Abstract
Ragweed (Ambrosia spp.) is an annually flowering plant whose pollen bears high allergenic potential. Ragweed-induced allergic rhinoconjunctivitis has long been seen as a major immunologic condition in Northern America with high exposure and sensitization rates in the general population. The invasive occurrence of ragweed (A. artemisiifolia) poses an increasing challenge to public health in Europe and Asia as well. Possible explanations for its worldwide spread are climate change and urbanization, as well as pollen transport over long distances by globalized traffic and winds. Due to the increasing disease burden worldwide, and to the lack of a current and comprehensive overview, this study aims to review the current and emerging treatment options for ragweed-induced rhinoconjunctivitis. Sound clinical evidence is present for the symptomatic treatment of ragweed-induced allergic rhinoconjunctivitis with oral third-generation H1-antihistamines and leukotriene antagonists. The topical application of glucocorticoids has also been efficient in randomized controlled clinical trials. Combined approaches employing multiple agents are common. The mainstay of causal treatment to date, especially in Northern America, is subcutaneous immunotherapy with the focus on the major allergen, Amb a 1. Beyond this, growing evidence from several geographical regions documents the benefit of sublingual immunotherapy. Future treatment options promise more specific symptomatic treatment and fewer side effects during causal therapy. Novel antihistamines for symptomatic treatment are aimed at the histamine H3-receptor. New adjuvants with toll-like receptor 4 activity or the application of the monoclonal anti-immunoglobulin E antibody, omalizumab, are supposed to enhance conventional immunotherapy. An approach targeting toll-like receptor 9 by synthetic cytosine phosphate–guanosine oligodeoxynucleotides promises a new treatment paradigm that aims to modulate the immune response, but it has yet to be proven in clinical trials.
Keywords: ambrosia, seasonal allergic rhinitis, allergic conjunctivitis
Specifics of ragweed-induced rhinoconjunctivitis
Botanical characteristics and ecologic factors
Ragweed (genus Ambrosia in the family Asteraceae) is an annual herbaceous flowering plant that is originally native to Northern America. Worldwide, the two most widespread species are common (or short) ragweed (A. artemisiifolia) and great ragweed (A. trifida), which are, in turn, clinically the most relevant for their high potential to cause allergic rhinitis. The distribution of the ragweed species is, however, dependent upon the geographical region. For example, the species causing the most severe symptoms in allergic patients in Israel and parts of the Mediterranean region is A. maritime.1
Ragweed is an invasive species with a growing occurrence in different world regions. Presumably because of climate change, ragweed has reportedly shown an increasing length of pollen season in Northern America,2 implying a potential increasing disease burden for atopic patients in affected areas. A rise in atmospheric carbon dioxide concentrations and air temperature, brought about not only by climate change but also by urbanization, are conditions that stimulate ragweed growth, which is signified by biomass production and flowering date.3 Since ragweed is becoming increasingly common in Europe, pollen load in several regions is also a growing health problem.4 Excluding mountain ranges, almost all of Western, Southern, and Eastern Europe are assumed to be viable to sustain ragweed occurrence.5
Globalization in trade and traffic has been identified as relevant to the distribution of ragweed seeds, contributing to the plant’s invasive characteristics. This is shown, for example, in the contamination of birdseeds with germinable ragweed seeds in France.6 Also, ragweed pollen has been estimated to be transported over long distances, with Eastern Europe being a possible major source.7–10 This has important implications since it makes containment more difficult, while it increases the potential exposure of atopic populations.
Allergens and epidemiology
Presumably, due to high nicotinamide adenine dinucleotide phosphate oxidase,11 as well as serine and cysteine protease activity in its pollen,12 ragweed carries high potential to cause allergic rhinitis. The major allergens of ragweed, Amb a 1 and Amb a 2, belong to the pectate-lyase family and are secreted, acidic, nonglycosylated, single-chain proteins. Minor allergens include Amb a 3 (secreted basic glycoprotein, copper-binding domain), Amb a 5 (secreted basic protein, homologous to Amb t 5 and Amb p 5), Amb a 6 (secreted basic protein, homologous to nonspecific lipid transfer proteins), and Amb a 7 (basic protein, plastocyanin domain).13 As recently as 2010, Amb a 4 has been described for the first time; it is a glycoprotein with a defensin-like domain and is highly homologous to Art v 1, the sunflower protein P22357, and the feverfew allergen Par h 1.14 Further, minor allergens of ragweed include Amb a 11 (cysteine protease)15,16 and Amb a CPI (a cysteine protease inhibitor).17,18 Amb a 8 (profilin), Amb a 9 (polcalcin), and Amb a 10 (polcalcin) are known pan-allergens.13,19 Amb a 6 and Amb a 8 (with potential homologues Cuc m2 and Mus xp 1) are candidates for the cross-reactive structures involved in the clinical manifestation of the supposed ragweed–melon–banana association, which is a pollen–food syndrome.20
With respect to patients allergic to ragweed, in an in vitro study, all patients displayed serum reactivity to Amb a 1, while 30% were monosensitized against Amb a 1 alone.19 A high prevalence of cosensitivity to mugwort (Artemisia vulgaris) has been noted in patients hypersensitive to ragweed; 93% of mugwort-sensitized patients were sensitized to ragweed, whereas 38% of ragweed-hypersensitive patients were sensitized to mugwort.21 This might partially be explained by the homology of Amb a 4 to Art v 1, the major allergen of mugwort.14
In Northern America, ragweed allergy has long been recognized as a major health problem. Sensitization rates are estimated to be as high as 10%–26% in the common population.22,23 Recently, attention has also been drawn to ragweed in Europe, since a rising prevalence of ragweed sensitization has been observed beginning in the 1990s.24 Ragweed sensitization rates in the general population in Europe are given as 10%–30%, as assessed by pollen extracts of ragweed,25,26 and 1.9% for purified Amb a 1.26 In atopic patients, a great variety of sensitization rates has been observed, with values reported from 7.9%–70.0%.27–31 Comparable to the situation in Europe, pollen count and sensitization rates for ragweed in Asia have been increasing for several decades now, as exemplified by the situation in Korea.32 For distinct Asian populations, up to 21%–29% of atopic patients were reported to be sensitized to a specific ragweed species.1 Due to the high cross-reactivity between ragweed and mugwort,21,33,34 however, data on the sensitization rates for ragweed have to be interpreted with care since extracts, and not recombinant allergens, are often used and the values could, at least in part, reflect an effect of allergen homology.
