Abstract
Background
Deficits in balance control are one of the most common and serious mobility challenges facing individuals with lower limb loss. Yet, dynamic postural balance control among indivdiuals with lower limb loss remains poorly understood. Here we examined the kinematics and kinetics of dynamic balance in individuals with unilateral transtibial limb loss.
Methods
Five individuals with unilateral transtibial limb loss, and five age- and gender-matched controls completed a series of randomly applied multi-directional support surface translations. Whole-body metrics, e.g. peak center-of-mass displacement and net center-of-pressure displacement were compared across cohorts. Stability margin was computed as the difference between peak center-of-pressure and center-of-mass displacement. Additionally, center-of-pressure and ground reaction force magnitude and direction were compared between the prosthetic, intact, and control legs.
Findings
Peak center-of-mass displacement and stability margin did not differ between individuals with transtibial limb loss and controls for all perturbation directions except those loading only the prosthetic leg; in such cases the stability margin was actually larger than controls. Despite similar center-of-mass displacement, greater center-of-pressure displacement was observed in the intact leg during anterior-posterior perturbations, and under the prosthetic leg in medial-lateral perturbations. Further, in the prosthetic leg, ground reaction forces were smaller and spanned fewer directions.
Interpretation
Deficits in balance control among indivdiuals with transtibial limb loss may be due to their inability to use their prosthetic leg to generate forces that are equal in magnitude and direction to those of unimpaired adults. Targeting this force-generating deficit through technological or rehabilitation innovations may improve balance control.
Keywords: posture, stability, amputee, artificial limb, feedback, ground reaction force
1.0 Introduction
One of the most commonly cited functional mobility limitations facing individuals with lower limb loss (LLL) is a deficit in balance control [1]. Over half of community-living individuals with LLL report having fallen in the past year and 49.2% report a fear of falling [2]. Ulger et al. (2010) found that 80% of geriatric amputees had fallen within the past year, with 64% falling more than once [3]. These impairments in balance control carry an immense cost to an individual’s functional mobility at home and in the community [4,5], lowering their health-related quality of life [6]. With LLL affecting more than 1.6 million individuals living in the United States, and forecast to increase to more than 3.6 million individuals by the year 2050 [7], the impact of balance impairments will continue to exact an increasing toll on the healthcare system. Although falling is clearly a common and serious problem among individuals with LLL [8], very little is known regarding the challenges and mechanisms underlying deficits in balance control among individuals with LLL [9].
To date, research investigating postural balance control in individuals with LLL has generally been limited to unperturbed standing [1,10]. These posturography studies have revealed increases in measures of postural sway including center of pressure velocity and displacement compared to age-matched controls [11–16]. These differences in postural sway are influenced by age [14,17], cause of limb loss [14], residual limb length [18], level of limb loss [11,15], and time since limb loss [19], but not prosthetic alignment [16,20]. Additionally, the intact leg may contribute substantially more to maintaining static postural stability than the prosthetic leg [21].
In contrast to unperturbed standing balance, dynamic postural balance in response to internal or external perturbations has received much less attention among individuals with LLL, despite offering several advantages to other means of balance assessment [22]. Aruin et al., (1997) studied anticipatory postural adjustments among a cohort of individuals with unilateral transtibial limb loss (TTLL) in response to rapid arm raises. They found that individuals with TTLL exhibited asymmetry in anticipatory changes of background muscle activity. These responses were much larger on the intact side of the body, and frequently absent on the side affected by limb loss [23]. In response to external perturbations, individuals with unilateral TTLL exhibit delayed muscle responses in both the intact and prosthetic leg [24], as well as greater reliance on the intact versus prosthetic leg to generate corrective responses (i.e. CoP displacement, ankle torque) [25–27].
In these prior studies, perturbations were often limited to the sagittal plane [9,24–26]. Sagittal plane perturbations load both legs, allowing participants to compensate for sensorimotor impairment in one leg by using the contralateral leg more. Conversely, medial-lateral perturbations do not allow contralateral leg compensation, providing a direct assessment of each leg’s capabilities. Medial-lateral balance control is also known to present greater fall risk [28,29], impose greater processing demands on the central nervous system [30], and be particularly challenging to individuals with LLL [31]. Furthermore, stance width [25], perturbation parameters [26], and cause of limb loss [9,25–27], features known to influence postural responses [14,32,33], were not standardized across subjects in previous studies. Additionally, several of the studies examining dynamic postural balance responses among individuals with LLL have used continuous [25] or predictable perturbations [9,27]. Lastly, little has been reported regarding the ability of individuals with unilateral TTLL to generate ground reaction forces of appropriate magnitude and direction in response to multi-directional perturbations.
