Abstract
Background and Purpose
The important pathological consequences of ischaemic heart disease arise from the detrimental effects of the accumulation of long-chain acylcarnitines in the case of acute ischaemia-reperfusion. The aim of this study is to test whether decreasing the L-carnitine content represents an effective strategy to decrease accumulation of long-chain acylcarnitines and to reduce fatty acid oxidation in order to protect the heart against acute ischaemia–reperfusion injury.
Key Results
In this study, we used a novel compound, 4-[ethyl(dimethyl)ammonio]butanoate (Methyl-GBB), which inhibits γ-butyrobetaine dioxygenase (IC50 3 μM) and organic cation transporter 2 (OCTN2, IC50 3 μM), and, in turn, decreases levels of L-carnitine and acylcarnitines in heart tissue. Methyl-GBB reduced both mitochondrial and peroxisomal palmitate oxidation rates by 44 and 53% respectively. In isolated hearts treated with Methyl-GBB, uptake and oxidation rates of labelled palmitate were decreased by 40%, while glucose oxidation was increased twofold. Methyl-GBB (5 or 20 mg·kg−1) decreased the infarct size by 45–48%. In vivo pretreatment with Methyl-GBB (20 mg·kg−1) attenuated the infarct size by 45% and improved 24 h survival of rats by 20–30%.
Conclusions and Implications
Reduction of L-carnitine and long-chain acylcarnitine content by the inhibition of OCTN2 represents an effective strategy to protect the heart against ischaemia–reperfusion-induced damage. Methyl-GBB treatment exerted cardioprotective effects and increased survival by limiting long-chain fatty acid oxidation and facilitating glucose metabolism.
Tables of Links
| LIGANDS |
|---|
| L-Carnitine |
| FABP, fatty acid binding protein |
| Glucose |
| Lactic acid |
| Palmitic acid |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Ischaemic heart disease (IHD) is a major medical problem causing disability and death for millions of people annually (Lavie et al., 2009). The important pathological consequences of IHD arise from the detrimental effects of the accumulation of long-chain fatty acids (LCFAs) in the case of acute ischaemia-reperfusion (Wang and Lopaschuk, 2007; Liepinsh et al., 2013b). Therefore, pharmacological intervention that targets LCFA accumulation has been suggested for the development of novel treatment strategies to improve the clinical outcomes of patients with IHD (Horowitz et al., 2010; Lopaschuk et al., 2010). The main advantages of reduced LCFA oxidation are the reduction of energy waste through mitochondrial uncoupling induced by LCFA overload as well as the reduction of direct damage by LCFA metabolites on glucose metabolism and insulin signalling (Zhang et al., 2011). In addition, pharmacological manipulation of energy metabolism does not directly influence the haemodynamic parameters of the heart and could be combined with existing cardiovascular therapies.
LCFAs supply a major part of the energy that is required for heart function (Wang and Lopaschuk, 2007). However, under conditions of ischaemia-reperfusion, a reduced use of fatty acids and a greater use of glucose oxidation as the energy source lead to better functional recovery of the myocardium. Carnitine palmitoyltransferase I (CPT I) is the most important rate-limiting step in LCFA uptake and oxidation by mitochondria (Sebastian et al., 2009; Shriver and Manchester, 2011). L-carnitine is a cofactor required by CPT I-dependent LCFA transport, and a decreased cardiac content of L-carnitine limits LCFA oxidation in heart mitochondria (Liepinsh et al., 2011a; Kuka et al., 2012). Decreased availability of L-carnitine can be achieved by the administration of 3-(2,2,2-trimethylhydrazinium)-propionate (meldonium) (Liepinsh et al., 2011a,b). As a result of decreased L-carnitine content and inhibited CPT I-dependent LCFA oxidation, an increase in glucose oxidation was observed. Thus, several studies demonstrate that a long-term decrease in L-carnitine availability is beneficial for the regulation of energy metabolism and treatment of heart diseases and atherosclerosis (Liepinsh et al., 2006; 2009; 2011c,,; Vilskersts et al., 2009; Kuka et al., 2012)
In this study, we describe a novel compound, 4-[ethyl(dimethyl)ammonio]butanoate (Methyl-GBB) (Kalvinsh et al., 2011), which is more potent than meldonium as an inhibitor of γ-butyrobetaine dioxygenase (BBOX) and the organic cation transporter 2 (OCTN2) and significantly decreases L-carnitine content in heart tissue. To determine the effects of Methyl-GBB on LCFA and glucose oxidation, we measured the oxidation rate of labelled palmitate and glucose in isolated hearts. Taking into account previous concerns about heart functional alterations in models of genetically or pharmacologically decreased fatty acid metabolism, we performed measurements of heart function in isolated heart perfusions along with in vivo echocardiography. To evaluate the cardioprotective properties of Methyl-GBB, we measured infarct size (IS) in isolated hearts and in vivo. The purpose of the current study was to test whether decreasing L-carnitine content represents an effective strategy to protect the heart against the injury induced by acute ischaemia–reperfusion.
Methods
Animals and treatment
All animal care and experimental procedures complied with the guidelines of the European Community and local laws and policies and were approved by the Latvian Animal Protection Ethical Committee, Food and Veterinary Service, Riga, Latvia. Studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010). A total of 92 animals were used in the experiments described here.
Eighty male Wistar rats weighing 250–300 g were housed under standard conditions (21–23°C, 12 h light-dark cycle) with unlimited access to food (R70 diet, Lantmännen Lantbruk, Malmö, Sweden) and water. Twelve male ICR mice weighing 25–28 g were housed under standard conditions (21–23°C, 12 h light-dark cycle) with unlimited access to food (R70 diet) and water. Animals (rats and mice) were obtained from the Laboratory of Experimental animals, Riga Stradins University (Riga, Latvia). The rats were adapted to local conditions for 2 weeks before the start of treatment. Methyl-GBB at doses of 1, 5, 10 and 20 mg·kg−1 was administered p.o. by gavage daily for 3–14 days. Meldonium was administered at a dose of 100 mg·kg−1 for 14 days. Control rats received water. The rats were used for experiments 24 h after the last administration.
Group sizes were selected according to previous experience or pilot experiments. According to the animal welfare ‘reduction’ principle, we used the minimal number of animals to reach statistical significance. Rats were randomized before the beginning of treatment. In vivo and ex vivo myocardial infarction experiments were performed without knowledge of the treatments, by both surgeon and researcher who measured IS by planimetric analysis.
Measurement of levels of L-carnitine, Methyl-GBB and acylcarnitines by UPLC/MS/MS
Determination of L-carnitine and Methyl-GBB in heart tissue, plasma and urine samples was performed by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC/MS/MS) using the positive ion electrospray mode. The previously described UPLC/MS/MS method (Dambrova et al., 2008) was slightly modified. Determination of the acylcarnitines in the mitochondrial and heart tissue samples was performed by UPLC/MS/MS method (Makrecka et al., 2014). The sample preparation was performed as previously described (Blachnio-Zabielska et al., 2011) with modifications (Liepinsh et al., 2013b). The concentrations of acylcarnitines were measured using a 7-point standard curve of palmitoylcarnitine (10–1000 ng·mL−1).
Mitochondrial and peroxisomal fatty acid oxidation (FAO)
The mitochondrial and peroxisomal rates of palmitate oxidation were determined as described previously (Degrace et al., 2004), with the exception being that corresponding concentrations of L-carnitine were used. The reaction was started by the addition of 100 μM palmitate (supplemented with 1 μCi [1-14C]palmitate, specific activity 60 Ci·mmol−1) bound to fatty-acid-free BSA. After 60 min of incubation at 37°C, the samples were treated with 10% perchloric acid. To measure peroxisomal palmitate oxidation, the mitochondrial β-oxidation activity was inhibited by preincubating the samples with 75 μM antimycin, 10 μM rotenone and 250 μM KCN. The rate of peroxisomal palmitate oxidation was calculated from the radioactivity of the acid-soluble products and was expressed as pmol of palmitate per min per mg protein. The rate of mitochondrial palmitate oxidation was expressed as the difference between total palmitate oxidation (without inhibitors) and peroxisomal oxidation rate.