In epidemiologic studies, a high rate of comorbidity of allergic rhinitis, chronic rhinosinusitis, and asthma bronchiale is well established today.35 Specific to ragweed-induced allergic rhinitis, this correlation with concomitant maxillary sinusitis during the allergy season has been confirmed in a clinical study.36 A foundation for this was brought forward in an experimental setting by showing that an intranasal challenge with ragweed allergens leads to an inflammatory reaction of the maxillary sinus.37
Diagnosis
Diagnostic approaches in ragweed-induced allergic rhinitis include obtaining the patient’s precise medical history, especially in terms of potential sources of exposure, seasonal symptoms of nasal inflammation, and relevant comorbidities like asthma. Following this, a skin prick test38 and nasal provocation are the main clinical tests applied today. In vitro testing allows for the subclassification of allergens and cross-reactions.39
Symptomatic treatment options
Several agents in topical and systemic dosage forms are available for the symptomatic treatment of ragweed-induced rhinoconjunctivitis. In clinical trials, first- and second-generation H1-antihistamines and leukotriene receptor antagonists dispensed orally, as well as topical glucocorticoids, were studied alone and in various combinations.
For the symptomatic treatment of conjunctival affection caused by ragweed allergy, an uncontrolled, nonblinded, randomized clinical trial attributed benefit to the topical use of the mast cell stabilizer, nedocromil, with oral application of the third-generation H1-antihistamine, fexofenadine.40 Due to the lack of a proper control group, however, the level of evidence for this finding is considerably low.
H1-antihistamines are the traditional mainstay for the symptomatic treatment of allergies. A longstanding debate has argued whether a reduction in vigilance observed in patients suffering from nasal allergies is caused by the condition itself or by first-generation antihistamines as a possible sedating medication.41 Evidence exists from an experimental study that attributed vigilance reduction to the condition itself in ragweed-induced allergic rhinitis.42 Regarding antihistamines, it was shown that for the symptomatic treatment of ragweed allergy, the first-generation H1-antagonist, diphenhydramine, and the third-generation H1-antagonist, desloratadine, effected a comparable reduction in nasal allergy symptoms compared to placebo.43 However, vigilance and cognitive function were significantly more reduced by diphenhydramine, confirming the value of newer antihistaminic agents.
Olopatadine hydrochloride acts as an antihistamine with anticholinergic and mast cell stabilizing effects. In topical dosage forms, it reduced nasal symptoms, as assessed by a nasal symptom score, when compared to placebo in a 1-day trial.44 During and after exposure in an allergen challenge chamber, a concentration of 0.6% proved to be the most efficient.44 Of note, this was the only study that validated the results obtained in the environmental exposure chamber against previous studies on natural exposure.45,46
The relative benefit of different classes of symptomatic medication is essentially a question of clinical relevance, and therefore the comparison of different agents among each other and against placebo have been the subject of randomized controlled trials. Therefore, the oral application of the third-generation H1-antagonist, levocetirizine, was tested against the leukotriene receptor antagonist, montelukast, and placebo.47 Efficacy was shown in the reduction of nasal symptoms for 5 mg of levocetirizine once daily, while 10 mg of montelukast once daily failed to cause significant mitigation of symptoms in a 2-day trial. The investigation took place in an allergen challenge chamber,47 thereby providing only an approximation of the situation in the natural environment with potentially different allergen characteristics.
Combination treatments with antihistamines are increasingly investigated. No statistically significant differences were found when the second-generation H1-antagonist, fexofenadine, in combination with the sympathomimetic decongestant, pseudoephedrine, was compared to a combination of the second-generation H1-antagonist, loratadine, and the leukotriene receptor antagonist, montelukast.48 Both interventions improved symptoms from baseline. However, without an a priori stated primary endpoint, this study bears a high risk of bias.48 It should be noted that this was the only study available for ragweed-induced rhinoconjunctivitis where the Rhinoconjunctivitis Quality of Life Questionnaire was used as the validated outcome measure for quality of life.49 In another trial, the second-generation H1-antagonist, loratadine, in combination with the leukotriene receptor antagonist, montelukast, as a tablet and solution reduced nasal congestion significantly in patients with ragweed-induced allergic rhinoconjunctivitis in comparison to placebo, while the common over-the-counter decongestant, phenylephrine (an alpha 1-adrenergic receptor agonist), failed to prove efficacy in a parallel arm of the study.50
Increasing interest focuses on topical glucocorticoids for the symptomatic treatment of allergic rhinitis. In patients with ragweed-induced allergic rhinoconjunctivitis, the synthetic glucocorticoid, fluticasone furoate, was shown to be superior with 110 mg applied once daily in a 15-day trial during the allergy season versus placebo, with a symptom score as the primary endpoint.51
Taken together, there is sound evidence to support the clinical application of oral antihistamines and topical glucocorticoids for the symptomatic treatment of ragweed allergy. Leukotriene receptor antagonists were effective in combination with antihistamines, but they have yet to be proven as monotherapy. An overview of the recent prospective, randomized, controlled, double-blind Phase III studies for the symptomatic treatment of ragweed allergy is given in Table 1.
Table 1.
Recent prospective, randomized, controlled, double-blind Phase III studies for the symptomatic treatment of seasonal allergic rhinitis caused by ragweed pollen
| Reference | Randomized participants | Geographic region* | Multicenter | Agent | Study design | Primary endpoint | Validated outcome measure? | Significant difference of primary endpoint? |
|---|---|---|---|---|---|---|---|---|
| Kaiser et al51 | 299 | Northern America | Yes | Topical fluticasone furoate | 15 days (during local allergy season), two arms: 1 10 mg versus placebo | Symptom score | No | Yes |
| Patel et al44 | 320 | Northern America | No | Topical olopatadine | 1 day (allergen challenge chamber), four arms: once at 0.2%; 0.4%; or 0.6% versus placebo | Symptom score | Environmental exposure chamber was validated against natural exposure45,46 | Yes (0.6%) |
| Patel and Patel47 | 403 | Northern America | No | Oral levocetirizine versus montelukast versus placebo | 2 days (allergen challenge chamber), three arms: levocetirizine 5 mg once daily versus montelukast 10 mg once daily versus placebo | Symptom score | No | Yes (levocetirizine) |
| Day et al50 | 379 | Northern America | Yes | Oral loratadine and montelukast versus phenylephrine versus placebo | 2 days (allergen challenge chamber), three arms: loratadine 10 mg and montelukast 10 mg once daily versus phenylephrine 10 mg once daily versus placebo | Peak nasal flow | No | Yes (loratadine and montelukast) |
| Moinuddin et al48 | 72 | Northern America | No | Oral fexofenadine and pseudoephedrine versus loratadine and montelukast | 2 weeks (during local allergy season), two arms: fexofenadine 60 mg and pseudoephedrine 120 mg twice daily versus loratadine, 10 mg and montelukast, 10 mg once daily | Not specifically designated | Rhinoconjunctivitis Quality of Life Questionnaire45 | Not applicable |
Note:
Geographical subregion as defined by the United Nations Organization.
