y, Tashiro N, Uchimura H. Ceruletide inhibits phencyclidine-induced dopamine and serotonin release in rat prefrontal cortex. Pharmacol Biochem Behav. 1998;61:427–434. doi: 10.1016/s0091-3057(98)00128-2. [DOI] [PubMed] [Google Scholar]
- Guidali C, Viganò D, Petrosino S, Zamberletti E, Realini N, Binelli G, et al. Cannabinoid CB1 receptor antagonism prevents neurochemical and behavioural deficits induced by chronic phencyclidine. Int J Neuropsychopharmacol. 2011;14:17–28. doi: 10.1017/S1461145710000209. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Barret-Grévoz C, Bruins Slot L, Newman-Tancredi A. Novel antipsychotic agents with 5-HT1A agonist properties: role of 5-HT1A receptor activation in attenuation of catalepsy induction in rats. Neuropharmacology. 2005;49:135–143. doi: 10.1016/j.neuropharm.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Large CH. Do NMDA receptor antagonist models of schizophrenia predict the clinical efficacy of antipsychotic drugs? J Psychopharmacol. 2007;21:283–301. doi: 10.1177/0269881107077712. [DOI] [PubMed] [Google Scholar]
- Lucki I, Singh A, Kreiss DS. Antidepressant-like behavioral effects of serotonin receptor agonists. Neurosci Biobehav Rev. 1994;18:85–95. doi: 10.1016/0149-7634(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Ma YL, Weston SE, Whalley BJ, Stephens GJ. The phytocannabinoid D9-tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. Br J Pharmacol. 2008;154:204–215. doi: 10.1038/bjp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Carlsson ML, Hjorth S. Systemic PCP treatment elevates brain extracellular 5-HT: a microdialysis study in awake rats. Neuroreport. 1998;9:2985–2988. doi: 10.1097/00001756-199809140-00012. [DOI] [PubMed] [Google Scholar]
- Martin RS, Secchi RL, Sung E, Lemaire M, Bonhaus DW, Hedley LR, et al. Effects of cannabinoid receptor ligands on psychosis-relevant behavior models in the rat. Psychopharmacology (Berl) 2003;165:128–135. doi: 10.1007/s00213-002-1240-x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Massey BW, Horiguchi M. Serotonin receptors as targets for drugs useful to treat psychosis and cognitive impairment in schizophrenia. Curr Pharm Biotechnol. 2012;13:1572–1586. doi: 10.2174/138920112800784880. [DOI] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Neill JC, Harte MK, Haddad PM, Lydall ES, Dwyer DM. Acute and chronic effects of NMDA receptor antagonists in rodents, relevance to negative symptoms of schizophrenia: a translational link to humans. Eur Neuropsychopharmacol. 2014;24:822–835. doi: 10.1016/j.euroneuro.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A. The importance of 5-HT1A receptor agonism in antipsychotic drug action: rationale and perspectives. Curr Opin Investig Drugs. 2010;11:802–812. [PubMed] [Google Scholar]
- Ohno Y. Therapeutic role of 5-HT1A receptors in the treatment of schizophrenia and Parkinson's disease. CNS Neurosci Ther. 2011;17:58–65. doi: 10.1111/j.1755-5949.2010.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci. 2005;76:1307–1324. doi: 10.1016/j.lfs.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A, Stevenson LA, Ross RA, Varvel SA, Lichtman AH, et al. The psychoactive plant cannabinoid, D9-tetrahydrocannabinol, is antagonized by D8- and D9-tetrahydrocannabivarin in mice in vivo. Br J Pharmacol. 2007;150:586–594. doi: 10.1038/sj.bjp.0707124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MR, Baillie GL, Thomas A, Stevenson LA, Easson M, Goodwin R, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495. doi: 10.1124/mol.105.016162. [DOI] [PubMed] [Google Scholar]