Measurements of substrate oxidation in rat isolated heart
The rates of radiolabelled glucose, lactate and palmitate oxidation were measured in different sets of hearts from Wistar rats, as previously described (Lopaschuk and Barr, 1997), with certain modifications indicated below. The energy metabolism measurements were performed according to the Langendorff perfusion technique. The hearts were retrogradely perfused (perfusion pressure 70 mmHg) with Krebs-Henseleit (KH) buffer solution ‘high insulin’ supplemented with 10 mM glucose, 0.3 mM sodium palmitate bound to 2% BSA, 2 mM lactate, 0.2 mM pyruvate and 3 ng·mL−1 insulin, or with KH buffer solution ‘low insulin’ supplemented with 5 mM glucose, 1.2 mM sodium palmitate bound to 2% BSA, 1 mM lactate, 0.1 mM pyruvate and 0.3 ng·mL−1 insulin. After 10 min, the perfusate was switched to the respective (‘high’ or ‘low insulin’) oxygenated radiolabelled KH buffer solution for 10 min at a constant flow of 10 mL·min−1. Then the hearts were switched back to the respective non-labelled KH perfusion solution and perfused for 10 min at the constant flow prior to the global ischaemia. Global no-flow ischaemia was induced for 20 min followed by 60 min reperfusion. At the end of the reperfusion, the perfusate was switched to the respective (‘high’ or ‘low insulin’) oxygenated radiolabelled KH buffer solution for 10 min at a constant flow of 10 mL·min–1. Glucose and lactate oxidation rates were determined by measuring the 14CO2 released from the metabolism of [U-14C]glucose (specific activity, 300 mCi·mmol−1) or [1-14C] lactate (specific activity, 55 mCi·mmol−1) respectively. Palmitate oxidation was determined by measuring 3H2O released from [9,10-3H]palmitate (specific activity, 60 Ci·mmol−1). Palmitate uptake in the heart was calculated from the amount of radiolabelled palmitate oxidized during the perfusion and the amount found in the cardiac tissue at the end of the perfusion.
Measurements of palmitate uptake and oxidation in vivo
To determine the palmitate uptake and oxidation in vivo, 1 μCi of [9,10-3H]palmitate (specific activity, 60 Ci·mmol−1) per 25 g of body weight was administered intravenously to the mice. After 10 min, the mice were killed by cervical dislocation, and heart and muscle tissue homogenates (1:5, w/v in Milli-Q water) were prepared. Samples were treated in the same manner as in the isolated heart experiment.
Mitochondrial respiration measurements
The mitochondria were isolated from cardiac tissues, and mitochondrial respiration was measured at 37°C using a Clark-type electrode (Microelectrodes Inc., Bedford, MA, USA) as previously described (Kuka et al., 2012). To determine the L-carnitine-dependent oxidation of fatty acids, 10 μM palmitoyl-CoA and specified L-carnitine concentrations were used for the respiration measurements. Palmitoylcarnitine (10 μM) was used as a substrate to determine L-carnitine-independent FAO.
To assess mitochondrial function after ischaemia-reperfusion injury, rat isolated hearts were subjected to 20 min of no-flow ischaemia following 120 min reperfusion, and then cardiac fibres were prepared as previously described (Kuka et al., 2012). Respiration rates of cardiac fibres were measured at 37°C with Clark-type electrode using 6 mM pyruvate/6 mM malate as substrates. ADP-stimulated respiration (state 3) was achieved by adding 0.2 mM ADP. To determine the uncoupling of oxidative phosphorylation, state 4 respiration was measured after the addition of 5 μM carboxyatractyloside.
mRNA isolation and quantitative RT-PCR
Total RNA from heart tissue in normoxia and after 30 min of no-flow ischaemia followed by 60 min reperfusion was isolated using the TRI Reagent (Sigma, St. Louis, MO, USA) according to the manufacturer's protocol. First-strand cDNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, Foster City, CA, USA) following the manufacturer's instructions. Quantitative RT-PCR analysis for genes was performed by mixing synthesized cDNA, forward and reverse primers specific for long-chain fatty acid CoA synthetase (ACSL), CPT1A, CPT1B, acyl-CoA oxidase (ACOX), fatty acid translocase (CD36), FABP and β-actin, and SYBR® Green Master Mix (Applied Biosystems), and run in the Applied Biosystems Prism 7500 according to the manufacturer's protocol. The transcript levels for the constitutive housekeeping gene product β-actin were quantitatively measured for each sample, and RT-PCR data were reported as the number of transcripts per number of β-actin mRNA molecules. To avoid genomic DNA contamination, the primers were designed to span an intron. The primer sequences used for the quantitative RT-PCR analysis are listed in Supporting Information Table S1.
Rat isolated heart infarction study
The infarction was performed according to the Langendorff technique as described previously (Kuka et al., 2012), with some modifications. For the infarction studies, the hearts were perfused with KH buffer solution at a constant perfusion pressure of 60 mmHg. The isolated hearts were allowed to stabilize for 20 min, and the left anterior descending coronary artery (LAD) was subsequently occluded for 40 min followed by 120 min of reperfusion. Occlusion was confirmed by the 40% drop in coronary flow. The IS was determined as described previously (Kuka et al., 2012; Liepinsh et al., 2013a). Briefly, at the end of the reperfusion, the LAD was re-occluded, and the heart was perfused with 0.1% methylene blue dissolved in KH buffer solution. Afterwards, the ventricles of the heart were transversely cut into 2-mm-thick slices stained by 2,3,5-triphenyltetrazolium chloride (TTC) and photographed. Computerized planimetric analyses of the stained ventricle slice photographs were performed using Image-Pro Plus v6.3 (Media Cybernetics) software to determine the area at risk (AAR) and the area of necrosis (AN), and each area was expressed as a percentage of the left ventricle area. The obtained values were then used to calculate the IS as a percentage of the risk area, according to the formula IS = AN/AAR × 100%.
Heart infarction study in vivo
Before evaluation of Methyl-GBB in our experimental model of LAD occlusion and reperfusion in vivo, we validated this model. In the control group, the IS values were more variable after 20 min, than after 30 min of occlusion and we therefore chose a 30 min occlusion period for further experiments. As mentioned by Hearse et al. (1988), at least several hours are necessary for development of necrosis after occlusion. Therefore, in our experiments, we chose to reperfuse 24h after occlusion to ensure that all the damaged tissue was measured. Several studies have used the same procedure: 30 min occlusion of the LAD followed by 24 h reperfusion (Deuchar et al., 2007; Mohan et al., 2009; Ertracht et al., 2011). In contrast to Hearse et al. (1988), we observed very minor wall thinning after 24 h of reperfusion in both control and treatment groups. We could confirm the positive staining for leukocytes (with TTC) in the AN, as observed by Hearse. However, in properly processed high-quality digital photomicrographs, this staining is pale pink to pink and differs substantially from viable red to dark red tissue (Liepinsh et al., 2013a).
Before the induction of anaesthesia, experimental animals received s.c. injections of atropine sulfate (50 μg·kg−1), tramadol (20 mg·kg−1) and sodium benzylpenicillin (150 mg·kg−1).