The choice of meaningful outcome measures in clinical trials of the symptomatic application of topical glucocorticoids has recently been evaluated. In a prospective clinical evaluation,52 a nasal challenge was effected by the topical delivery of allergens. Sneeze count, symptom scores, and albumin level in nasal lavage were validated as being reproducibly influenced by the intervention, and they were therefore deemed to be suitable outcome parameters. The further candidate, lysozyme level in nasal lavage, was not influenced by glucocorticoid application, while kinin level was not reproducible.52 For the design of future studies, these results should be honored.
Causal treatment options
Subcutaneous immunotherapy is the traditional mainstay for the causal treatment of allergic disorders. Subcutaneous immunotherapy for ragweed is considerably well documented in Northern America. A prospective, randomized clinical trial53 from Southern Europe also reported some benefit for the efficacy of this treatment option in another geographical region. However, without an a priori stated primary endpoint, this study is at high risk of biased results.
Sublingual immunotherapy is generally gaining interest due to its supposed lower risk of side effects and potentially better compliance.54,55 However, a considerable number of questions has remained open regarding the mechanisms of the desired effect and the optimal dosage.56 As will be detailed, several randomized, controlled clinical trials have therefore been conducted to investigate the efficacy and safety of sublingual immunotherapy for ragweed allergy.
Testing a formulation with a continuous dose applied as a solid tablet, André et al57 found significant benefit of an oral immunotherapy of a standardized ragweed extract in a multicenter study from Western Europe. However, this study did not include an a priori defined primary endpoint and thus also bears considerable risk of bias.
Two prospective clinical trials from Northern America failed to show a significant difference in the primary endpoint for efficacy, which included the improvement of a symptom score58 or improvements in combined symptom and medication scores,59 during the respective local ragweed pollen season. However, both formulations of ragweed extract used for sublingual application were deemed to be safe for further clinical investigations. As a reason for not being able to demonstrate efficacy, a possibly polysensitized patient cohort58 and variations in pollen load between the study centers59 were hypothesized.
Recently, two studies with considerably high numbers of randomized patients (565 patients60 and 784 patients61) were undertaken to investigate the effect of a sublingual allergy immunotherapy tablet. Both studies showed the efficacy of this treatment in Northern America,60 as well as in Northern America and Eastern Europe.61 In both cases, the primary endpoint was a combined symptom and medication score, while the effective doses were 6 μg and 12 μg60 or 12 μg61 of the major allergen Amb a 1 in each single tablet.
Taken together, growing evidence supports the clinical value of sublingual immunotherapy in ragweed allergies. An overview of the recent prospective, randomized, controlled, double-blind Phase III studies for the causal treatment of ragweed allergy is given in Table 2.
Table 2.
Recent prospective, randomized, controlled, double-blind Phase III studies for the causal treatment of seasonal allergic rhinitis caused by ragweed pollen
| Reference | Randomized participants | Geographic region* | Multi center | Agent | Study design | Primary endpoint | Validated outcome measure? | Significant difference of primary endpoint? |
|---|---|---|---|---|---|---|---|---|
| Creticos et al61 | 784 | Northern America and Eastern Europe | Yes | Sublingual allergy immunotherapy tablet (MK-3641; Merck and Co, Inc., Whitehouse Station, NJ, USA) | 52 weeks, four arms: l.5 μg;6 μg; 12 μg; or placebo | Combined symptom and medication score | No | Yes(12 μg) |
| Nolte et al60 | 565 | Northern America | Yes | Sublingual allergy immunotherapy tablet (MK-3641; Merck and Co, Inc.) | 52 weeks, three arms: 6 μg; 12 μg; or placebo | Combined symptom and medication score | No | Yes (6 μg and 12 μg) |
| Skoner et al58 | 115 | Northern America | Yes | Sublingual liquid (standardized glycerinated short ragweed pollen allergenic extract; Greer Laboratories, Inc., Lenoir, NC, USA) | 17 weeks, three arms: 4.8 μg; 48 μg; or placebo | Symptom score | No | No |
| Bowen et al59 | 83 | Northern America | Yes | Sublingual liquid (active product standardized ragweed allergen extract; Staloral; Stallergenes SA, Antony, France) | Ragweed season, two arms: increasing doses or placebo | Combined symptom and medication score | No | No |
| Mirone et al53 | 32 | Southern Europe | Yes | Subcutaneous injection (ragweed pollen extract absorbed with aluminum hydroxide/phenolated saline solution; ALK-Abellò, Hørsholm, Denmark) | 12 months, two arms: increasing doses or placebo | Not specifically designated | No | Not applicable |
| André et al57 | 110 | Western Europe | Yes | Sublingual liquid or tablet (standardized ragweed extract; Stallergenes SA) | 7.5 months, two arms: increasing doses or placebo | Not specifically designated | No | Not applicable |
Note:
Geographical subregion as defined by the United Nations Organization.
Future perspectives
In the future, an important challenge in Europe will be the reduction of pollen exposure to the population by limitation of ragweed expansion. As a potential basic measure, repetitive mowing during the annual life cycle of the plant has been shown to be effective in the reduction of pollen load.62 Beyond this, combined efforts of manual uprooting, herbicides, and mowing have been proposed according to local conditions.63 Furthermore, advanced knowledge of pollen load might help affected patients avoid allergen exposure. Numerous studies investigated region-specific pollen counts,64–66 helping to develop several forecasting models.67–69
