Abstract
Colon cancer is associated with a high incidence and a poor prognosis. The aim of the present study was to determine whether Fourier transform infrared (FTIR) microspectroscopy can be used to monitor the chemotherapy drug-induced apoptosis of SW620 colon cancer cells. The 50% inhibitory concentration (IC50) of 5-fluorouracil (5-FU), the main chemotherapeutic agent used for the treatment of colorectal cancer, was determined as the inhibition of growth of the SW620 cells using an MTT assay. Cell starvation and 5-FU treatment synergized to arrest the cells in the G1 and S phases of the cell cycle. FTIR combined with fluorescence activated cell sorting (FACS) analysis were used to analyze the SW620 cells following treatment with 5-FU for 12, 24 and 48 h. The apoptotic cells had several spectral characteristics. The relative peak intensity ratio (I1740/I1460) was significantly increased (P<0.05), the I1740/I1460 ratio, associated with a band of amino acid residues at 1,410 cm−1 was significantly increased at the early and late phases of cell death (P<0.05), the peaks at 1,240 cm−1 increased in wave number, a band at 1,040 cm−1, associated with polysaccharides, appeared at 24 and 48 h and then moved to a higher wave number and the I1040/I1460 ratio increased at the late stage of apoptosis. These results demonstrated that FTIR can be used as a label-free technique to monitor cancer cell apoptosis and to understand the spectral fingerprints of apoptotic cells. This suggested that FTIR spectral features have potential as a powerful tool to monitor cancer cell apoptosis.
Keywords: colon cancer, cell cycle, apoptosis, Fourier transform infrared, chemotherapy
Introduction
Cancer remains one of the major life threatening diseases in humans. Each year, almost 70,000 individuals between the ages of 15 and 40 years are diagnosed with cancer in the USA (1). Amongst all types of cancer, colorectal cancer is the second most frequent cause of cancer-associated mortality in developed countries and is the fifth leading cause of mortality in China (2,3). Surgery and chemotherapy are the main techniques used in the treatment of colorectal cancer and 5-fluorouracil (5-FU) is the chemotherapeutic agent of choice for its treatment (4). It has been widely used for the treatment of solid tumors, including colorectal, breast and head and neck cancer (5). On entering the tumor cell, 5-FU can exert cytotoxic effects via the inhibition of thymidylate synthetase (TS) or through incorporation into RNA and DNA, which lead to the activation of apoptosis (6,7). Apoptosis occurs as part of various normal and pathological processes and can be triggered by chemotherapeutic drugs (8). Apoptosis is the process by which cells self-destruct without evoking an inflammatory response and is characterized by nuclear and cytoplasmic shrinkage, chromatin condensation, internucleosomal DNA cleavage and plasma membrane blebbing (9,10).
At present, colorectal cancer is monitored following chemotherapy using techniques, including electronic colon endoscopy, computer-assisted tomography and B ultrasonic imaging. The disadvantage of these approaches is of that auxiliary examination can only monitor the level of morphological changes, but not molecular changes, including gene mutations. Although molecular biological techniques focusing on the molecular mechanism of colorectal cancer have improved, there are few specific biomarkers and these techniques are time-consuming (11). Thus, it is important to develop an accurate, simple, rapid and non-invasive technique for the real-time monitoring of colon cancer chemotherapy at the molecular level.
Following advances in vibrational spectroscopy techniques, their application in medical biology is increasing (12). Fourier transform infrared spectroscopy (FTIR) has been used as an analytical tool in chemistry for several years. In addition, FTIR can be applied as a simple, rapid and non-invasive method to detect and identify cancer with minimal sample preparation and can be used for the qualitative identification and quantitative analysis of various components in a complex mixture (13). Few studies have monitored the morphology of cancer cells using vibrational spectroscopy (14–17) and, although several spectroscopic studies have examined the cell apoptosis, cell cycle, differentiation and proliferation of different cell lines and tissues (18,19), there has been no detailed analysis of colon cancer cell apoptosis following treatment with chemotherapeutic agents. In the present study, the feasibility of FTIR spectroscopy analysis to identify different stages of tumor cells apoptosis was assessed to attempt to provide a theoretical basis for the application of FTIR spectroscopy in the monitoring of clinical chemotherapy.
Materials and methods
Cell lines and cell culture
The human colon carcinoma cell line SW620, also termed CCL227, which overexpresses TS, was purchased from the China Center for Type Culture Collection, (Wuhan, China) (20). The cells were grown in high glucose RPMI medium (Hyclone Laboratories, Inc., Logan, UT, USA) supplemented with 10% fetal calf serum (Gibco-BRL, Carlsbad, CA, USA) with penicillin and streptomycin (100 U/ml each; Sigma-Aldrich, St. Louis, MO, USA) in a humidified incubator with 5% CO2 at 37°C. All experiments were performed on exponentially growing cells. All cells were incubated with 5-FU (Sigma-Aldrich) for 12, 24 and 48 h to induce apoptosis.