Our objective was to study reactive postural balance responses to multi-directional support surface translations in individuals with unilateral TTLL. We examined net center of pressure (CoP) and center of mass (CoM) displacement, as well as CoP displacement and ground reaction forces (GRF) in each leg. Reactive balance control was quantified using the stability margin, the difference between the peak net CoP and peak CoM displacement during a perturbation [34,35]. The smaller the stability margin, the more likely the individual is to lose their balance [34]. Based on reported deficits in balance control in individuals with lower limb loss [2,3], we hypothesized that individuals with unilateral TTLL would have a smaller stability margin compared to unimpaired age-matched controls. We further hypothesized that individuals with unilateral TTLL would display greater asymmetries between legs in maintaining their stability margin.
2.0 Methods
2.1 Recruitment
Inclusion criteria for individuals with unilateral TTLL included: age greater than 18 years, time since limb loss greater than one year, cause of limb loss non-dysvascular, at least 8 hours of prosthesis wear per day, and self-reported ability to ambulate with variable cadence, equivalent to K3 Medicare functional mobility level (i.e. ability to walk at variable cadences). Exclusion criteria were medical conditions, assessed by self-report, which could result in impaired balance or sensory loss, including significant musculoskeletal, neurologic, or cardiopulmonary conditions. All participants with unilateral TTLL used their own prosthesis. Institutional Review Boards of Georgia Tech and Emory University approved all protocols. Written informed consent was obtained from each participant prior to enrollment.
2.2 Experimental Protocol
Responses to balance perturbations were studied using a series of ramp-and-hold support surface translations in 12 evenly distributed and randomly applied directions in the horizontal plane (0–330 degrees, every 30 degrees), (Fig. 1A) [36]. Each perturbation totaled 9 cm in total displacement, 35 cm/s peak velocity and 0.5 g (~490cm/s2) peak acceleration. Four perturbations were given in each direction, for a total of 48 perturbations. During perturbations, participants were instructed to cross their arms over their chest and maintain balance without stepping, if possible. If a stepping response was required, the trial was excluded and the perturbation direction was repeated. Only perturbations in the four cardinal directions were analyzed.
Figure 1.
Reactive postural response paradigm. A: Coordinate system for support surface translations in 12 evenly spaced directions along the horizontal plane with corresponding sway directions. B: Example of postural responses in the right leg to a forward translation of the support surface. Ground reaction forces, center of pressure, and center of mass displacement were examined during the active force production period, 160–300 ms after platform onset, of the automatic postural response (shaded area).
Stance width was standardized based on subject-specific anthropometrics. Specifically, the centers of the subject’s heels were positioned such that stance width equaled their inter-ASIS distance, which approximates the distance between the hip joints [37]. The prescribed stance width was maintained during the protocol by marking the position of the subject’s feet on the platform. To accommodate the built-in prosthetic heel height and standardize footwear across subjects, all subjects wore a standard shoe with a 3/8″ heel height.
2.3 Data Collection
Twenty-five reflective markers were placed on participants’ bony landmarks according to the Vicon Plug-in-Gait model. The position of markers on the prosthetic limb matched those of the sound limb. Three-dimensional marker coordinate data were collected at 120 Hz using an eight-camera motion capture system (Vicon, Centennial, CO) and synchronized with ground reaction force (GRF) data collected from force plates (AMTI, Watertown, MA) under each foot at 1080Hz.
2.4 Data Processing and Analysis
Marker coordinate data were filtered with a third-order Butterworth 30 Hz low-pass cut-off frequency and combined with participant-specific anthropometric data to calculate whole-body center of mass (CoM) position using the weighted sum approach. Differences in body segment parameters for the prosthetic foot were not taken into account. Ground reaction forces (GRF) were filtered with a third-order Butterworth 100 Hz low-pass cut-off frequency and used to calculate the CoP for individual force plates. The net CoP was calculated as the sum of the CoP from individual force plates multiplied by the percentage of body weight on each foot [38].
For each trial, whole-body reactive balance responses were quantified by calculating peak CoM and peak net CoP displacement from initial position, as well as the stability margin for each trial. Stability margin is the difference between peak CoP and CoM displacements during a perturbation [34,35]. Peak net CoP, CoM and stability margin were analyzed from 160–300 ms following perturbation onset (Fig. 1B). This time period is referred to as the active force production period [32,36]. 160 ms post-perturbation reflects the point in time when active force production begins, accounting for the excitation-contraction coupling time period. The ending time of 300 ms, which is prior to peak platform deceleration, was chosen to minimize the effect of the additional unwanted counterforce on the body caused by the deceleration of the platform.