Allergies to different ragweed species might carry different clinical profiles. A recent study70 compared the symptoms elicited during the natural pollen season in a region where giant ragweed is more prevalent to the symptoms elicited in an allergen challenge chamber by short ragweed pollen. It thereby identified clusters with three distinct response profiles among the patients tested, signifying low and high responses during the natural season and experimental challenge. This implies the possibility of subtypes in ragweed pollinosis that could bear consequences for treatment.70
Several nonpharmacological treatment approaches have been explored to treat ragweed-induced allergic rhinoconjunctivitis. Prospective open clinical studies explored the value of intranasal phototherapy three times a week during the ragweed pollen season. The defined dose of a combination of ultraviolet (UV)-B, UV-A, and visible light led to improvements in a nasal symptom score,71 even in comparison to the second-generation H1-antagonist, fexofenadine.72 Another open study found a beneficial effect of photochemotherapy by irradiation of the nasal cavity with UV-A light after topical sensitization with a psoralen (psoralen with ultraviolet A light [PUVA]) in terms of a symptom score.73 A simple barrier method like nasal filters was also described as being effective in reducing symptoms during exposure to ragweed allergens.74
New agents are nearing clinical application. Proposals for symptomatic treatments include selective histamine-H3 receptor antagonists, with the compounds JNJ-39220675 and PF-03654746 being principally able to relieve nasal allergy symptoms.75,76 In preclinical investigations, new target structures are being evaluated for their value in the treatment of ragweed-induced allergies. While traditional leukotriene receptor antagonists like montelukast block the cysteinyl leukotriene receptor 1, the leukotriene B4 receptor antagonist ONO-4057 was able to inhibit itching after a ragweed challenge in an experimental animal model of conjunctivitis.77 In a comparable model, the selective DP2 receptor antagonist, AM 156, has been shown to reduce the symptoms of an allergen challenge.78
Improvements of subcutaneous immunotherapy include the addition of adjuvants or omalizumab, an anti-immunoglobulin (Ig)E antibody, as well as modified allergens.79 Considerable effort is being put into the development of toll-like receptor agonists, with toll-like receptors 4 and 9 as the most promising target structures.80
The monoclonal antibody, omalizumab, has been approved for clinical use in several countries now. It is able to block IgE binding to the high-affinity IgE receptor, Fc epsilon RI (FCER1), on mast cells and basophils by inactivating soluble and membrane-bound IgE81 and reducing the overall serum level of IgE after continuous therapy.82 It acts as a mast cell stabilizing agent81 and has the potential to limit the effects of aeroallergen exposure mediated by basophil83 or dendritic cells.84 Therefore, the expression of FCER1 on immune cells is reduced.82 A randomized controlled clinical trial was able to show that omalizumab pretreatment was able to ameliorate the side effects of subcutaneous rush immunotherapy for ragweed seasonal allergic rhinitis,85 while it has also been shown that this was caused by the inhibition of allergen-specific IgE binding.86 However, an issue that still needs to be resolved for ragweed-induced allergic rhinoconjunctivitis is the cost effectiveness of omalizumab. For allergic asthma, the benefits of omalizumab have been calculated to outweigh the cost, but only in severely affected subgroups of patients.87
An entirely new approach is the toll-like receptor 9-based inhibition of immune responses mediated by type 2 T-helper cells, which modulate dendritic cells and mononuclear cells.88 This has been attempted by an immunostimulatory conjugate of the ragweed major allergen, Amb a 1, and an oligodeoxyribonucleotide DNA sequence containing a CpG motif. Favorable redirection of the immunoreaction has been shown,89–94 in addition to a reduction of symptoms and an improvement in quality of life for up to two consecutive seasons, following a regime containing only six injections in a pilot clinical trial.95 However, further treatments were not able to prove clinical benefit, and there are currently no new data on this approach.79
New treatment regimens and formulations for subcutaneous immunotherapy are also being evaluated. An ultrashort treatment course applying an allergoid with the toll-like receptor 4 agonist, monophosphoryl lipid A, as an adjuvant was demonstrated to be safe,96 and it had effected a significant improvement in the symptom score.97
A field that is currently not employed for the treatment of allergic rhinitis is cytokine signaling. In studies employing an animal model of experimental conjunctivitis, a role for interleukin-1098,99 and interleukin-16100 has been described in the development of the reaction in response to an allergen challenge. Correspondingly, suppressors of cytokine signaling (SOCS)3 and SOCS5 have been reported to bear the potential to regulate allergic responses.101 Taken together, there are several more promising targets for the treatment of allergic rhinitis currently still outside clinical investigation.
Methods
The search strategy on MEDLINE via PubMed applied the medical subject heading (MeSH) terms “Ambrosia” in combination with either “Rhinitis, Allergic, Seasonal”, “Rhinitis, Allergic, Perennial”, “Conjunctivitis, Allergic”, or “Allergic Rhinitis [Supplementary Concept]”.
The search was restricted to English- and German-language publications and yielded 182 single entries as of September 2014. The review was limited to publications published since 2002 (170 entries). Obtained publications were systematically screened by the abstract. For the evaluation of symptomatic and causal treatment options, only publications reporting Phase III randomized, controlled, double-blind clinical trials were included. This resulted in five studies reporting symptomatic and six studies reporting causal treatments.
This low number of studies presents a considerable risk for publication bias. The variety in study designs and primary outcome measures found in the literature, as well as the lack of validated outcome measures, did not allow us to pool the study results for the meta-analysis. A flow diagram of the review process according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement102 is shown in Figure 1.
Figure 1.