5-FU treatment
The cells in 100 μl culture medium per well were seeded into 96-well plates (Gibco-BRL) and cultured at 37°C for 24 h. The culture medium was then replaced with serum-free medium and various concentrations of 5-FU (6.25, 12.5, 25, 50, 100, 200 and 400 μM) were adjusted in the wells. Following additional incubation at 37°C for 24 h, 10 μl MTT (Sigma-Aldrich) dissolved in phosphate-buffered saline (PBS) at a concentration of 5 mg/ml was added to each well and the plates were incubated at 37°C for 4 h. The medium was removed and 150 μl dimethylsulfoxide (Sigma-Aldrich) was added to each well followed by agitation of the plates for 5 min. The absorbance was then measured at 570 nm in a scanning spectrophotometer (Spectronic 20D; Milton Roy, Rochester, NY, USA). A total of six wells were used for each drug concentration and the experiment was repeated three times. The 50% inhibitory concentration (IC50) was then calculated from the survival curves using GraphPad Prism 5 (San Diego, CA, USA).
Cell cycle analysis by FACS
The tumor cells were seeded (2×105 cells/well) into six-well plates. Following overnight incubation, the medium was replaced with serum-free medium containing 50 μM 5-FU for 24 h for serum starvation and incubated for 12, 24 or 48 h. Starving the cells of serum prior to treatment arrests them at the G1/S phase of the cell cycle for synchronization in order to minimize spectral differences at 1,080 cm−1, which can be attributed to cells being in different phases of the cell cycle (21–23). The cells were then harvested and stained with propidium iodide (PI) for cell cycle and apoptotic analysis using standard FACS techniques with a FACSort flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. The results were analyzed and expressed as percentages of the total gated cells using Modifit LT™ software (BD Biosciences).
Isolation of nuclei
The SW620 cells were harvested by scraping the plates in cold PBS and were collected by centrifugation at 666 × g. The cells were then resuspended in three volumes with respect to the cell pellet of the hypotonic buffer CelLyticTM NuCLEARTM Extraction kit (Sigma, USA) (24). Following incubation on ice for 15 min, 5% Triton X-100 was added and a cytosolic fraction was prepared by centrifuging the sample for 30 sec at 4°C at 1,498 × g and subsequently clarified by centrifugation at 666 × g for 15 min at 4°C. The nuclear pellets were washed twice in hypotonic buffer to remove any residual cytosolic contamination and then resuspended in one volume of nuclear lysis buffer. The cells were then incubated for 30 min at 4°C with gentle agitation and finally centrifuged for 30 min at 4°C at 1,498 × g.
FTIR microspectroscopy (MSP) collection
FTIR-MSP is similar to visible light microscopy; however, it does not use glass refractive elements (glass is opaque to infrared light of λ>5 μm) (12). For this reason, microtransmission FTIR requires samples to be deposited on optical windows, such as CaF2, which do not absorb infared light (25,26). The FTIR spectrometer was equipped with a liquid nitrogen-cooled mercury cadmium telluride (MCT) detector and a KBr beam splitter (Thermo Nicolet 6700; Thermo Fisher Scientific, Waltham, MA, USA). All cells were grown on a CaF2 window film. A blank BaF2 window-film was scanned as background and the sample was then scanned. To collect the data for each spectrum, 32 scans were performed in the mid-infrared range (wave number, 4,000-900 cm−1) at a resolution of 8 cm−1.
Statistical analysis
OMNIC 8.0 (Thermo Fisher Scientific) was used as data-processing software for FTIR spectrum analysis (27). The five point moving average smoothing method was used for each spectrum to reduce random noise. The intensity and peak position of each band was measured using Spapro version 2.2 software (College of Chemistry and Molecular Engineering, Peking University, Beijing, China). All experiments were repeated at least three times. The statistical significance of the differences were evaluated by one way analysis of variance and P<0.05 was considered to indicate a statistically significant difference.
Results
Concentration of 5-FU
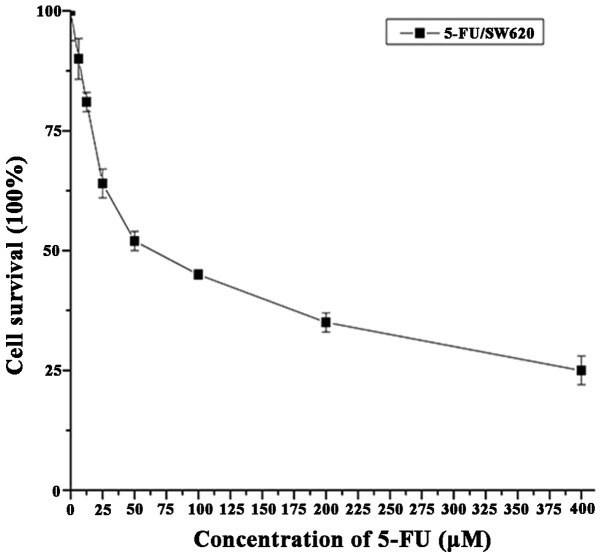
Following treatment of the SW620 cells with increasing concentrations of 5-FU, cell growth was inhibited to different degrees and concentration-dependent cell toxicity was observed (Fig. 1). The IC50 data indicated the 5-FU-resistance levels of the SW-620 cells at IC50 50 μM (Fig. 1). Therefore, 50 μM 5-FU was selected as the concentration for use in the subsequent experiments.