Individual leg contributions to whole-body reactive balance responses were assessed for each perturbation direction by calculating leg-specific measures including: peak CoP displacement, mean vertical, anterior-posterior (AP) and medial-lateral (ML) GRF magnitude, and the direction of the resultant horizontal GRF vector. Mean background GRFs were calculated during quiet stance, 250-50 ms prior to perturbation onset. Mean active GRF magnitude and the resultant horizontal force directions were computed over the first 60 ms time window of the active force production period (160–220 ms post-perturbation) as the change in force from background levels. All data was analyzed using custom MATLAB™ (MathWorks, Natick, MA) code.
2.5 Statistical Analysis
To limit the number of statistical comparisons, four cardinal directions (forward, backward, right and left) of the 12 perturbation directions were subjected to statistical analysis. To determine the effect of group (TTLL versus control) and sway direction (4 directions) on the dependent variables peak COM displacement, peak net CoP displacement, and stability margin, a 2-way mixed-design ANOVA (1-between group and 1-repeated measures factors) was performed for each variable. To determine the effect of leg (prosthetic, intact, matched control) and sway direction (4 directions) on leg-specific peak CoP displacement, a 2-way mixed-design ANOVA (1-between group and 1-repeated measures factors) was performed. Vertical, ML and AP GRFs were compared between leg conditions (prosthetic, intact, matched control) with a 1-way ANOVA. For background GRFs, the mean across all directions was compared. For active forces, the peak mean GRF across all directions was compared. Net and leg-specific active resultant horizontal GRF directions were plotted against sway direction for visual analysis. For all tests the level of significance was set at α = 0.05. Post-hoc testing used the Sidak test. All statistical tests were conducted using SPSS (V.19; SPSS, Inc., Chicago IL).
3.0 Results
3.1 Participant Demographics
Five males with unilateral TTLL and five age- and gender-matched unimpaired adults were recruited to participate in the study (Table 1).
Table 1.
Participant Demographics
| Cohort | Height (m) | Mass (kg) | Age (years) | Gender | Etiology | Prosthetic Foot Type | Years Since Limb Loss | |
|---|---|---|---|---|---|---|---|---|
| TTLLa (n=5) | Mean (SD) | 1.76 (0.25) | 81.5 (14.8) | 43.9 (13.7) | 5 Male | Trauma (4) RSDb (1) | NA ESRc (4) Hydraulic ESR (1) | 6.33 (6.88) |
| Controls (n=5) | Mean (SD) | 1.71 (0.33) | 84.1 (16.0) | 44.2 (15.5) | 5 Male | N/A | N/A | N/A |
TTLL – Transtibial limb loss
RSD = Reflex Sympathetic Dystrophy
NA ESR = Non-articulated energy storage and response
3.2 Differences in peak CoM, peak net CoP, and stability margin between groups
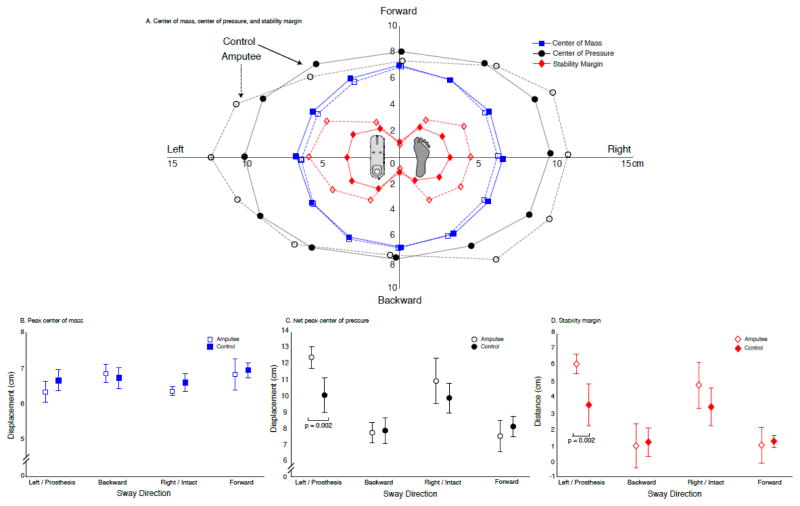
Although CoM displacement and net CoP magnitude did not differ between groups, we found directionally specific differences between individuals with TTLL and controls in net CoP displacement and stability margin. Peak CoM displacement did not differ between groups (group effect: P = 0.052), across direction of sway (direction effect: P = 0.16), nor were there significant interaction effects (interaction: P = 0.345) (Fig. 2A–B). The magnitude of net CoP displacement did not differ between groups, while the stability margin did (group effect: net CoP P = 0.062; stability margin P = 0.038). Net CoP displacement and stability margin varied across directions (direction effect: net CoP P < 0.001; stability margin P < 0.001), with a significant interaction between group and direction (interaction: net CoP P = 0.001; stability margin P = 0.003). Specifically, individuals with TTLL had significantly larger net CoP displacement (P = 0.002) and stability margin (P = 0.002) than controls when swaying towards and loading their prosthesis (leftward for controls) (Figure 2A, C–D). Controls and individuals with TTLL had significantly larger peak net CoP displacements during medial-lateral than anterior-posterior perturbations (controls P < 0.029; TTLL P < 0.001) (Fig. 2A, C). These same directionally-sensitive differences in stability margin were only observed in individuals with TTLL (P < 0.003), with controls demonstrating limited differences in stability margin across directions, leftward versus anterior-posterior sway (P < 0.011) (Fig. 2D).