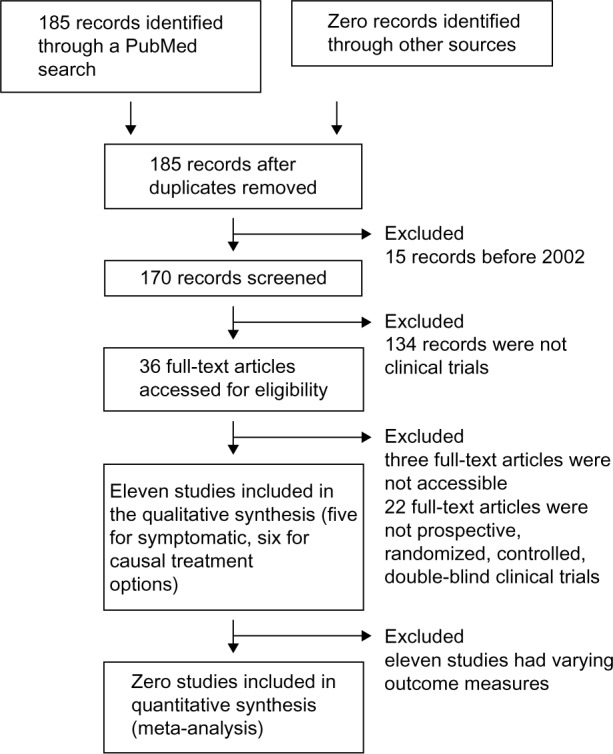
Flow diagram of the review process compliant to the PRISMA statement.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Conclusion
Ragweed-induced allergic rhinoconjunctivitis is prevalent in Northern America and Europe. For symptomatic treatment, clinical evidence exists for antihistamines, leukotriene antagonists, and glucocorticoids. Options for causal treatment include subcutaneous and sublingual immunotherapy. Novel antihistamines, refined adjuvants, and combination therapy with the anti-IgE antibody, omalizumab, as well as an immunomodulatory approach involving toll-like receptor 9 are promising for the future.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Waisel Y, Eshel A, Keynan N, Langgut D. Ambrosia: a new impending disaster for the Israeli allergic population. Isr Med Assoc J. 2008;10(12):856–857. [PubMed] [Google Scholar]
- 2.Ziska L, Knowlton K, Rogers C, et al. Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proc Natl Acad Sci U S A. 2011;108(10):4248–4251. doi: 10.1073/pnas.1014107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG. Cities as harbingers of climate change: common ragweed, urbanization, and public health. J Allergy Clin Immunol. 2003;111(2):290–295. doi: 10.1067/mai.2003.53. [DOI] [PubMed] [Google Scholar]
- 4.Ridolo E, Albertini R, Giordano D, Soliani L, Usberti I, Dall’Aglio PP. Airborne pollen concentrations and the incidence of allergic asthma and rhinoconjunctivitis in northern Italy from 1992 to 2003. Int Arch Allergy Immunol. 2007;142(2):151–157. doi: 10.1159/000096441. [DOI] [PubMed] [Google Scholar]
- 5.Chapman DS, Haynes T, Beal S, Essl F, Bullock JM. Phenology predicts the native and invasive range limits of common ragweed. Glob Chang Biol. 2014;20(1):192–202. doi: 10.1111/gcb.12380. [DOI] [PubMed] [Google Scholar]
- 6.Thibaudon M, Colonnello C, Besancenot JP, Toloba Y, François H, Caillaud D. Can birdseed contribute to the spread of ragweed? J Investig Allergol Clin Immunol. 2012;22(3):234–236. [PubMed] [Google Scholar]
- 7.Kasprzyk I. Non-native Ambrosia pollen in the atmosphere of Rzeszów (SE Poland); evaluation of the effect of weather conditions on daily concentrations and starting dates of the pollen season. Int J Biometeorol. 2008;52(5):341–351. doi: 10.1007/s00484-007-0129-0. [DOI] [PubMed] [Google Scholar]
- 8.Stach A, Smith M, Skjøth CA, Brandt J. Examining Ambrosia pollen episodes at Poznań (Poland) using back-trajectory analysis. Int J Biometeorol. 2007;51(4):275–286. doi: 10.1007/s00484-006-0068-1. [DOI] [PubMed] [Google Scholar]
- 9.Cecchi L, Lorenzo C, Morabito M, et al. Long distance transport of ragweed pollen as a potential cause of allergy in central Italy. Ann Allergy Asthma Immunol. 2006;96(1):86–91. doi: 10.1016/s1081-1206(10)61045-9. [DOI] [PubMed] [Google Scholar]
- 10.Puc M. Ragweed pollen in the air of Szczecin. Ann Agric Environ Med. 2004;11(1):53–57. [PubMed] [Google Scholar]
- 11.Dharajiya N, Boldogh I, Cardenas V, Sur S. Role of pollen NAD(P) H oxidase in allergic inflammation. Curr Opin Allergy Clin Immunol. 2008;8(1):57–62. doi: 10.1097/ACI.0b013e3282f3b5dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunawan H, Takai T, Ikeda S, Okumura K, Ogawa H. Protease activity of allergenic pollen of cedar, cypress, juniper, birch and ragweed. Allergol Int. 2008;57(1):83–91. doi: 10.2332/allergolint.O-07-507. [DOI] [PubMed] [Google Scholar]
- 13.Gadermaier G, Dedic A, Obermeyer G, Frank S, Himly M, Ferreira F. Biology of weed pollen allergens. Curr Allergy Asthma Rep. 2004;4(5):391–400. doi: 10.1007/s11882-004-0090-5. [DOI] [PubMed] [Google Scholar]
- 14.Léonard R, Wopfner N, Pabst M, et al. A new allergen from ragweed (Ambrosia artemisiifolia) with homology to art v 1 from mugwort. J Biol Chem. 2010;285(35):27192–27200. doi: 10.1074/jbc.M110.127118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radauer C, Nandy A, Ferreira F, et al. Update of the WHO/IUIS Allergen Nomenclature Database based on analysis of allergen sequences. Allergy. 2014;69(4):413–419. doi: 10.1111/all.12348. [DOI] [PubMed] [Google Scholar]
- 16.Mari A, Rasi C, Palazzo P, Scala E. Allergen databases: current status and perspectives. Curr Allergy Asthma Rep. 2009;9(5):376–383. doi: 10.1007/s11882-009-0055-9. [DOI] [PubMed] [Google Scholar]
- 17.Rogers BL, Pollock J, Klapper DG, Griffith IJ. Sequence of the proteinase-inhibitor cystatin homologue from the pollen of Ambrosia artemisiifolia (short ragweed) Gene. 1993;133(2):219–221. doi: 10.1016/0378-1119(93)90641-f. [DOI] [PubMed] [Google Scholar]
- 18.Popovic MM, Milovanovic M, Burazer L, et al. Cysteine proteinase inhibitor Act d 4 is a functional allergen contributing to the clinical symptoms of kiwifruit allergy. Mol Nutr Food Res. 2010;54(3):373–380. doi: 10.1002/mnfr.200900035. [DOI] [PubMed] [Google Scholar]
- 19.Gadermaier G, Wopfner N, Wallner M, et al. Array-based profiling of ragweed and mugwort pollen allergens. Allergy. 2008;63(11):1543–1549. doi: 10.1111/j.1398-9995.2008.01780.x. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006;61(4):461–476. doi: 10.1111/j.1398-9995.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 21.Asero R, Wopfner N, Gruber P, Gadermaier G, Ferreira F. Artemisia and Ambrosia hypersensitivity: co-sensitization or co-recognition? Clin Exp Allergy. 2006;36(5):658–665. doi: 10.1111/j.1365-2222.2006.02477.x. [DOI] [PubMed] [Google Scholar]
- 22.Gergen PJ, Turkeltaub PC, Kovar MG. The prevalence of allergic skin test reactivity to eight common aeroallergens in the US population: results from the second National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 1987;80(5):669–679. doi: 10.1016/0091-6749(87)90286-7. [DOI] [PubMed] [Google Scholar]
- 23.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116(2):377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Burbach GJ, Heinzerling LM, Röhnelt C, Bergmann KC, Behrendt H, Zuberbier T, GA(2)LEN study Ragweed sensitization in Europe – GA(2)LEN study suggests increasing prevalence. Allergy. 2009;64(4):664–665. doi: 10.1111/j.1398-9995.2009.01975.x. [DOI] [PubMed] [Google Scholar]
- 25.Boehme MW, Gabrio T, Dierkesmann R, et al. Sensitization to airborne ragweed pollen – a cause of allergic respiratory diseases in Germany? Dtsch Med Wochenschr. 2009;134(28–29):1457–1463. doi: 10.1055/s-0029-1225300. German. [DOI] [PubMed] [Google Scholar]