Figure 1.
Proliferative activity of the 5-FU-treated SW620 cells at 5-FU concentrations of 6.25, 12.5, 25, 50, 100, 200 and 400 μM, assessed after 24 h using an MTT assay. The y-axis represents the cell survival, calculated as the ratio between the 5-FU-treated and control cells. 5-FU, 5-fluorouracil.
Sw620 cells arrest at the G1 and S phase
The SW620 cells were serum-starved and analyzed by flow cytometry following PI staining, which resulted in S and G1 phase cell cycle arrest with 90% cells in the G1+S phases as compared with 77% in the control (Fig. 2). Compared with the control, S and G1 phase arrest in the serum-starved SW-620 cells was significantly increased (P<0.05).
Figure 2.
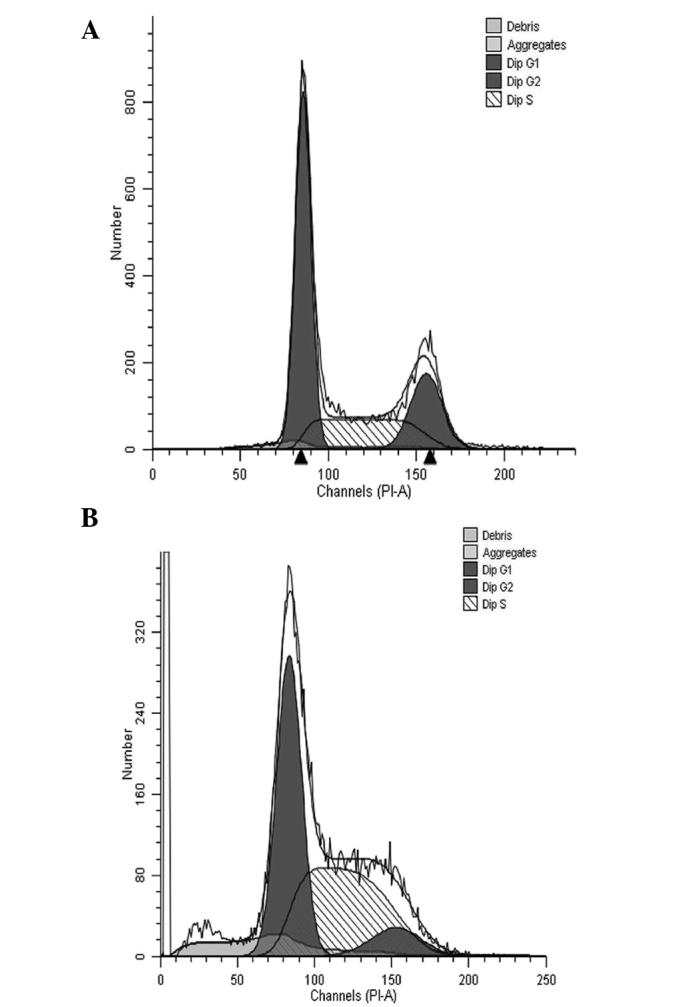
Cell cycle arrest at the G1/S phase was evaluated by fluorescence activated cell sorting using Annexin V/PI staining in the SW620 cells in the (A) control and (B) following serum-starving. PI, propidium iodide.
Apoptosis induced by 5-FU
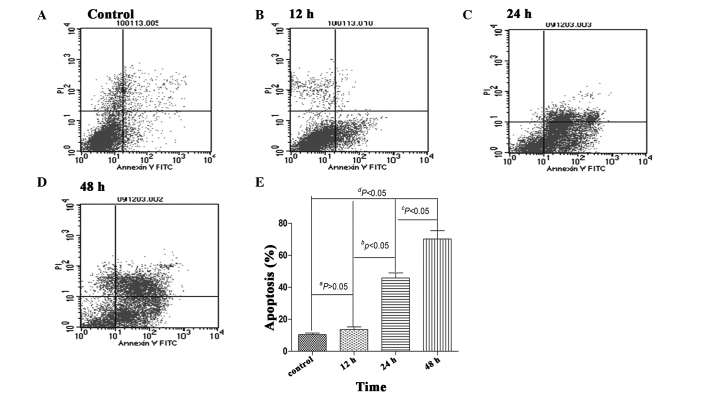
Treatment with 5-FU induced SW620 cell apoptosis. The population of apoptotic cells was evaluated by FACS using Annexin V/PI staining following 5-FU treatment at 50 μM for 12, 24 and 48 h. Annexin V is a Ca2+-dependent phospholipid binding protein, which can combine with the apoptotic cell valgus membrane, and PI is a nucleic acid-binding dye. PI can distinguish between early and late stage apoptosis using FACS. The percentage of Annexin V-positive apoptotic cells increased following 5-FU treatment compared with that of the control (10.04±2.1%; Fig. 3A), for 12 h (Fig. 3B; 13.74±3.2%), 24 h (Fig. 3C; 64.77±7.5%) and 48 h (Fig. 3D; 70.41±9.2%). As shown in Fig. 3E, a significant increase in apoptosis was observed between the control and 12 h, 12 and 24 h (early apoptosis) and between 24 and 48 h (late apoptosis).