Anaesthesia was induced by i.p. injection of a mixture of ketamine and xylazine (100 and 10 mg·kg−1 respectively). After the loss of nociceptive reflexes, the experimental animals were intubated using 16G intravenous catheters (Venflon, Becton Dickinson, Franklin Lakes, NJ, USA), ventilated with room air by a UB 7025 rodent ventilator (Hugo Sachs Elektronik-Harvard Apparatus GmbH, March-Hugstetten, Germany) at a tidal volume of 1.5 mL/100 g animal and a rate of 55 strokes per minute. The chest was opened between ribs 5 and 6, a 5-0 polypropylene thread (SURGIPRO™ II; Covidien, Dublin, Ireland) was placed around the left coronary artery at the level of the left atrium, and a polypropylene ligature was passed through a piece of plastic tubing. The ECG was recorded from a standard lead (II) using PowerLab systems (ADInstruments, Oxford, UK). After surgery, the experimental animals were allowed to stabilize for 10 min. The coronary artery was occluded by applying tension to the plastic tube-polypropylene string arrangement. Successful occlusion was confirmed by ischaemia-induced alterations in the ECG. After 30 min of occlusion, reperfusion was initiated by removing the clamp from the plastic tube. Afterwards, the chest was closed using 3-0 silk threads (SOFSILK II; Covidien), and the skin was closed using 4-0 nylon threads (MONOSOF; Covidien). When the animals started to breathe spontaneously, they were extubated.
Twenty-four hours following the induction of reperfusion, the experimental animals received an i.p. injection of sodium pentobarbital (60 mg·kg−1) and heparin (1000 IU·kg−1). After the onset of anaesthesia, the heart was excised, connected via the aorta to the Langendorff apparatus and washed with KH buffer solution. The coronary artery was re-occluded; staining and quantification of necrotic tissues were performed as in an isolated heart infarction set-up.
Data analysis
Results are presented as the mean ± SEM. Statistically significant differences in the mean values were evaluated using an unpaired Student's t-test, chi-square test or a one-way anova with Tukey's test. The differences were considered significant when P < 0.05. The data were analysed using GraphPad Prism 3 statistical software (GraphPad Inc., La Jolla, CA, USA).
Materials
Methyl-GBB was synthesized according to the method described previously (Kalvinsh et al., 2011; Tars et al., 2014). Meldonium was a kind gift from JSC Grindeks, Riga, Latvia. [U-14C]glucose, [1-14C]palmitate, [9,10-3H]palmitate and [1-14C]lactate were supplied by American Radiolabeled Chemicals, St. Louis, USA. Antimycin, carboxyatractyloside, L-carnitine, rotenone and sodium palmitate were from Sigma-Aldrich, St. Louis, USA. Palmitoylcarnitine and palmitoyl-CoA were from Larodan, Malmo, Sweden.
Results
Content of L-carnitine in heart, blood plasma and urine
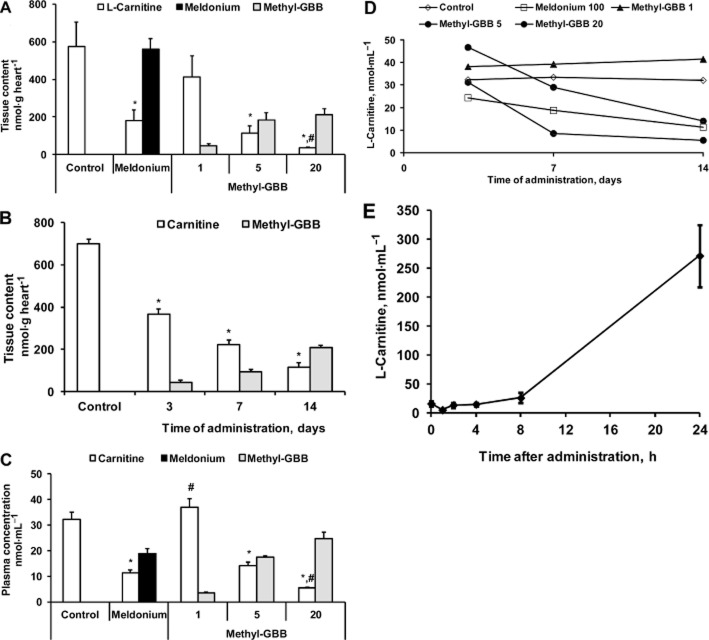
The effects of Methyl-GBB on the L-carnitine system can be explained by inhibition of BBOX and OCTN2 (Table 2013a). In comparison to meldonium, Methyl-GBB was eight times more potent than meldonium at inhibiting L-carnitine synthesis by BBOX (IC50 3 μM vs. 26 μM) and 20 times more potent at inhibiting L-carnitine transport by OCTN2 (IC50 3 μM vs. 62 μM) (Liepinsh et al., 2014). The plasma concentrations of Methyl-GBB reached their highest values after 3–7 days of treatment and remained unchanged for the remainder of the 14 day treatment (Supporting Information Fig. S2). After 3 days of the treatment at doses of 5 and 20 mg·kg−1, plasma concentration of Methyl-GBB exceeded 10 μM and according to measurements in vitro (Supporting Information Fig. S1) almost completely inhibited L-carnitine transport by OCTN2. Methyl-GBB not only inhibits carnitine transport by OCTN2, but is also itself transported by OCTN2. Therefore, a high affinity of methyl-GBB for OCTN2 ensures that methyl-GBB has excellent bioavailability in tissues. After the 14 day treatment at doses of 1, 5 and 20 mg·kg−1, Methyl-GBB concentrations in the heart were 47, 185 and 221 nmol·g−1 respectively (Figure 1A). The concentration of Methyl-GBB in the heart increased in a time-dependent manner (Figure 1B), reaching a maximum after 14 days of treatment. We previously found that to induce significant changes in energy metabolism, the L-carnitine content in heart tissue should be decreased by at least 60% (Kuka et al., 2012). As shown in Figure 1A, treatment with meldonium for 2 weeks at a dose of 100 mg·kg−1 induced a significant decrease in the L-carnitine heart content by 75%. In comparison, Methyl-GBB treatment at 1, 5 and 20 mg·kg−1 decreased the L-carnitine content in the heart by 42, 84 and 95% respectively.
Figure 1.
Content of L-carnitine, meldonium and Methyl-GBB in heart (A) and blood plasma (C), after 14 days of treatment by meldonium (100 mg·kg−1) or Methyl-GBB (1, 5 and 20 mg·kg−1). Content of L-carnitine and Methyl-GBB in heart tissue (B) and blood plasma (D,) after 3, 7 and 14 days of treatment by Methyl-GBB at a dose of 5 mg·kg–1. Effect of Methyl-GBB (5 mg·kg−1) on the L-carnitine content in urine after single administration (E). Data shown are the means ± SEM of 5–10 animals. *P < 0.05, significantly different from control group; #P < 0.05, significantly different from meldonium group; Tukey's test.
Table 1.
Structural formulas and corresponding IC50 values of inhibitors of BBOX and OCTN2
| Substance | Formula | BBOX IC50, μM | OCTN2 IC50, μM |
|---|---|---|---|
| GBB | 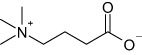 |
– | 3.9 ± 0.1 |
| Meldonium | 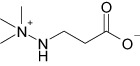 |
26 ± 2 | 62 ± 5 |
| Methyl-GBB | 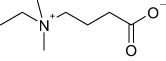 |
2.8 ± 0.6 | 3 ± 0.3 |
To study the time-dependent effects of Methyl-GBB on L-carnitine content, we measured the concentration of L-carnitine in plasma samples after 1, 3, 7 and 14 day administrations of Methyl-GBB at a dose of 5 mg·kg−1. As shown in Figure 1B, Methyl-GBB decreased L-carnitine content in the heart in a time-dependent manner. Meanwhile, after short-term (up to 7 days) treatment with Methyl-GBB, the blood plasma concentration of L-carnitine did not correlate with the changes in L-carnitine content in the heart (Figure 1B and D). Thus, in contrast to the effects of Methyl-GBB on L-carnitine content in the heart, a maximal reduction of plasma L-carnitine concentration was observed only after 14 days of the treatment. Unexpectedly, Methyl-GBB (1 mg·kg−1) did not decrease L-carnitine concentration in blood plasma even after 14 days of treatment, while in heart tissue, a significant reduction in L-carnitine content was observed (Figure 1A and C). Furthermore, as a result of the OCTN2 inhibition, Methyl-GBB significantly increased the L-carnitine concentration in urine (Figure 1E).