- 26.Boehme MW, Kompauer I, Weidner U, Piechotowski I, Gabrio T, Behrendt H. Respiratory symptoms and sensitization to airborne pollen of ragweed and mugwort of adults in Southwest Germany. Dtsch Med Wochenschr. 2013;138(33):1651–1658. doi: 10.1055/s-0033-1343330. German. [DOI] [PubMed] [Google Scholar]
- 27.Ruëff F, Przybilla B, Walker A, et al. Sensitization to common ragweed in southern Bavaria: clinical and geographical risk factors in atopic patients. Int Arch Allergy Immunol. 2012;159(1):65–74. doi: 10.1159/000335192. [DOI] [PubMed] [Google Scholar]
- 28.Canis M, Becker S, Gröger M, Kramer MF. IgE reactivity patterns in patients with allergic rhinoconjunctivitis to ragweed and mugwort pollens. Am J Rhinol Allergy. 2012;26(1):31–35. doi: 10.2500/ajra.2012.26.3698. [DOI] [PubMed] [Google Scholar]
- 29.Tosi A, Wüthrich B, Bonini M, Pietragalla-Köhler B. Time lag between Ambrosia sensitisation and Ambrosia allergy: a 20-year study (1989–2008) in Legnano, northern Italy. Swiss Med Wkly. 2011;141:w13253. doi: 10.4414/smw.2011.13253. [DOI] [PubMed] [Google Scholar]
- 30.Testi S, Carabelli A, Cecchi L, et al. Multicenter investigation to assess the prevalence of ambrosia pollen allergy in Tuscany. J Investig Allergol Clin Immunol. 2009;19(3):251–252. [PubMed] [Google Scholar]
- 31.Ackermann-Liebrich U, Schindler C, Frei P, et al. Sensitisation to Ambrosia in Switzerland: a public health threat on the wait. Swiss Med Wkly. 2009;139(5–6):70–75. doi: 10.4414/smw.2009.12489. [DOI] [PubMed] [Google Scholar]
- 32.Kim JH, Oh JW, Lee HB, et al. Changes in sensitization rate to weed allergens in children with increased weeds pollen counts in Seoul metropolitan area. J Korean Med Sci. 2012;27(4):350–355. doi: 10.3346/jkms.2012.27.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberhuber C, Ma Y, Wopfner N, et al. Prevalence of IgE-binding to Art v 1, Art v 4 and Amb a 1 in mugwort-allergic patients. Int Arch Allergy Immunol. 2008;145(2):94–101. doi: 10.1159/000108134. [DOI] [PubMed] [Google Scholar]
- 34.Han D, Lai X, Gjesing B, Zhong N, Zhang L, Spangfort MD. The specific IgE reactivity pattern of weed pollen-induced allergic rhinitis patients. Acta Otolaryngol. 2011;131(5):533–538. doi: 10.3109/00016489.2010.539265. [DOI] [PubMed] [Google Scholar]
- 35.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67(1):91–98. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 36.Baroody FM, Mucha SM, deTineo M, Naclerio RM. Evidence of maxillary sinus inflammation in seasonal allergic rhinitis. Otolaryngol Head Neck Surg. 2012;146(6):880–886. doi: 10.1177/0194599811435972. [DOI] [PubMed] [Google Scholar]
- 37.Baroody FM, Mucha SM, Detineo M, Naclerio RM. Nasal challenge with allergen leads to maxillary sinus inflammation. J Allergy Clin Immunol. 2008;121(5):1126–1132. doi: 10.1016/j.jaci.2008.02.010. e7. [DOI] [PubMed] [Google Scholar]
- 38.Gungor A, Houser SM, Aquino BF, et al. A comparison of skin endpoint titration and skin-prick testing in the diagnosis of allergic rhinitis. Ear Nose Throat J. 2004;83(1):54–60. [PubMed] [Google Scholar]
- 39.Jiang XD, Li GY, Dong Z, Zhu DD. Correlation analysis of two serum-specific immunoglobulin E test systems and skin-prick test in allergic rhinitis patients from northeast China. Am J Rhinol Allergy. 2011;25(2):116–119. doi: 10.2500/ajra.2011.25.3572. [DOI] [PubMed] [Google Scholar]
- 40.Alexander M, Patel P, Allegro S, Hicks A. Supplementation of fexofenadine therapy with nedocromil sodium 2% ophthalmic solution to treat ocular symptoms of seasonal allergic conjunctivitis. Clin Experiment Ophthalmol. 2003;31(3):206–212. doi: 10.1046/j.1442-9071.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- 41.Druce HM. Performance impairment revisited. Ann Allergy Asthma Immunol. 2002;89(4):344–345. doi: 10.1016/S1081-1206(10)62031-5. [DOI] [PubMed] [Google Scholar]
- 42.Wilken JA, Berkowitz R, Kane R. Decrements in vigilance and cognitive functioning associated with ragweed-induced allergic rhinitis. Ann Allergy Asthma Immunol. 2002;89(4):372–380. doi: 10.1016/S1081-1206(10)62038-8. [DOI] [PubMed] [Google Scholar]
- 43.Wilken JA, Kane RL, Ellis AK, et al. A comparison of the effect of diphenhydramine and desloratadine on vigilance and cognitive function during treatment of ragweed-induced allergic rhinitis. Ann Allergy Asthma Immunol. 2003;91(4):375–385. doi: 10.1016/S1081-1206(10)61685-7. [DOI] [PubMed] [Google Scholar]
- 44.Patel P, Roland PS, Marple BF, et al. An assessment of the onset and duration of action of olopatadine nasal spray. Otolaryngol Head Neck Surg. 2007;137(6):918–924. doi: 10.1016/j.otohns.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Ratner PH, Hampel FC, Amar NJ, et al. Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis to mountain cedar. Ann Allergy Asthma Immunol. 2005;95(5):474–479. doi: 10.1016/S1081-1206(10)61174-X. [DOI] [PubMed] [Google Scholar]
- 46.Meltzer EO, Hampel FC, Ratner PH, et al. Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95(6):600–606. doi: 10.1016/S1081-1206(10)61025-3. [DOI] [PubMed] [Google Scholar]
- 47.Patel P, Patel D. Efficacy comparison of levocetirizine vs montelukast in ragweed sensitized patients. Ann Allergy Asthma Immunol. 2008;101(3):287–294. doi: 10.1016/S1081-1206(10)60494-2. [DOI] [PubMed] [Google Scholar]
- 48.Moinuddin R, deTineo M, Maleckar B, Naclerio RM, Baroody FM. Comparison of the combinations of fexofenadine-pseudoephedrine and loratadine-montelukast in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2004;92(1):73–79. doi: 10.1016/S1081-1206(10)61713-9. [DOI] [PubMed] [Google Scholar]
- 49.Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clin Exp Allergy. 1991;21(1):77–83. doi: 10.1111/j.1365-2222.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 50.Day JH, Briscoe MP, Ratz JD, Danzig M, Yao R. Efficacy of loratadine-montelukast on nasal congestion in patients with seasonal allergic rhinitis in an environmental exposure unit. Ann Allergy Asthma Immunol. 2009;102(4):328–338. doi: 10.1016/S1081-1206(10)60339-0. [DOI] [PubMed] [Google Scholar]