Figure 3.
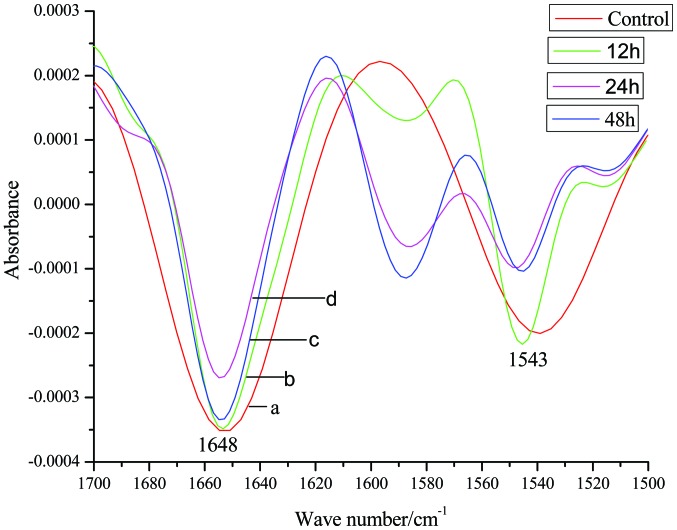
Apoptosis in the (A) control SW620 cells and in SW620 cells induced by 5-FU (50 μM) for (B) 12 h (C) 24 h and (D) 48 h compared with the control. (E) Quantification of apoptosis in the SW620 cells. a12 h, vs. control; b24 h, vs. 12 h; c48 h, vs. 24 h; d48 and 24 h vs. 12 h and control. 5-FU, 5-fluorouracil.
FTIR-MSP spectral analysis
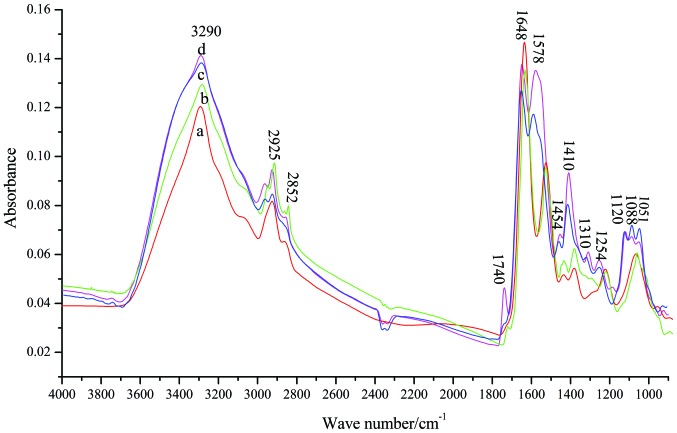
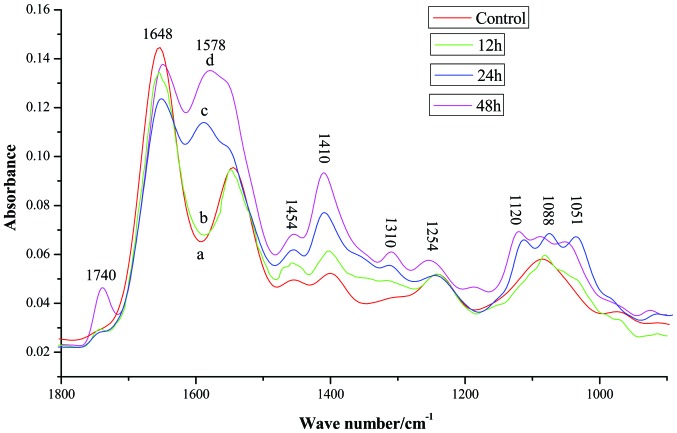
The absorbance spectra of the SW620 cells from the control group and the cells treated with 5-FU for 12, 24 and 48 h are shown in Fig. 4. The appearance of two new bands occurred at 24 h (Fig. 4C) at 1,120 cm−1 and 1,080 cm−1, respectively. Relevant absorbance bands in the FTIR spectra are shown in Fig. 5, between the fingerprint region of 1,800 and 900 cm−1. The new bands, which appeared at 24 h, were further examined by extraction of the nuclei of the SW620 cells to confirm whether 1,120 cm−1, representing threonine (Thr) and serine (Ser) and 1,080 cm−1 (nucleic acid molecule polysaccharide) were present in the nuclei (Fig. 6). The band assignments are shown in Table I. The second derivative spectra of 1,700-1,400 cm−1 in Fig. 7 were used to investigate amide I and amide II and the FTIR results were analyzed between the 4,000 and 900 cm−1 spectral regions.