Content of acylcarnitines in heart
In Methyl-GBB-treated hearts, the acylcarnitine content was significantly decreased both in heart and mitochondria (Figure 2A and B). Methyl-GBB treatment induced up to 50-fold reduction in the content of long-chain acylcarnitines. Thus, the link between decrease in the content of L-carnitine and preserved mitochondrial function during reperfusion is related to decreased content of long-chain acylcarnitines.
Figure 2.
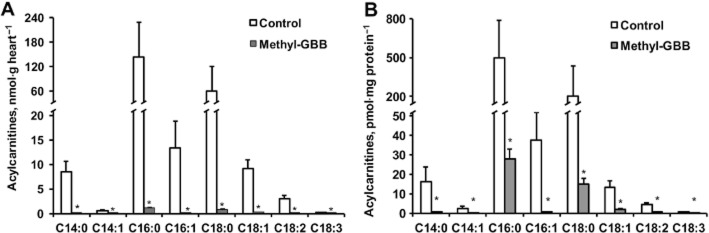
Effects of long-term treatment with Methyl-GBB (10 mg·kg−1) on cardiac (A) and mitochondrial (B) content of acylcarnitine. Data shown are the means ± SEM of at least five animals. *P < 0.05, significantly different from control group; Student's t-test.
LCFAs and glucose oxidation
To test the changes in energy metabolism induced by Methyl-GBB treatment, we determined the amount of FAO in the isolated heart and its organelles. Initially, we tested the effects of Methyl-GBB on the metabolism of LCFAs in mitochondria and peroxisomes. The treatment with Methyl-GBB significantly reduced both mitochondrial and peroxisomal palmitate oxidation rates by 44 and 53% respectively (Figure 3A). To elucidate the role of decreased L-carnitine content in the regulation of FAO, we measured both the L-carnitine-dependent and L-carnitine-independent mitochondrial respiration rates. After 14 days of Methyl-GBB treatment, the L-carnitine-dependent mitochondrial respiration rate with palmitoyl-CoA was decreased by 27% (Figure 3B), but the treatment had no effect on the L-carnitine-independent mitochondrial respiration rate with palmitoylcarnitine (Figure 3C). We then determined the effects of Methyl-GBB on the uptake and oxidation of LCFA in an isolated heart. In contrast to the previously observed effects of meldonium (Liepinsh et al., 2013b), the oxidation rate of labelled palmitate in the isolated heart was decreased by 40% (Figure 4A). An increase in LCFA oxidation was observed after reperfusion in Methyl-GBB-treated group (Figure 4A). In addition, the Methyl-GBB treatment reduced the labelled palmitate concentration in the mitochondria and, thus, also reduced the risk of LCFA accumulation (Figure 4B). Additionally, we measured the uptake and metabolism of labelled palmitate in mice in vivo (Figure 4C). In hearts and skeletal muscle from Methyl-GBB-treated mice, we observed a twofold decrease in the labelled palmitate uptake and oxidation rate, which coincides with the results observed in the isolated heart and organelles.
Figure 3.
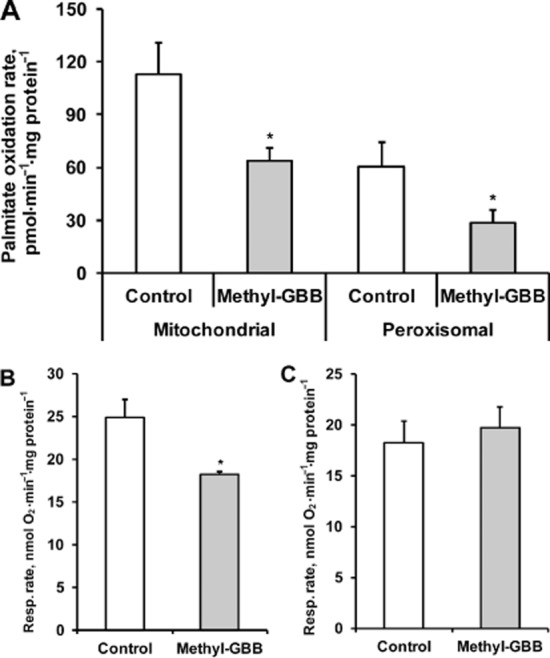
Effects of long-term treatment with Methyl-GBB (10 mg·kg−1) on [1-14C]-palmitate oxidation in isolated mitochondria and peroxisomes (A) and mitochondrial L-carnitine dependent respiration on 10 μM palmitoyl-CoA (B) and L-carnitine independent oxidation on 10 μM palmitoylcarnitine (C). Data shown are the means ± SEM of five to six animals. *P < 0.05, significantly different from control group; Student's t-test.
Figure 4.

Effects of long-term treatment with Methyl-GBB (10 mg·kg−1) on [9,10-3H]-palmitate oxidation in isolated heart (A). In (B), mitochondrial [9,10-3H]-palmitate content is shown. In (C), results shown are for free palmitate (FA) and for labelled acid-soluble metabolites in heart and skeletal muscles in vivo. Data shown are the means ± SEM of five to six animals. *P < 0.05, significantly different from control group; Student's t-test.
Treatment with Methyl-GBB induced a reduction in LCFA oxidation and in turn stimulated glucose oxidation (Figure 5A). Glucose oxidation was doubled in the Methyl-GBB-treated isolated hearts perfused with low and high concentrations of insulin. Similarly, Methyl-GBB treatment increased lactate uptake and oxidation rates in isolated hearts (Figure 5B and C). Thus, Methyl-GBB treatment switched a part of the energy production from LCFA to glucose oxidation.
Figure 5.
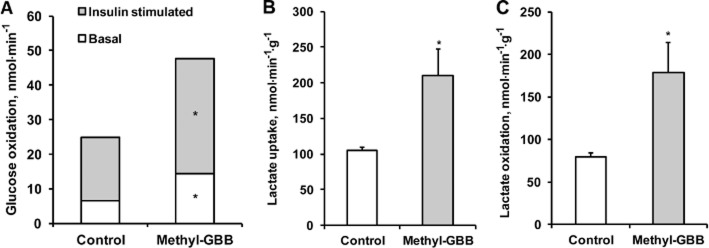
Effects of long-term treatment with Methyl-GBB (10 mg·kg−1) on basal and insulin-stimulated [U-14C]-glucose oxidation (A) [1-14C], lactate uptake (B) and oxidation (C) in isolated heart. Data shown are the means ± SEM of five to six animals. *P < 0.05, significantly different from control group; Student's t-test.
Expression of genes related to FAO
To detect the changes in the expression of the PPAR-α target genes involved in LCFA metabolism, mRNA was isolated from cardiac tissues in normoxia and after no-flow ischaemia followed by reperfusion. Treatment with Methyl-GBB (10 mg·kg−1) for 14 days had no effect on gene expression in normoxia (data not shown), while it stimulated the expression of the genes related to LCFA uptake and metabolism in the AAR after 60 min of reperfusion (Figure 6). On average, long-term treatment with Methyl-GBB induced a 1.5- to 2.5-fold increase in the expression of the genes involved in LCFA uptake (CPT1A, CPT1B, FABP, CD36) and metabolism (ACSL, ACOX). These results suggest that treatment with Methyl-GBB activated the PPAR-α/PGC-1α signalling pathway and stimulated the corresponding target genes, during reperfusion.
Figure 6.