- 51.Kaiser HB, Naclerio RM, Given J, Toler TN, Ellsworth A, Philpot EE. Fluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;119(6):1430–1437. doi: 10.1016/j.jaci.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 52.Proud D, Riker DK, Togias A. Reproducibility of nasal allergen challenge in evaluating the efficacy of intranasal corticosteroid treatment. Clin Exp Allergy. 2010;40(5):738–744. doi: 10.1111/j.1365-2222.2010.03466.x. [DOI] [PubMed] [Google Scholar]
- 53.Mirone C, Albert F, Tosi A, et al. Efficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double-blind, placebo-controlled study. Clin Exp Allergy. 2004;34(9):1408–1414. doi: 10.1111/j.1365-2222.2004.02056.x. [DOI] [PubMed] [Google Scholar]
- 54.Canonica GW, Passalacqua G. Noninjection routes for immunotherapy. J Allergy Clin Immunol. 2003;111(3):437–448. doi: 10.1067/mai.2003.129. ; quiz 449. [DOI] [PubMed] [Google Scholar]
- 55.Leatherman BD, Owen S, Parker M, et al. Sublingual Immunotherapy: Past, present, paradigm for the future? A review of the literature. Otolaryngol Head Neck Surg. 2007;136(3 Suppl):S1–S20. doi: 10.1016/j.otohns.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 56.Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006;117(5):1021–1035. doi: 10.1016/j.jaci.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 57.André C, Perrin-Fayolle M, Grosclaude M, et al. A double-blind placebo-controlled evaluation of sublingual immunotherapy with a standardized ragweed extract in patients with seasonal rhinitis. Evidence for a dose-response relationship. Int Arch Allergy Immunol. 2003;131(2):111–118. doi: 10.1159/000070926. [DOI] [PubMed] [Google Scholar]
- 58.Skoner D, Gentile D, Bush R, Fasano MB, McLaughlin A, Esch RE. Sublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollen. J Allergy Clin Immunol. 2010;125(3):660–666. doi: 10.1016/j.jaci.2009.12.931. , 666. e1–e666. e4. [DOI] [PubMed] [Google Scholar]
- 59.Bowen T, Greenbaum J, Charbonneau Y, et al. Canadian trial of sub-lingual swallow immunotherapy for ragweed rhinoconjunctivitis. Ann Allergy Asthma Immunol. 2004;93(5):425–430. doi: 10.1016/S1081-1206(10)61408-1. [DOI] [PubMed] [Google Scholar]
- 60.Nolte H, Hébert J, Berman G, et al. Randomized controlled trial of ragweed allergy immunotherapy tablet efficacy and safety in North American adults. Ann Allergy Asthma Immunol. 2013;110(6):450–456. doi: 10.1016/j.anai.2013.03.013. e4. [DOI] [PubMed] [Google Scholar]
- 61.Creticos PS, Maloney J, Bernstein DI, et al. Randomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adults. J Allergy Clin Immunol. 2013;131(5):1342–1349. doi: 10.1016/j.jaci.2013.03.019. e6. [DOI] [PubMed] [Google Scholar]
- 62.Simard MJ, Benoit DL. Effect of repetitive mowing on common ragweed (Ambrosia artemisiifolia L.) pollen and seed production. Ann Agric Environ Med. 2011;18(1):55–62. [PubMed] [Google Scholar]
- 63.Gajnik D, Peternel R. Methods of intervention in the control of ragweed spread (Ambrosia artemisiifolia L.) in the area of Zagreb County and the city of Zagreb. Coll Antropol. 2009;33(4):1289–1294. [PubMed] [Google Scholar]
- 64.Laaidi M, Laaidi K, Besancenot JP, Thibaudon M. Ragweed in France: an invasive plant and its allergenic pollen. Ann Allergy Asthma Immunol. 2003;91(2):195–201. doi: 10.1016/S1081-1206(10)62177-1. [DOI] [PubMed] [Google Scholar]
- 65.Peternel R, Music Milanovic S, Srnec L. Airborne ragweed (Ambrosia artemisiifolia L.) pollen content in the city of Zagreb and implications on pollen allergy. Ann Agric Environ Med. 2008;15(1):125–130. [PubMed] [Google Scholar]
- 66.Sikoparija B, Radisic P, Pejak T, Simic S. Airborne grass and ragweed pollen in the southern Panonnian Valley – consideration of rural and urban environment. Ann Agric Environ Med. 2006;13(2):263–266. [PubMed] [Google Scholar]
- 67.Csépe Z, Makra L, Voukantsis D, et al. Predicting daily ragweed pollen concentrations using Computational Intelligence techniques over two heavily polluted areas in Europe. Sci Total Environ. 2014;476–477:542–552. doi: 10.1016/j.scitotenv.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 68.Howard LE, Levetin E. Ambrosia pollen in Tulsa, Oklahoma: aerobiology, trends, and forecasting model development. Ann Allergy Asthma Immunol. 2014;113(6):641–646. doi: 10.1016/j.anai.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 69.Laaidi M, Thibaudon M, Besancenot JP. Two statistical approaches to forecasting the start and duration of the pollen season of Ambrosia in the area of Lyon (France) Int J Biometeorol. 2003;48(2):65–73. doi: 10.1007/s00484-003-0182-2. [DOI] [PubMed] [Google Scholar]
- 70.Jacobs RL, Harper N, He W, et al. Responses to ragweed pollen in a pollen challenge chamber versus seasonal exposure identify allergic rhinoconjunctivitis endotypes. J Allergy Clin Immunol. 2012;130(1):122–127. doi: 10.1016/j.jaci.2012.03.031. e8. [DOI] [PubMed] [Google Scholar]
- 71.Koreck AI, Csoma Z, Bodai L, et al. Rhinophototherapy: a new therapeutic tool for the management of allergic rhinitis. J Allergy Clin Immunol. 2005;115(3):541–547. doi: 10.1016/j.jaci.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Garaczi E, Boros-Gyevi M, Bella Z, Csoma Z, Kemény L, Koreck A. Intranasal phototherapy is more effective than fexofenadine hydrochloride in the treatment of seasonal allergic rhinitis: results of a pilot study. Photochem Photobiol. 2011;87(2):474–477. doi: 10.1111/j.1751-1097.2010.00882.x. [DOI] [PubMed] [Google Scholar]
- 73.Csoma Z, Koreck A, Ignacz F, et al. PUVA treatment of the nasal cavity improves the clinical symptoms of allergic rhinitis and inhibits the immediate-type hypersensitivity reaction in the skin. J Photochem Photobiol B. 2006;83(1):21–26. doi: 10.1016/j.jphotobiol.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 74.O’Meara TJ, Sercombe JK, Morgan G, Reddel HK, Xuan W, Tovey ER. The reduction of rhinitis symptoms by nasal filters during natural exposure to ragweed and grass pollen. Allergy. 2005;60(4):529–532. doi: 10.1111/j.1398-9995.2005.00741.x. [DOI] [PubMed] [Google Scholar]
- 75.Barchuk WT, Salapatek AM, Ge T, D’Angelo P, Liu X. A proof-of-concept study of the effect of a novel H3-receptor antagonist in allergen-induced nasal congestion. J Allergy Clin Immunol. 2013;132(4):838–846. doi: 10.1016/j.jaci.2013.05.001. e1–e6. [DOI] [PubMed] [Google Scholar]
- 76.Stokes JR, Romero FA, Jr, Allan RJ, et al. The effects of an H3 receptor antagonist (PF-03654746) with fexofenadine on reducing allergic rhinitis symptoms. J Allergy Clin Immunol. 2012;129(2):409–412. doi: 10.1016/j.jaci.2011.11.026. , 412. e1–e2. [DOI] [PubMed] [Google Scholar]