Figure 4.
Fourier transform infrared spectra at wave numbers 4,000 and 900 cm−1. (a) Untreated SW620 cells (control). (b, c and d) SW620 cells treated with 50 μM 5-FU for 12, 24 and 48 h, respectively.
Figure 5.
Fourier transform infrared spectra in the 1,800 and 900 cm−1 wave number region in the (a) control SW620 cells and (b-d) in the SW620 cells treated with 5-fluorouracil for (b) 12 h, (c) 24 h and (d) 48 h.
Figure 6.
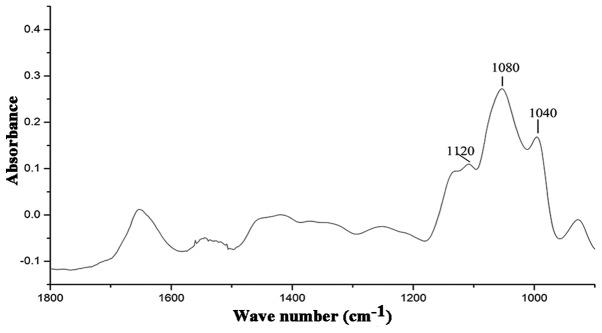
Fourier transform infrared spectra of the SW620 cell nuclei in the 1,800-900 cm−1 wave number region.
Table I.
Band assignments of major absorptions in the Fourier transform infrared spectra of the SW620 cells in the 4,000-900 cm−1 region.
| Peak number | Frequency (cm−1) | Assignments |
|---|---|---|
| 1 | 3,290 | νO-H, νN-H (water, protein) |
| 2 | 2,925 | C-H stretching bands νas CH2 (lipid) |
| 3 | 2,852 | νs CH2 of lipids |
| 4 | 1,740 | C=O stretching bands (lipids) |
| 5 | 1,640 | Amide I (of proteins in α-helix conformation) and water |
| 6 | 1,540–1,580 | Amide II (an N-H bending vibration coupled to C-N stretching) |
| 7 | 1,454 | CH3 bending vibration (lipids and proteins) |
| 8 | 1,410 | δC-H, δC-O-H, amino acid residues |
| 9 | 1,310–1,320 | Amide III band components of proteins |
| 10 | 1,240 | Asymmetric PO2− stretching in RNA |
| 11 | 1,121 | C-O(H) stretching bands, threonine and tyrosine |
| 12 | 1,080–1,085 | C-O-C stretching (nucleic acids and phospholipids) indicates a degree of oxidative damage to DNA |
| 13 | 1,040 | C-O stretching bands, nucleic acid molecule polysaccharide |
Figure 7.
Derivative spectra of the 1,700-1,400 cm−1 region. (a) Control SW620 cells and (b–d) in the SW620 cells treated with 5-fluorouracil for (b) 12 h, (c) 24 h and (d) 48 h.
Band changes are associated with lipids
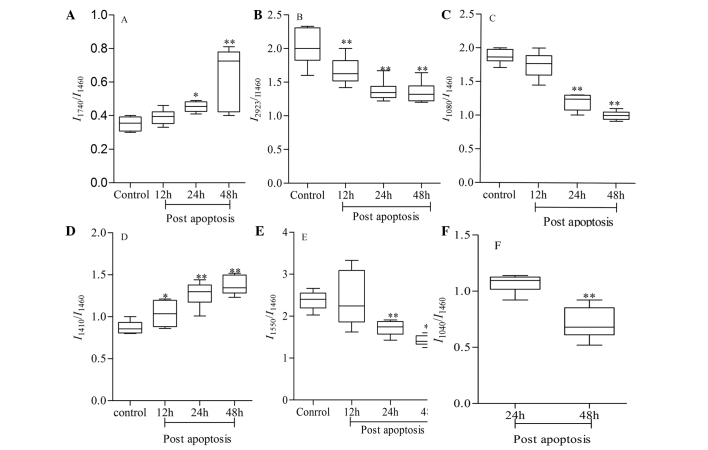
The changes in the relative intensity ratios of the main functional groups are shown in Fig. 8. The bands associated with cell lipids occurred mainly at 2,925, 2,852 and 1,740 cm−1. The 2,925 and 2,852 cm−1 spectral features were absorption bands of asymmetric and symmetric C-H stretching vibrations of CH2 and CH3 methylene groups, which are contained in fatty acids in cellular membranes (28). The band at 1,740 cm−1 is a C=O stretching vibration. Changes in these bands can reflect lipid changes in tissues and cells (29). Late apoptosis (48 h) was associated with large lipid-associated peaks (1,743 cm−1), the relative intensity ratios I1740/I1460 were significantly increased after 48 h compared with those of the control, 12 and 24 h groups, with an increase between 0.34±0.03 and 0.77±0.04 (P<0.001; Fig. 8A), whereas the relative intensity ratio I2923/11460 was significantly decreased after 48 h compared with those of the control and the 12 and 24 h groups, which may indicate that the degree of apoptotic cell methylation decreased (Fig. 8B).