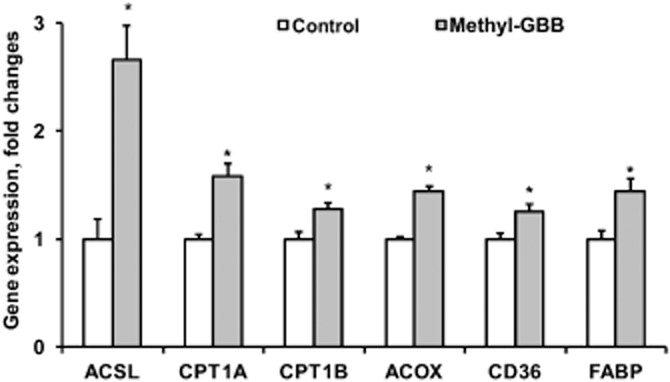
Effects of long-term treatment with Methyl-GBB (10 mg·kg−1) on gene expression in reperfusion [ACSL, carnitine palmitoyltransferase I, liver isoform (CPT-1A) and muscle isoform (CPT-1B), acyl-CoA oxidase 1 (ACOX), fatty acid translocase (CD36), fatty acid binding protein (FABP)]. mRNA isolated from rat heart tissue after 30 min ischaemia/60 min reperfusion injury. Data shown are the means ± SEM of eight animals. *P < 0.05, significantly different from control group; Student's t-test.
Ex vivo and in vivo cardiac function at baseline
To determine the functional consequences of Methyl-GBB treatment under normal physiological conditions, we monitored the function of isolated hearts before ischaemia and performed echocardiography on anaesthetized rats. Measures of systolic and diastolic functions were not altered after treatment by Methyl-GBB (20 mg·kg−1). In fact, none of the variables measured were different between groups, including left ventricular developed pressure, heart rate (HR), contraction, relaxation and coronary flow in isolated heart and ejection fraction, fractional shortening, isovolumic relaxation time and E/A ratio in vivo (Figure 7 and Supporting Information Figs S3 and S4). Consistent with unchanged cardiac function, hearts from Methyl-GBB-treated rats had no structural or morphological abnormalities: left ventricular internal diameter in end diastole, left ventricular internal diameter in end systole, interventricular septum thickness in diastole, interventricular septum thickness in systole, left ventricular posterior wall thickness in diastole, left ventricular posterior wall thickness in systole where unchanged (Supporting Information Fig. S4). Together, these data indicate that despite alterations in substrate metabolism, hearts from Methyl-GBB-treated rats were neither functionally compromised nor had any compensatory structural remodelling.
Figure 7.
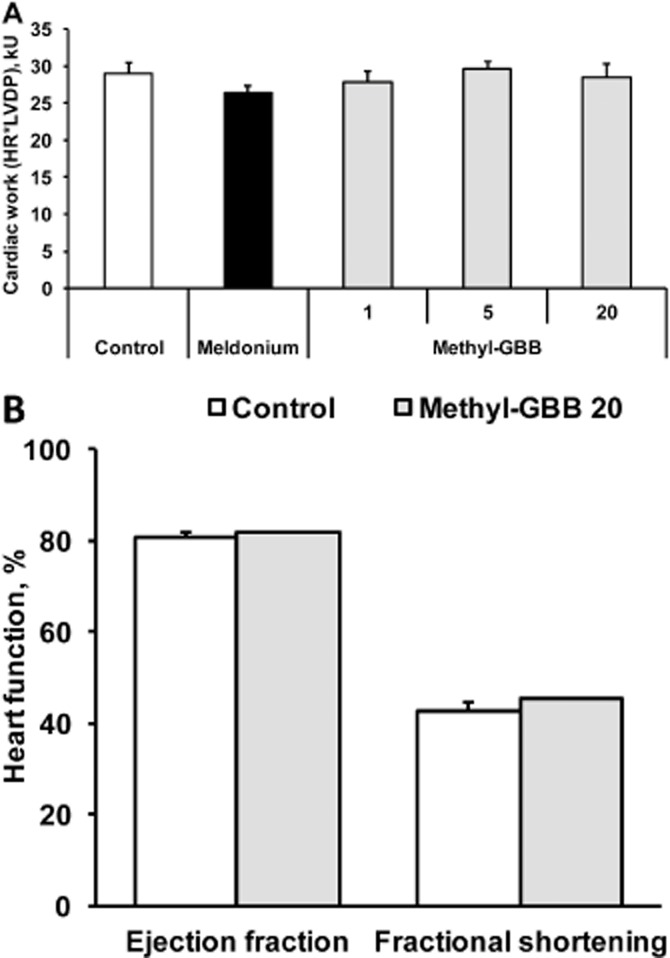
Heart work [heart rate × left ventricular developed pressure (LVDP)] (A) was measured in isolated hearts after 14 days of treatment by meldonium (100 mg·kg−1) or Methyl-GBB (1, 5 and 20 mg·kg−1). Heart function in vivo (B) was measured by echocardiography after 14 days of treatment by meldonium (100 mg·kg−1) or Methyl-GBB (1, 5 and 20 mg·kg−1). Data shown are the means ± SEM of 5–10 animals.
Infarction models in rat isolated heart and in vivo
The anti-infarction effects of Methyl-GBB and meldonium were investigated in both rat isolated hearts with in vitro ischaemia-reperfusion, and in vivo. In rat isolated spontaneously beating hearts, these compounds did not affect HR, peak left ventricle developed pressure (LVDP), coronary flow, and LV contractility (+dp/dt) or LV relaxation (−dp/dt) either before (Figure 7 and Supporting Information Fig. S3), during or after ischaemia compared with the control group. During LAD occlusion, the coronary flow in all experimental groups was decreased by 39–44% and a fall in LVDP by 29–36% was also observed. No changes in the HR during the occlusion period were observed. In the reperfusion stage, coronary flow recovered up to 77–87% of the baseline values. The values for the AAR were similar in the hearts of all the experimental groups, and the AAR was approximately 45–50% of the area of the LV. As shown in Figure 8A, meldonium treatment (100 mg·kg−1) decreased the IS by 25% (P = 0.03) of the control group. Methyl-GBB (5 and 20 mg·kg−1) was more effective and decreased the IS by 45–48%. Thus, Methyl-GBB with a dose 20 times lower was almost twice as protective as meldonium.
Figure 8.
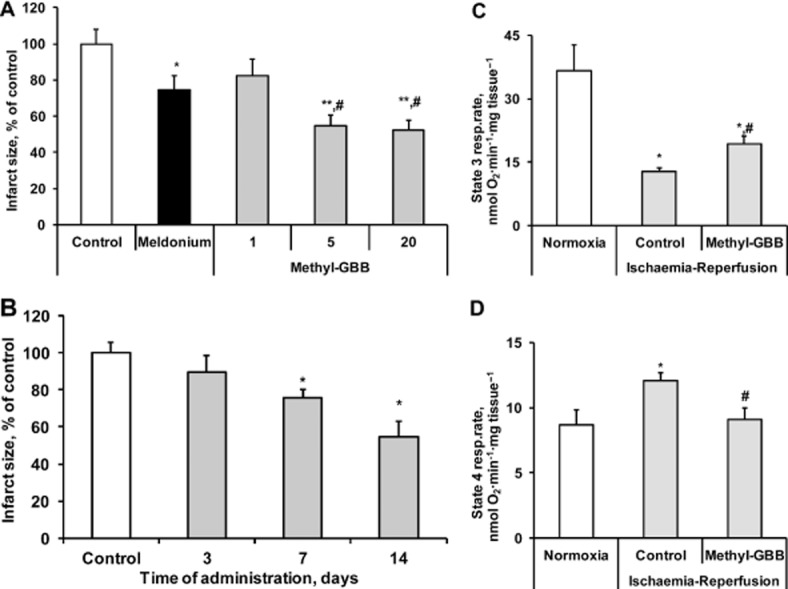
Effects of meldonium (100 mg·kg−1) and Methyl-GBB (1, 5 and 20 mg·kg−1) on the infarct size after 14 days of treatment (A). Effects of Methyl-GBB (5 mg·kg−1) on the infarct size after 3, 7 and 14 days of treatment (B). Effects of Methyl-GBB (20 mg·kg−1) treatment on ADP-stimulated mitochondrial respiration (state 3) (C) and uncoupling of oxidative phosphorylation (state 4) (D) in cardiac fibres (expressed as mg tissue), isolated after ischaemia-reperfusion. Myocardial infarction experiments were performed in the isolated rat hearts. Data shown are the means ± SEM of at least 5–10 animals. *P < 0.05, significantly different from control group;< #P < 0.05, significantly different from meldonium group; Tukey's test.
