Abstract
Several polypeptides contained in the coat and the core of adenovirus type 2 particles have been identified as virus gene products. They are synthesized in vitro by a cell-free protein-making system programmed by adenovirus type 2 messenger RNA that has been hybridized with and eluted from virus DNA. Fractionation by size yields subpopulations of viral messenger RNA that differ in their coding specificities. The procedures outlined in this study may be used to establish a genetic map of adenovirus type 2.
Keywords: polysomal RNA, oligo(dT)-cellulose, RNA·DNA hybridization, Krebs II ascites system
Full text
PDF
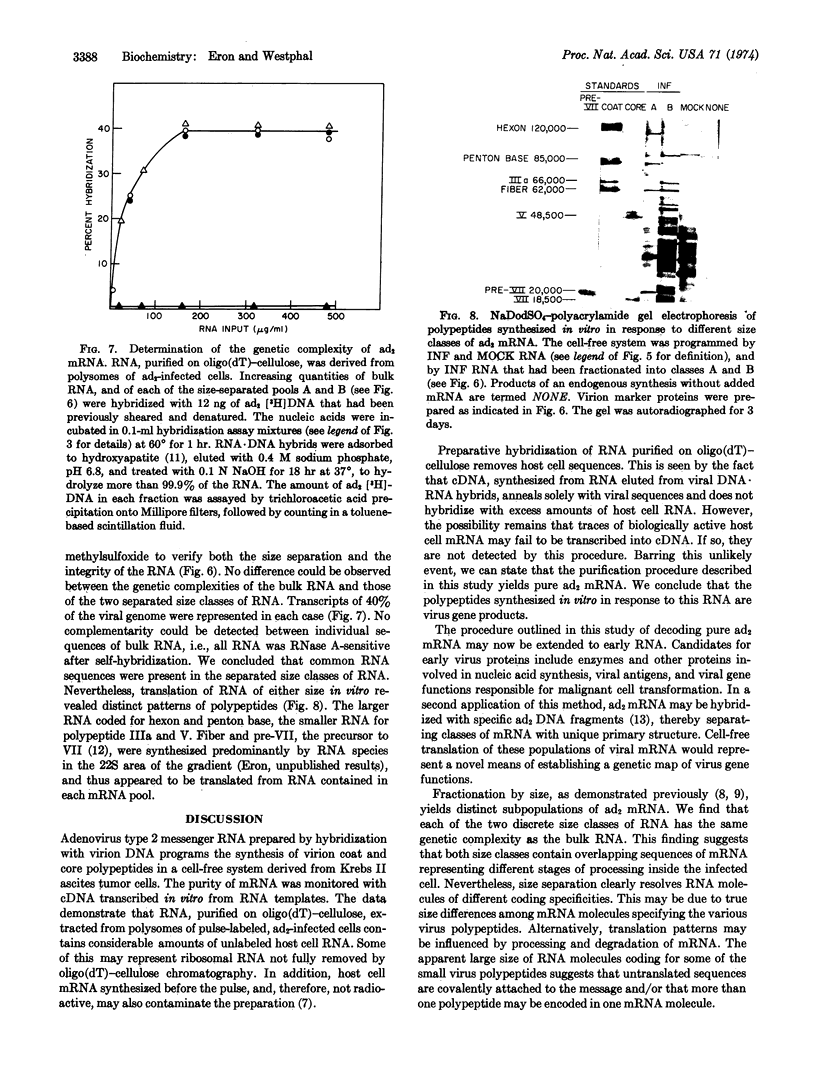

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaduri S., Raskas H. J., Green M. Procedure for the preparation of milligram quantities of adenovirus messenger ribonucleic acid. J Virol. 1972 Dec;10(6):1126–1129. doi: 10.1128/jvi.10.6.1126-1129.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinowitz M., Green M. The effect of infection with adenovirus 2 on the transcription of cellular RNA. Virology. 1972 Dec;50(3):619–629. doi: 10.1016/0042-6822(72)90416-3. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Manteuil S., Zamansky M. H., Gros F. DNA-RNA hybridization at low temperature in the presence of urea. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1080–1087. doi: 10.1016/0006-291x(70)90196-8. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T., Philipson L. Isolation and characterization of adenovirus messenger ribonucleic acid in productive infection. J Virol. 1972 Nov;10(5):909–919. doi: 10.1128/jvi.10.5.909-919.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Ross J., Aviv H., Scolnick E., Leder P. In vitro synthesis of DNA complementary to purified rabbit globin mRNA (RNA-dependent DNA polymerase-reticulocyte-hemoglobin-density gradient centrifugation-oligo(dT) primer). Proc Natl Acad Sci U S A. 1972 Jan;69(1):264–268. doi: 10.1073/pnas.69.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]