Figure 8.
Statistical analysis of Fourier transform infrared relative intensity ratios (/I1460) of the control SW620 cells and the cells treated with 5-fluorouracil for 12, 24 and 48 h using one-way analysis of variance. (A) I1740/I1460, (B) I2923/I1460, (C) I1080/I1640, (D) I1410/I1640, (E) I1550/I1640 and (F) I1040/I1640. Boxes and error bars represent median, interquartile range and range, respectively.*P<0.05, compared with the control; **P<0.001, compared with 12 and 24 h.
Band changes are associated with nucleic acids
The two major bands in the regions of 1,080 and 1,240 cm−1 are mainly due to the symmetric and asymmetric stretching modes of the phosphodiester groups (30,31). These two bands are associated with the nucleic acid content of a cell (32). The ratio of intensities at the absorption I1080/I1640 began to decrease at 12 h and the ratio was significantly lower at 48 h (P<0.001; Fig. 8C), with late apoptotic DNA degraded into small fragments. The morphological results of the apoptotic bodies from the phagocytosis of DNA fragments morphological results coincided with this. The band at the 1,080 cm−1 absorption peak shifted to lower wave numbers at 12 and 24 h; however, the shift to a higher wavenumber at 48 h may have been due to enhancement of hydrogen bonds in the late apoptotic stage (Fig. 9A). Further investigation is required to investigate the underlying causes.
Figure 9.
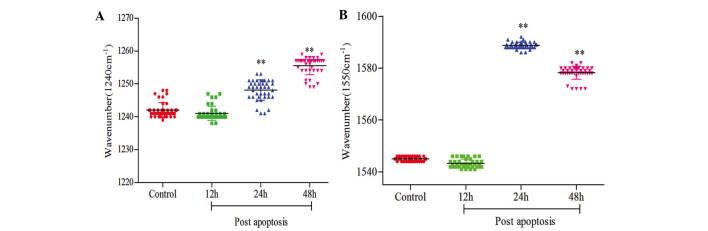
(A and B) Fourier transform infrared spectrum peak position comparisons between the control cells and the cells treated with 5-fluorouracil for 12, 24 and 48 h using one-way analysis of variance. **P<0.001 compared with the control.
Band changes are associated with amino acids
The vibrational bands at 1,120 cm−1 appeared at 24 h (early apoptosis) and increased at 48 h (late apoptosis) compared with those in the other groups. The 1,120 cm−1 absorption band was mainly due to ser, thr and tyrosine C-O (H) stretching vibration (Figs. 4C and D and 5). FACS can detect apoptotic cells as Annexin V has a high affinity for phosphatidylserine. Fig. 3E shows Annexin V binding at the different stages of apoptosis (24 and 48 h). Annexin V binding to phosphatidylserine increased over time, indicating that ser increased the exposure of phosphatidylserine on the outer lipid layer, consistent with the FTIR spectra results.
The band at the 1,410 cm−1 peak, which represents C-H stretching-associated amino acid residues, shifted to higher wave numbers compared with those in the other groups (Fig. 8D).
Band changes are associated with amide I and II
In all spectra, major peaks were observed for absorptions in the amide I and amide II regions at 1,640 and 1,550 cm−1, respectively. The vibrational band at 1,640 cm−1 was primarily characterized by the α-helix secondary structure of the proteins (33) and the absorption bands at 1,550 cm−1 were attributed to the β-sheet secondary structure of proteins (34). As shown in Fig. 7, the spectral intensity at 1,640 and 1,550 cm−1 decreased in the second order derivative spectra of the region of 1,700-1,400 cm−1 at early and late stages of apoptosis. This decrease in intensity indicated that the α-helix and β-sheet contents of the apoptotic cells decreased. The relative peak intensity ratio I1543/I1460 increased significantly, indicating that the hydrogen bond constraint was reduced (Fig. 8E) and the band at 1,550 cm−1 shifted to higher wave numbers at 24 and 48 h (Fig. 9B).
Band changes are associated with polysaccharides
The absorption band at 1,040 cm−1 was mainly due to the polysaccharide C-O stretching vibration arising in early apoptosis (35); however, the peak intensity decreased in late apoptosis (Fig. 8F), suggesting that polysaccharides decreased in late apoptotic cells.
Discussion
The present study demonstrated that the FTIR-MSP spectral pattern of cells of the SW620 human colon cancer cell line reflects their apoptotic stage. On comparison of the spectral analysis with the FACS data, the most obvious differences were observed between 24 h (early apoptosis) and 48 h (late apoptosis). Flow cytometric data revealed that the number of apoptotic cells increased significantly after 24 h and 48 h compared with the cells in the control group and at 12 h.