In earlier work, meldonium exhibited significant cardioprotective effects only after 14 days of treatment (Liepinsh et al., 2006). We therefore assessed the time-course of the effects of Methyl-GBB. As shown in Figure 8B, after 7 days of treatment, Methyl-GBB significantly decreased the IS by 24%. Overall, as seen with meldonium, long-term treatment with Methyl-GBB was required to provide maximal cardioprotection.
The ischaemia-reperfusion induced a 2.8-fold decrease in state 3 respiration and a 40% increase in state 4 respiration (Figure 8C and D). Methyl-GBB treatment protected against the mitochondrial dysfunction induced by ischaemia-reperfusion. Mitochondria respiration rate at state 3 was increased by 50% compared with ischaemic control (Figure 8C) and, for state 4 respiration, Methyl-GBB completely protected against ischaemia-reperfusion-induced mitochondrial uncoupling (Figure 8D).
In the in vivo myocardial infarction model, the effects of a 14 day pretreatment with Methyl-GBB were evaluated. Values of the AAR were comparable in all experimental groups (Figure 9A). Pretreatment with Methyl-GBB (10 and 20 mg·kg−1) attenuated the IS by 18 and 45% (P = 0.001), respectively, in Wistar rats undergoing LAD occlusion and reperfusion in vivo (Figure 9B). Moreover, Methyl-GBB pretreatment improved the 24 h survival of rats by 20–30% (Figure 9C). Thus, treatment with Methyl-GBB provided cardioprotective effects in isolated hearts ex vivo and in vivo.
Figure 9.
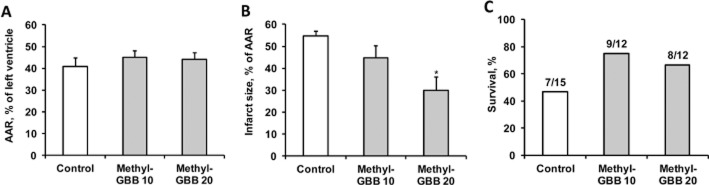
Effects of Methyl-GBB (10 and 20 mg·kg−1) on the AAR (A) infarct size (B) and rat 24 h survival (C). Methyl-GBB was administered daily for 14 days. Data shown are the means ± SEM of at least eight animals. *P < 0.05, significantly different from control group; Tukey's test.
Discussion
This study demonstrates that Methyl-GBB, a novel inhibitor of BBOX and OCTN2, protected the myocardium from ischaemia and reperfusion-induced damage in rat isolated hearts and in vivo. Methyl-GBB effectively reduced content of acylcarnitines in heart and mitochondria, limited LCFA oxidation and, consequently, stimulated glucose oxidation in the heart tissues by lowering L-carnitine availability. Despite these significant changes in myocardial metabolism, no evidence of cardiac dysfunction was observed in isolated hearts ex vivo or by echocardiography in rats in vivo. Different approaches have been used to partly inhibit LCFA oxidation and switch energy metabolism from LCFA to glucose oxidation (Lopaschuk et al., 1989; Kantor et al., 2000; Dyck et al., 2004). CPT I is considered to be a rate-limiting enzyme for LCFA transport into mitochondria and is often targeted to achieve inhibition of LCFA metabolism (Lopaschuk et al., 1989; Unger et al., 2005; Bentebibel et al., 2006). In addition to the direct inhibition of CPT I or to an increase in malonyl-CoA content, a decrease in L-carnitine concentration in heart tissues was found to be one of the most effective ways to decrease the activity of CPT I. For a long time, the only non-toxic and effective compound that decreased the concentration of L-carnitine and consequently had protective effects against cardiovascular diseases was meldonium (Dambrova et al., 2002; Schurch et al., 2010). In comparison, the new compound Methyl-GBB is 10–20 times more potent as an inhibitor of BBOX and OCTN2, and it was more effective in reducing the L-carnitine concentration in blood plasma and tissues. Thus, the same decrease of L-carnitine in cardiac tissues was induced by Methyl-GBB at a dose 20 times lower than that of meldonium. Previously, long-term meldonium treatment was shown to decrease the plasma concentrations of L-carnitine through the inhibition of renal re-uptake (Liepinsh et al., 2011b). Similarly, Methyl-GBB increased L-carnitine content in the urine even after a few hours of administration. This effect is attributed to a highly efficient inhibition of OCTN2 which results in rapid L-carnitine excretion. Overall, the decreased L-carnitine content in tissues after Methyl-GBB was the result of a combination of decreased L-carnitine transport into tissues and decreased reabsorption in the kidneys.
As we have demonstrated previously, the preconditioning-like cardioprotective effects of meldonium depend on a decreased L-carnitine content in the heart tissues (Kuka et al., 2012). In the present study, the time-dependent reduction in L-carnitine content also correlated with the cardioprotective effect induced by Methyl-GBB, although some significant differences between the actions of Methyl-GBB and meldonium were observed. After only 7 days of treatment, Methyl-GBB reached the same maximal IS-limiting effect induced by meldonium, while after 14 days of treatment with Methyl-GBB, the size of the infarcted area in the isolated heart model was half that in the meldonium-treated hearts. The effect of Methyl-GBB on limiting the IS was also confirmed in the in vivo myocardial infarction model. As observed in isolated hearts, the effects of limiting the IS induced by Methyl-GBB in the in vivo model were almost twice the effects induced by meldonium (Sesti et al., 2006). In addition, Methyl-GBB treatment improved rat survival at 24 h after myocardial infarction in vivo. The overall cardioprotective actions of Methyl-GBB were greater than those of meldonium.
In previous studies, we have observed an increase in the nuclear content of PPAR-α and PGC-1α, as a response to decreased L-carnitine content (Liepinsh et al., 2011c; 2013b,). The compensatory changes in the expression of the genes related to FAO and the redirection of LCFA flux from the mitochondria to the peroxisomes are clearly beneficial to preserve mitochondrial function and recovery after heart ischaemia (Liepinsh et al., 2013b). In this study, we observed that the decrease in L-carnitine content did not induce substantial changes in gene expression involved in LCFA metabolism in normoxic hearts. Therefore, Methyl-GBB treatment induced a decrease in L-carnitine availability, acylcarnitine production and reduced palmitate oxidation in isolated mitochondria, peroxisomes, both in isolated hearts and in vivo. In previous studies using isolated mitochondria or cultured cells, palmitoylcarnitine, an intermediate in LCFA metabolism, affected mitochondrial membrane potential, the mitochondrial permeability transition pore, activity of the respiratory chain and the generation of reactive oxygen species (Abdul-Ghani et al., 2008; Tominaga et al., 2008; Seifert et al., 2010). Therefore, the main advantage of the pharmacological decrease of L-carnitine might be the reduction of direct damage by the long-chain acylcarnitines on mitochondria in an ischaemic heart. In contrast, in reperfusion, the metabolism of LCFA in Methyl-GBB-treated hearts was not decreased because the up-regulated PPARα/PGC-1α pathway-dependent gene expression compensated for the reduced L-carnitine content. Thus, while the protection against long-chain acylcarnitine accumulation-induced damage is beneficial in ischaemia, when the oxygen supply is limited, the activation of LCFA metabolism in reperfusion would help restore energy production in the heart.