- Realini N, Vigano' D, Guidali C, Zamberletti E, Rubino T, Parolaro D. Chronic URB597 treatment at adulthood reverted most depressive-like symptoms induced by adolescent exposure to THC in female rats. Neuropharmacology. 2011;60:235–243. doi: 10.1016/j.neuropharm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Rock EM, Goodwin JM, Limebeer CL, Breuer A, Pertwee RG, Mechoulam R, et al. Interaction between non-psychotropic cannabinoids in marihuana: effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology (Berl) 2011;215:505–512. doi: 10.1007/s00213-010-2157-4. [DOI] [PubMed] [Google Scholar]
- Rock EM, Bolognini D, Limebeer CL, Cascio MG, Anavi-Goffer S, Fletcher PJ, et al. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT1A somatodendritic autoreceptors in the dorsal raphe nucleus. Br J Pharmacol. 2012;165:2620–2634. doi: 10.1111/j.1476-5381.2011.01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA, Gibson TM, Stevenson LA, Saha B, Crocker P, Razdan RK, et al. Structural determinants of the partial agonist-inverse agonist properties of 6′-azidohex-2′-yne-Δ8-tetrahydrocannabinol at cannabinoid receptors. Br J Pharmacol. 1999b;128:735–743. doi: 10.1038/sj.bjp.0702836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Effects of continuous D-amphetamine and phencyclidine administration on social behaviour, stereotyped behaviour, and locomotor activity in rats. Neuropsychopharmacology. 1998;19:18–25. doi: 10.1016/S0893-133X(97)00200-5. [DOI] [PubMed] [Google Scholar]
- Scorza MC, Castañé A, Bortolozzi A, Artigas F. Clozapine does not require 5-HT1A receptors to block the locomotor hyperactivity induced by MK-801 Clz and MK-801 in KO1A mice. Neuropharmacology. 2010;59:112–120. doi: 10.1016/j.neuropharm.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int J Neuropsychopharmacol. 2010;13:373–386. doi: 10.1017/S146114570999023X. [DOI] [PubMed] [Google Scholar]
- Shams TA, Müller DJ. Antipsychotic induced weight gain: genetics, epigenetics, and biomarkers reviewed. Curr Psychiatry Rep. 2014;16:473–480. doi: 10.1007/s11920-014-0473-9. [DOI] [PubMed] [Google Scholar]
- Shimizu S1, Mizuguchi Y, Ohno Y. Improving the treatment of schizophrenia: role of 5-HT receptors in modulating cognitive and extrapyramidal motor functions. CNS Neurol Disord Drug Targets. 2013;12:861–869. doi: 10.2174/18715273113129990088. [DOI] [PubMed] [Google Scholar]
- Snigdha S, Neill JC. Improvement of phencyclidine-induced social behaviour deficits in rats: involvement of 5-HT1A receptors. Behav Brain Res. 2008;191:26–31. doi: 10.1016/j.bbr.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Thiemann G, Di Marzo V, Molleman A, Hasenöhrl RU. The CB(1) cannabinoid receptor antagonist AM251 attenuates amphetamine-induced behavioural sensitization while causing monoamine changes in nucleus accumbens and hippocampus. Pharmacol Biochem Behav. 2008;89:384–391. doi: 10.1016/j.pbb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, et al. Evidence that the plant cannabinoid D9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist. Br J Pharmacol. 2005;146:917–926. doi: 10.1038/sj.bjp.0706414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaseghi G, Rabbani M, Hajhashemi V. The CB(1) receptor antagonist, AM281, improves recognition loss induced by naloxone in morphine withdrawal mice. Basic Clin Pharmacol Toxicol. 2012;111:161–165. doi: 10.1111/j.1742-7843.2012.00881.x. [DOI] [PubMed] [Google Scholar]
- Wargent ET, Zaibi MS, Silvestri C, Hislop DC, Stocker CJ, Stott CG, et al. The cannabinoid Δ(9)-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutr Diabetes. 2013;27:e68. doi: 10.1038/nutd.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamberletti E, Prini P, Speziali S, Gabaglio M, Solinas M, Parolaro D, et al. Gender-dependent behavioral and biochemical effects of adolescent delta-9-tetrahydrocannabinol in adult maternally deprived rats. Neuroscience. 2012;204:245–257. doi: 10.1016/j.neuroscience.2011.11.038. [DOI] [PubMed] [Google Scholar]