Apoptosis was characterized by changes in four IR biomarkers: Increased lipid content and absorbance of ser and thr at 1,740 cm−1 and decreased DNA absorbance, decreased α-helix and β-sheet secondary structures of total cellular proteins within the apoptotic cells, the appearance of new vibrational bands at 1,120 and 1,040 cm−1 at 24 h with a decrease in the band at 1,040 cm−1, associated with polysaccharide absorbance at 48 h and an increase in amino acid residues.
Despite several tools to monitor and assess the curative effect of chemotherapy on cancer, colon cancer remains the most frequent cause of mortality in cancer patients. Thus, the investigation of techniques to monitor colon cancer chemotherapy is of clinical importance.
Compared with established techniques, the IR method is relatively inexpensive, fast and can be automated with no reagents. In conclusion, the present study demonstrated that FTIR spectroscopy can distinguish cell apoptosis based on changes in DNA conformation and protein secondary structure, particularly alteration in amino acid residues. This may provide a promising novel tool to monitor cell death in cancer chemotherapy clinically.
Acknowledgements
The authors would like to thank the National Nature Science Foundation of China (nos. 81172362 and 81101874) and the Co-ordinative and Innovative Plan Projects of the Science and Technology Project of Shaanxi Province (no. 2013KTCQ03-08) for supporting this work. The authors would also like to thank Dr Yuanfu Zhang (College of Chemistry and Molecular Engineering, Peking University, Beijing, China) and Dr Shifu Weng for their assistance and advice during the collection and processing of samples.
References
- 1.Tricoli JV, Seibel NL, Blair DG, Albritton K, Hayes-Lattin B. Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. J Natl Cancer Inst. 2011;103:628–635. doi: 10.1093/jnci/djr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushi LH, Byers T, Doyle C, et al. American cancer society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–281. doi: 10.3322/canjclin.56.5.254. [DOI] [PubMed] [Google Scholar]
- 3.Zhao P, Dai M, Chen W, Li N. Cancer trends in china. Jpn J Clin Oncol. 2010;40:281–285. doi: 10.1093/jjco/hyp187. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki K, Tsuno NH, Sunami E, et al. Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer. 2010;10:370. doi: 10.1186/1471-2407-10-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folprecht G, Kohne CH. The role of new agents in the treatment of colorectal cancer. Oncology. 2004;66:1–17. doi: 10.1159/000076329. [DOI] [PubMed] [Google Scholar]
- 6.Stelling AL, Toher D, Uckermann O, et al. Infrared spectroscopic studies of cells and tissues: triple helix proteins as a potential biomarker for tumors. PLoS One. 2013;8:e58332. doi: 10.1371/journal.pone.0058332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyatt MD, Wilson DM., 3rd Participation of DNA repair in the response to 5-fluorouracil. Cell Mol Life Sci. 2009;66:788–799. doi: 10.1007/s00018-008-8557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudin CM, Thompson CB. Apoptosis and disease: regulation and clinical relevance of programmed cell death. Annu Rev Med. 1997;48:267–281. doi: 10.1146/annurev.med.48.1.267. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: the physiological suicide program revisited. Cell Mol Biol Lett. 2006;11:506–525. doi: 10.2478/s11658-006-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 11.Deng N, Zheng L, Liu F, Wang L, Duan H. crcTRP: a translational research platform for colorectal cancer. Comput Math Methods Med. 2013;2013:930362. doi: 10.1155/2013/930362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellisola G, Sorio C. Infrared spectroscopy and microscopy in cancer research and diagnosis. Am J Cancer Res. 2012;2:1–21. [PMC free article] [PubMed] [Google Scholar]
- 13.Cheheltani R, Rosano JM, Wang B, Sabri AK, Pleshko N, Kiani MF. Fourier transform infrared spectroscopic imaging of cardiac tissue to detect collagen deposition after myocardial infarction. J Biomed Opt. 2012;17:056014. doi: 10.1117/1.JBO.17.5.056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis PD, Lewis KE, Ghosal R, et al. Evaluation of FTIR spectroscopy as a diagnostic tool for lung cancer using sputum. BMC Cancer. 2010;10:640. doi: 10.1186/1471-2407-10-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor SE, Cheung KT, Patel II, et al. Infrared spectroscopy with multivariate analysis to interrogate endometrial tissue: a novel and objective diagnostic approach. Br J Cancer. 2011;104:790–797. doi: 10.1038/sj.bjc.6606094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banyay M, Sandbrink J, Stromberg R, Graslund A. Characterization of an RNA bulge structure by fourier transform infrared spectroscopy. Biochem Biophys Res Commun. 2004;324:634–639. doi: 10.1016/j.bbrc.2004.09.098. [DOI] [PubMed] [Google Scholar]