Enhanced glucose oxidation is beneficial during ischaemia because of the reduced proton production and less oxygen consumed per ATP produced (Ussher et al., 2012). In this study, we also observed that the decreased content of long-chain acylcarnitines and LCFA oxidation by Methyl-GBB treatment induced stimulation of glucose oxidation in the heart. These results suggest that reduced IS is at least partly associated with a stimulation of glucose and lactate oxidation and related beneficial effects.
In conclusion, these results provide additional evidence that the reduction of L-carnitine content by the inhibition of OCTN2 represents an effective strategy to protect the heart against ischaemia-reperfusion-induced damage. Methyl-GBB treatment leads to cardioprotective effects and increased survival by limiting LCFA oxidation and facilitating glucose metabolism.
Acknowledgments
This work was supported by the European Regional Development Fund Grant No. 2010/0234/2DP/2.1.1.1.0/10/APIA/VIAA/063 and State Research Program BIOMEDICINE.
Glossary
Abbreviations
- AAR
area at risk
- ACOX
acyl-CoA oxidase 1
- ACSL
long-chain fatty acid CoA synthetase
- AN
area of necrosis
- BBOX
γ-butyrobetaine dioxygenase
- CD36
fatty acid translocase
- CPT I
carnitine palmitoyltransferase I
- FABP
fatty acid binding protein
- FAO
fatty acid oxidation
- HR
heart rate
- IHD
ischaemic heart disease
- IS
infarct size
- KH
Krebs-Henseleit
- LAD
left anterior descending coronary artery
- LCFA
long-chain fatty acids
- LV
left ventricle
- LVDP
left ventricle developed pressure
- Methyl-GBB
4-[ethyl(dimethyl)ammonio]butanoate
- OCTN2
organic cation transporter 2
- PGC-1α
PPAR-γ coactivator 1α
- TTC
2,3,5-triphenyltetrazolium chloride
Author contributions
E. L., M. D., O. P. and I. K. participated in research design. M. M-K., J. K., R. V., E. M., D. L. and S. G. conducted the experiments. S. G., E. L. and D. L. performed organic synthesis and analytical chemistry. E. L., M. M-K., J. K. and E. M. performed data analysis. E. L., M. D., M. M-K. and J. K. wrote or contributed to the writing of the manuscript.
Conflict of interest
There is no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1 Effect of Methyl-GBB on OCTN2-mediated L-carnitine transport in HEK293 cells. Each value represents the mean ± SEM of three experiments. The experiment was performed as described by Dambrova et al. (2013, doi: 10.1002/jcph.135).
Figure S2 Plasma concentrations of meldonium and Methyl-GBB after 1, 3, 7 and 14 days of treatment with meldonium (100 mg·kg−1) or Methyl-GBB (1, 5 and 20 mg·kg−1). Each value represents the mean ± SEM of 5–10 animals.
Figure S3 Left ventricular developed pressure (a), heart rate (b) and coronary flow (c) of isolated hearts after 14 days of treatment by meldonium (100 mg·kg−1) and Methyl-GBB (1, 5 and 20 mg·kg−1). Each value represents the mean ± SEM of 10 animals.
Figure S4 Dimensions of the left chamber of the heart (a) and wall thickness (b) were measured by echocardiography after 14 days of treatment with Methyl-GBB (20 mg·kg−1). The mean values of left ventricular internal diameter in end diastole (LVIDD), left ventricular internal diameter in end systole (LVIDS), interventricular septum thickness in diastole (IVSd), interventricular septum thickness in systole (IVSs), left ventricular posterior wall thickness in diastole (LVPWd) and left ventricular posterior wall thickness in systole (LVPWs) represent the mean ± SEM of five animals.
Table S1 Sequences of primers used for quantitative RT-PCR.
References
- Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, et al. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295:678–685. doi: 10.1152/ajpendo.90287.2008. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013a;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Nuclear Hormone Receptors. Br J Pharmacol. 2013b;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentebibel A, Sebastian D, Herrero L, Lopez-Vinas E, Serra D, Asins G, et al. Novel effect of C75 on carnitine palmitoyltransferase I activity and palmitate oxidation. Biochemistry. 2006;45:4339–4350. doi: 10.1021/bi052186q. [DOI] [PubMed] [Google Scholar]
- Blachnio-Zabielska AU, Koutsari C, Jensen MD. Measuring long-chain acyl-coenzyme A concentrations and enrichment using liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Rapid Commun Mass Spectrom. 2011;25:2223–2230. doi: 10.1002/rcm.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambrova M, Liepinsh E, Kalvinsh I. Mildronate: cardioprotective action through carnitine-lowering effect. Trends Cardiovasc Med. 2002;12:275–279. doi: 10.1016/s1050-1738(02)00175-5. [DOI] [PubMed] [Google Scholar]
- Dambrova M, Cirule H, Svalbe B, Zvejniece L, Pugovichs O, Zorenko T, et al. Effect of inhibiting carnitine biosynthesis on male rat sexual performance. Physiol Behav. 2008;95:341–347. doi: 10.1016/j.physbeh.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Degrace P, Demizieux L, Gresti J, Tsoko M, Andre A, Demaison L, et al. Fatty acid oxidation and related gene expression in heart depleted of carnitine by mildronate treatment in the rat. Mol Cell Biochem. 2004;258:171–182. doi: 10.1023/b:mcbi.0000012853.20116.06. [DOI] [PubMed] [Google Scholar]
- Deuchar GA, Opie LH, Lecour S. TNFalpha is required to confer protection in an in vivo model of classical ischaemic preconditioning. Life Sci. 2007;80:1686–1691. doi: 10.1016/j.lfs.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Dyck JR, Cheng JF, Stanley WC, Barr R, Chandler MP, Brown S, et al. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res. 2004;94:e78–e84. doi: 10.1161/01.RES.0000129255.19569.8f. [DOI] [PubMed] [Google Scholar]
- Ertracht O, Liani E, Bachner-Hinenzon N, Bar-Am O, Frolov L, Ovcharenko E, et al. The cardioprotective efficacy of TVP1022 in a rat model of ischaemia/reperfusion. Br J Pharmacol. 2011;163:755–769. doi: 10.1111/j.1476-5381.2011.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearse DJ, Richard V, Yellon DM, Kingma JG., Jr Evolving myocardial infarction in the rat in vivo: an inappropriate model for the investigation of drug-induced infarct size limitation during sustained regional ischaemia. J Cardiovasc Pharmacol. 1988;11:701–710. [PubMed] [Google Scholar]
- Horowitz JD, Chirkov YY, Kennedy JA, Sverdlov AL. Modulation of myocardial metabolism: an emerging therapeutic principle. Curr Opin Cardiol. 2010;25:329–334. doi: 10.1097/HCO.0b013e328339f191. [DOI] [PubMed] [Google Scholar]
- Kalvinsh I, Dambrova M, Liepinsh E, Pugovics O, Vilskersts R, Kuka J, et al. 2011. Use of 4-[ethyl(dimethyl)ammonio]butanoate in the treatment of cardiovascular disease. WO/2011/048201.
- Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuka J, Vilskersts R, Cirule H, Makrecka M, Pugovics O, Kalvinsh I, et al. The cardioprotective effect of mildronate is diminished after co-treatment with L-carnitine. J Cardiovasc Pharmacol Ther. 2012;17:215–222. doi: 10.1177/1074248411419502. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Vilskersts R, Loca D, Kirjanova O, Pugovichs O, Kalvinsh I, et al. Mildronate, an inhibitor of carnitine biosynthesis, induces an increase in gamma-butyrobetaine contents and cardioprotection in isolated rat heart infarction. J Cardiovasc Pharmacol. 2006;48:314–319. doi: 10.1097/01.fjc.0000250077.07702.23. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Vilskersts R, Zvejniece L, Svalbe B, Skapare E, Kuka J, et al. Protective effects of mildronate in an experimental model of type 2 diabetes in Goto-Kakizaki rats. Br J Pharmacol. 2009;157:1549–1556. doi: 10.1111/j.1476-5381.2009.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh E, Kalvinsh I, Dambrova M. The regulation of energy metabolism pathways through L-carnitine homeostasis. In: Croniger C, editor. Role of the Adipocyte in Development of Type 2 Diabetes. Rijeka: InTech; 2011a. pp. 107–128. [Google Scholar]
- Liepinsh E, Konrade I, Skapare E, Pugovics O, Grinberga S, Kuka J, et al. Mildronate treatment alters gamma-butyrobetaine and l-carnitine concentrations in healthy volunteers. J Pharm Pharmacol. 2011b;63:1195–1201. doi: 10.1111/j.2042-7158.2011.01325.x. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Makrecka M, Kuka J, Cirule H, Makarova E, Sevostjanovs E, et al. Selective inhibition of OCTN2 is more effective than inhibition of gamma-butyrobetaine dioxygenase to decrease the availability of l-carnitine and to reduce myocardial infarct size. Pharmacol Res. 2014;85:33–38. doi: 10.1016/j.phrs.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Skapare E, Svalbe B, Makrecka M, Cirule H, Dambrova M. Anti-diabetic effects of mildronate alone or in combination with metformin in obese Zucker rats. Eur J Pharmacol. 2011c;658:277–283. doi: 10.1016/j.ejphar.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Kuka J, Dambrova M. Troubleshooting digital macro photography for image acquisition and the analysis of biological samples. J Pharmacol Toxicol Methods. 2013a;67:98–106. doi: 10.1016/j.vascn.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Skapare E, Kuka J, Makrecka M, Cirule H, Vavers E, et al. Activated peroxisomal fatty acid metabolism improves cardiac recovery in ischemia-reperfusion. Naunyn Schmiedebergs Arch Pharmacol. 2013b;386:541–550. doi: 10.1007/s00210-013-0849-0. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Barr RL. Measurements of fatty acid and carbohydrate metabolism in the isolated working rat heart. Mol Cell Biochem. 1997;172:137–147. [PubMed] [Google Scholar]
- Lopaschuk GD, McNeil GF, McVeigh JJ. Glucose oxidation is stimulated in reperfused ischemic hearts with the carnitine palmitoyltransferase 1 inhibitor, Etomoxir. Mol Cell Biochem. 1989;88:175–179. doi: 10.1007/BF00223440. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- Makrecka M, Svalbe B, Volska K, Sevostjanovs E, Liepins J, Grinberga S, et al. Mildronate, the inhibitor of l-carnitine transport, induces brain mitochondrial uncoupling and protects against anoxia-reoxygenation. Eur J Pharmacol. 2014;723:55–61. doi: 10.1016/j.ejphar.2013.12.006. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan IK, Khan M, Wisel S, Selvendiran K, Sridhar A, Carnes CA, et al. Cardioprotection by HO-4038, a novel verapamil derivative, targeted against ischemia and reperfusion-mediated acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H140–H151. doi: 10.1152/ajpheart.00687.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurch R, Todesco L, Novakova K, Mevissen M, Stieger B, Krahenbuhl S. The plasma carnitine concentration regulates renal OCTN2 expression and carnitine transport in rats. Eur J Pharmacol. 2010;635:171–176. doi: 10.1016/j.ejphar.2010.02.045. [DOI] [PubMed] [Google Scholar]
- Sebastian D, Guitart M, Garcia-Martinez C, Mauvezin C, Orellana-Gavalda JM, Serra D, et al. Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells. J Lipid Res. 2009;50:1789–1799. doi: 10.1194/jlr.M800535-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert EL, Estey C, Xuan JY, Harper ME. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem. 2010;285:5748–5758. doi: 10.1074/jbc.M109.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti C, Simkhovich BZ, Kalvinsh I, Kloner RA. Mildronate, a novel fatty acid oxidation inhibitor and antianginal agent, reduces myocardial infarct size without affecting hemodynamics. J Cardiovasc Pharmacol. 2006;47:493–499. doi: 10.1097/01.fjc.0000211732.76668.d2. [DOI] [PubMed] [Google Scholar]
- Shriver LP, Manchester M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep. 2011;1:79. doi: 10.1038/srep00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tars K, Leitans J, Kazaks A, Zelencova D, Liepinsh E, Kuka J, et al. Targeting carnitine biosynthesis: discovery of new inhibitors against gamma-butyrobetaine hydroxylase. J Med Chem. 2014;57:2213–2236. doi: 10.1021/jm401603e. [DOI] [PubMed] [Google Scholar]
- Tominaga H, Katoh H, Odagiri K, Takeuchi Y, Kawashima H, Saotome M, et al. Different effects of palmitoyl-L-carnitine and palmitoyl-CoA on mitochondrial function in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;295:105–112. doi: 10.1152/ajpheart.01307.2007. [DOI] [PubMed] [Google Scholar]
- Unger SA, Kennedy JA, Fadden-Lewis K, Minerds K, Murphy GA, Horowitz JD. Dissociation between metabolic and efficiency effects of perhexiline in normoxic rat myocardium. J Cardiovasc Pharmacol. 2005;46:849–855. doi: 10.1097/01.fjc.0000190488.77434.f1. [DOI] [PubMed] [Google Scholar]
- Ussher JR, Wang W, Gandhi M, Keung W, Samokhvalov V, Oka T, et al. Stimulation of glucose oxidation protects against acute myocardial infarction and reperfusion injury. Cardiovasc Res. 2012;94:359–369. doi: 10.1093/cvr/cvs129. [DOI] [PubMed] [Google Scholar]
- Vilskersts R, Liepinsh E, Mateuszuk L, Grinberga S, Kalvinsh I, Chlopicki S, et al. Mildronate, a regulator of energy metabolism, reduces atherosclerosis in apoE/LDLR-/- mice. Pharmacology. 2009;83:287–293. doi: 10.1159/000210015. [DOI] [PubMed] [Google Scholar]
- Wang W, Lopaschuk GD. Metabolic therapy for the treatment of ischemic heart disease: reality and expectations. Expert Rev Cardiovasc Ther. 2007;5:1123–1134. doi: 10.1586/14779072.5.6.1123. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C, Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res. 2011;89:148–156. doi: 10.1093/cvr/cvq266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effect of Methyl-GBB on OCTN2-mediated L-carnitine transport in HEK293 cells. Each value represents the mean ± SEM of three experiments. The experiment was performed as described by Dambrova et al. (2013, doi: 10.1002/jcph.135).
Figure S2 Plasma concentrations of meldonium and Methyl-GBB after 1, 3, 7 and 14 days of treatment with meldonium (100 mg·kg−1) or Methyl-GBB (1, 5 and 20 mg·kg−1). Each value represents the mean ± SEM of 5–10 animals.
Figure S3 Left ventricular developed pressure (a), heart rate (b) and coronary flow (c) of isolated hearts after 14 days of treatment by meldonium (100 mg·kg−1) and Methyl-GBB (1, 5 and 20 mg·kg−1). Each value represents the mean ± SEM of 10 animals.
Figure S4 Dimensions of the left chamber of the heart (a) and wall thickness (b) were measured by echocardiography after 14 days of treatment with Methyl-GBB (20 mg·kg−1). The mean values of left ventricular internal diameter in end diastole (LVIDD), left ventricular internal diameter in end systole (LVIDS), interventricular septum thickness in diastole (IVSd), interventricular septum thickness in systole (IVSs), left ventricular posterior wall thickness in diastole (LVPWd) and left ventricular posterior wall thickness in systole (LVPWs) represent the mean ± SEM of five animals.
Table S1 Sequences of primers used for quantitative RT-PCR.