- 77.Andoh T, Sakai K, Urashima M, Kitazawa K, Honma A, Kuraishi Y. Involvement of leukotriene B4 in itching in a mouse model of ocular allergy. Exp Eye Res. 2012;98:97–103. doi: 10.1016/j.exer.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Stebbins KJ, Broadhead AR, Musiyenko A, et al. DP2 (CRTh2) antagonism reduces ocular inflammation induced by allergen challenge and respiratory syncytial virus. Int Arch Allergy Immunol. 2012;157(3):259–268. doi: 10.1159/000328769. [DOI] [PubMed] [Google Scholar]
- 79.Casale TB, Stokes JR. Immunotherapy: what lies beyond. J Allergy Clin Immunol. 2014;133(3):612–619. doi: 10.1016/j.jaci.2014.01.007. : quiz 620. [DOI] [PubMed] [Google Scholar]
- 80.Aryan Z, Holgate ST, Radzioch D, Rezaei N. A new era of targeting the ancient gatekeepers of the immune system: toll-like agonists in the treatment of allergic rhinitis and asthma. Int Arch Allergy Immunol. 2014;164(1):46–63. doi: 10.1159/000362553. [DOI] [PubMed] [Google Scholar]
- 81.Chang TW, Shiung YY. Anti-IgE as a mast cell-stabilizing therapeutic agent. J Allergy Clin Immunol. 2006;117(6):1203–1212. doi: 10.1016/j.jaci.2006.04.005. ; quiz 1213. [DOI] [PubMed] [Google Scholar]
- 82.Lin H, Boesel KM, Griffith DT, et al. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol. 2004;113(2):297–302. doi: 10.1016/j.jaci.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 83.Saini S, Bloom DC, Bieneman A, Vasagar K, Togias A, Schroeder J. Systemic effects of allergen exposure on blood basophil IL-13 secretion and FcepsilonRIbeta. J Allergy Clin Immunol. 2004;114(4):768–774. doi: 10.1016/j.jaci.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 84.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112(6):1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Casale TB, Busse WW, Kline JN, et al. Immune Tolerance Network Group Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006;117(1):134–140. doi: 10.1016/j.jaci.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 86.Klunker S, Saggar LR, Seyfert-Margolis V, et al. Immune Tolerance Network Group Combination treatment with omalizumab and rush immunotherapy for ragweed-induced allergic rhinitis: Inhibition of IgE-facilitated allergen binding. J Allergy Clin Immunol. 2007;120(3):688–695. doi: 10.1016/j.jaci.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 87.Faria R, McKenna C, Palmer S. Optimizing the position and use of omalizumab for severe persistent allergic asthma using cost- effectiveness analysis. Value Health. 2014;17(8):772–782. doi: 10.1016/j.jval.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 88.Hayashi T, Raz E. TLR9-based immunotherapy for allergic disease. Am J Med. 2006;119(10):897. doi: 10.1016/j.amjmed.2005.12.028. e1–e6. [DOI] [PubMed] [Google Scholar]
- 89.Nayak AS, Tripathy I, Levitt D. Novel Amb a 1 CpG oligodeoxy-ribonucleotide conjugate ragweed vaccine administered to children. J Allergy Clin Immunol. 2006;117(2 Suppl):S159. [Google Scholar]
- 90.Simons FE, Shikishima Y, Van Nest G, Eiden JJ, HayGlass KT. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J Allergy Clin Immunol. 2004;113(6):1144–1151. doi: 10.1016/j.jaci.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 91.Simons FE, HayGlass KT. Immunotherapy with a ragweed vaccine. N Engl J Med. 2007;356(1):86–87. ; author reply 87. [PubMed] [Google Scholar]
- 92.Asai K, Foley SC, Sumi Y, et al. Amb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy increases CD4+CD25+ T cells in the nasal mucosa of subjects with allergic rhinitis. Allergol Int. 2008;57(4):377–381. doi: 10.2332/allergolint.O-07-528. [DOI] [PubMed] [Google Scholar]
- 93.Tulic MK, Christodoulopoulos P, Fiset PO, et al. Local induction of a specific Th1 immune response by allergen linked immunostimulatory DNA in the nasal explants of ragweed-allergic subjects. Allergol Int. 2009;58(4):565–572. doi: 10.2332/allergolint.09-OA-0108. [DOI] [PubMed] [Google Scholar]
- 94.Tulic MK, Fiset PO, Christodoulopoulos P, et al. Amb a 1- immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory response. J Allergy Clin Immunol. 2004;113(2):235–241. doi: 10.1016/j.jaci.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 95.Creticos PS, Schroeder JT, Hamilton RG, et al. Immune Tolerance Network Group Immunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitis. N Engl J Med. 2006;355(14):1445–1455. doi: 10.1056/NEJMoa052916. [DOI] [PubMed] [Google Scholar]
- 96.Baldrick P, Richardson D, Woroniecki SR, Lees B. Pollinex Quattro Ragweed: safety evaluation of a new allergy vaccine adjuvanted with monophosphoryl lipid A (MPL) for the treatment of ragweed pollen allergy. J Appl Toxicol. 2007;27(4):399–409. doi: 10.1002/jat.1223. [DOI] [PubMed] [Google Scholar]
- 97.Patel P, Holdich T, Fischer von Weikersthal-Drachenberg KJ, Huber B. Efficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollen. J Allergy Clin Immunol. 2014;133(1):121–129. doi: 10.1016/j.jaci.2013.05.032. e1–e2. [DOI] [PubMed] [Google Scholar]
- 98.Fukushima A, Sumi T, Fukuda K, Kumagai N, Nishida T, Ueno H. Endogenous interleukin-10 produced by antigen-irrelevant cells promotes the development of experimental murine allergic conjunctivitis. Int Arch Allergy Immunol. 2007;144(1):79–84. doi: 10.1159/000102618. [DOI] [PubMed] [Google Scholar]
- 99.Fukushima A, Sumi T, Fukuda K, et al. Interleukin 10 and transforming growth factor beta contribute to the development of experimentally induced allergic conjunctivitis in mice during the effector phase. Br J Ophthalmol. 2006;90(12):1535–1541. doi: 10.1136/bjo.2006.100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.El Bassam S, Pinsonneault S, Kornfeld H, Ren F, Menezes J, Laberge S. Interleukin-16 inhibits interleukin-13 production by allergen-stimulated blood mononuclear cells. Immunology. 2006;117(1):89–96. doi: 10.1111/j.1365-2567.2005.02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ozaki A, Seki Y, Fukushima A, Kubo M. The control of allergic conjunctivitis by suppressor of cytokine signaling (SOCS)3 and SOCS5 in a murine model. J Immunol. 2005;175(8):5489–5497. doi: 10.4049/jimmunol.175.8.5489. [DOI] [PubMed] [Google Scholar]
- 102.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]