- 17.Downes A, Mouras R, Elfick A. Optical spectroscopy for noninvasive monitoring of stem cell differentiation. J Biomed Biotechnol. 2010;2010:101864. doi: 10.1155/2010/101864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasparri F, Muzio M. Monitoring of apoptosis of HL60 cells by fourier-transform infrared spectroscopy. Biochem J. 2003;369:239–248. doi: 10.1042/BJ20021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelig U, Kapelushnik J, Moreh R, Mordechai S, Nathan I. Diagnosis of cell death by means of infrared spectroscopy. Biophys J. 2009;97:2107–2114. doi: 10.1016/j.bpj.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanciullino R, Giacometti S, Mercier C, et al. In vitro and in vivo reversal of resistance to 5-fluorouracil in colorectal cancer cells with a novel stealth double-liposomal formulation. Br J Cancer. 2007;97:919–926. doi: 10.1038/sj.bjc.6603970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flower KR, Khalifa I, Bassan P, et al. Synchrotron FTIR analysis of drug treated ovarian A2780 cells: an ability to differentiate cell response to different drugs? Analyst. 2011;136:498–507. doi: 10.1039/c0an00564a. [DOI] [PubMed] [Google Scholar]
- 22.Holman HY, Martin MC, Blakely EA, Bjornstad K, McKinney WR. IR spectroscopic characteristics of cell cycle and cell death probed by synchrotron radiation based fourier transform IR spectromicroscopy. Biopolymers. 2000;57:329–335. doi: 10.1002/1097-0282(2000)57:6<329::AID-BIP20>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Hammiche A, German MJ, Hewitt R, Pollock HM, Martin FL. Monitoring cell cycle distributions in MCF-7 cells using near-field photothermal microspectroscopy. Biophys J. 2005;88:3699–3706. doi: 10.1529/biophysj.104.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosino C, Tarallo R, Bamundo A, et al. Identification of a hormone-regulated dynamic nuclear actin network associated with estrogen receptor alpha in human breast cancer cell nuclei. Mol Cell Proteomics. 2010;9:1352–1367. doi: 10.1074/mcp.M900519-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehbe K, Filik J, Frogley MD, Cinque G. The effect of optical substrates on micro-FTIR analysis of single mammalian cells. Anal Bioanal Chem. 2013;405:1311–1324. doi: 10.1007/s00216-012-6521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bali R, Savino L, Ramirez DA, et al. Macroscopic domain formation during cooling in the platelet plasma membrane: an issue of low cholesterol content. Biochim Biophys Acta. 2009;1788:1229–1237. doi: 10.1016/j.bbamem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.German MJ, Hammiche A, Ragavan N, et al. Infrared spectroscopy with multivariate analysis potentially facilitates the segregation of different types of prostate cell. Biophys J. 2006;90:3783–3795. doi: 10.1529/biophysj.105.077255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyran N, Lasch P, Naumann D, Turan B, Severcan F. Early alterations in myocardia and vessels of the diabetic rat heart: an FTIR microspectroscopic study. Biochem J. 2006;397:427–436. doi: 10.1042/BJ20060171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogarty SW, Patel II, Trevisan J, et al. Sub-cellular spectrochemical imaging of isolated human corneal cells employing synchrotron radiation-based Fourier-transform infrared microspectroscopy. Analyst. 2013;138:240–248. doi: 10.1039/c2an36197c. [DOI] [PubMed] [Google Scholar]
- 30.Banyay M, Sarkar M, Graslund A. A library of IR bands of nucleic acids in solution. Biophys Chem. 2003;104:477–488. doi: 10.1016/S0301-4622(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 31.Mello ML, Vidal BC. Changes in the infrared microspectroscopic characteristics of DNA caused by cationic elements, different base richness and single-stranded form. PLoS One. 2012;7:e43169. doi: 10.1371/journal.pone.0043169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batard E, Jamme F, Boutoille D, et al. Fourier transform infrared microspectroscopy of endocarditis vegetation. Appl Spectrosc. 2010;64:901–906. doi: 10.1366/000370210792081172. [DOI] [PubMed] [Google Scholar]
- 33.Abbott GW, Ramesh B, Srai SK. Interaction between soluble and membrane-embedded potassium channel peptides monitored by fourier transform infrared spectroscopy. PLoS One. 2012;7:e49070. doi: 10.1371/journal.pone.0049070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantsch HH, Choo-Smith L-Pi, Shaw RA. Vibrational spectroscopy and medicine: an alliance in the making. Vib Spectrosc. 2002;30:31–41. doi: 10.1016/S0924-2031(02)00036-X. [DOI] [Google Scholar]
- 35.Gautam R, Chandrasekar B, Deobagkar-Lele M, et al. Identification of early biomarkers during acetaminophen-induced hepatotoxicity by fourier transform infrared microspectroscopy. PLoS One. 2012;7:e45521. doi: 10.1371/journal.pone.0045521. [DOI] [PMC free article] [PubMed] [Google Scholar]