Figure 2.
Whole-body reactive balance responses to multi-directional support surface translations. A: Peak center of mass displacement (blue squares), peak net center of pressure excursion (black circles), and stability margin (red diamonds) averaged across subjects (transtibial amputees: unfilled; controls: filled) for all 12 sway directions. B: Peak center of mass displacement (mean ± 1SD) across the four cardinal directions for transtibial amputees (unfilled) and controls (filled). There was no significant difference in the magnitude of whole-body displacement between individuals with limb loss and controls, or across directions within either cohort. C: Peak net center of pressure excursion (mean ± 1SD) across the four cardinal directions for individuals with transtibial limb loss (unfilled) and controls (filled). Individuals with transtibial limb loss had significantly larger net center of pressure excursion in response to lateral sway that loaded the prosthetic leg (p = 0.002) than controls. D: Stability margin (mean ± 1SD) across the four cardinal directions for individuals with transtibial limb loss (unfilled) and controls (filled). Individuals with transtibial limb loss had a significantly larger stability margin during lateral sway that loaded the prosthetic leg (p = 0.002) than controls.
3.3 Individual leg contributions to reactive balance responses (peak CoP, GRFs)
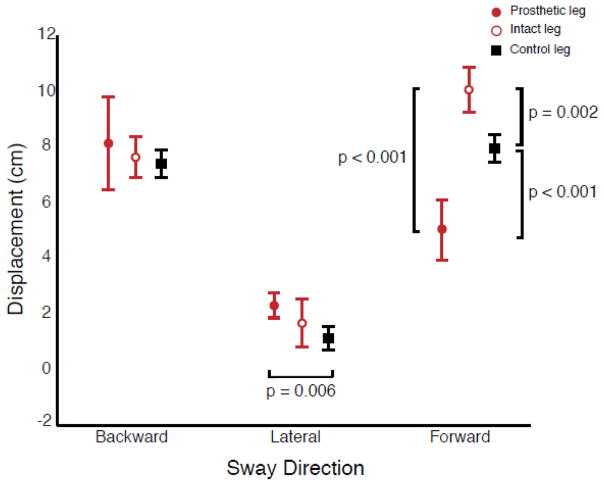
Individual leg force measurements revealed directionally specific differences in CoP displacement in the intact leg compared to the prosthetic and control legs. Peak CoP displacements for the right and left legs of controls were not significantly different (P = 0.491). Therefore the right leg was used for comparison to the peak CoP displacement of the prosthetic and intact legs of individuals with TTLL. Leg-specific peak CoP displacement (Fig. 3) differed between legs (leg effect: P = 0.004), as well as by sway direction (sway direction effect: P < 0.001), with a significant interaction between leg and sway direction (interaction: P < 0.001). During forward sway, the intact leg of individuals with TTLL had a significantly larger peak CoP displacement than the prosthetic leg (P < 0.001) or the control leg (P = 0.002). The control leg also had a significantly larger peak CoP displacement than the prosthetic leg (P < 0.001) (Fig. 3). During lateral sway (towards the prosthesis), the prosthetic leg of individuals with TTLL had larger peak CoP displacement that the control leg (P = 0.006). Additionally, peak CoP displacement under the intact and prosthetic leg of individuals with TTLL was significantly different across all sway directions (intact leg: P < 0.001, prosthetic leg: P < 0.001), while the peak CoP displacement among controls was only found to be significantly different between medial-lateral and anterior-posterior sway directions (P < 0.001).
Figure 3.
Leg specific peak center of pressure excursion (mean ± 1SD) for the prosthetic (filled circle), intact (unfilled circle), and control (filled square) legs. Peak center of pressure excursion on the intact leg of individuals with transtibial limb loss was significantly greater than that of the prosthetic (p < 0.001) and control (p = 0.002) legs during forward sway. Additionally, peak center of pressure excursion during lateral sway towards the prosthetic leg was significantly larger than in controls (p = 0.006) when the comparable leg was loaded.
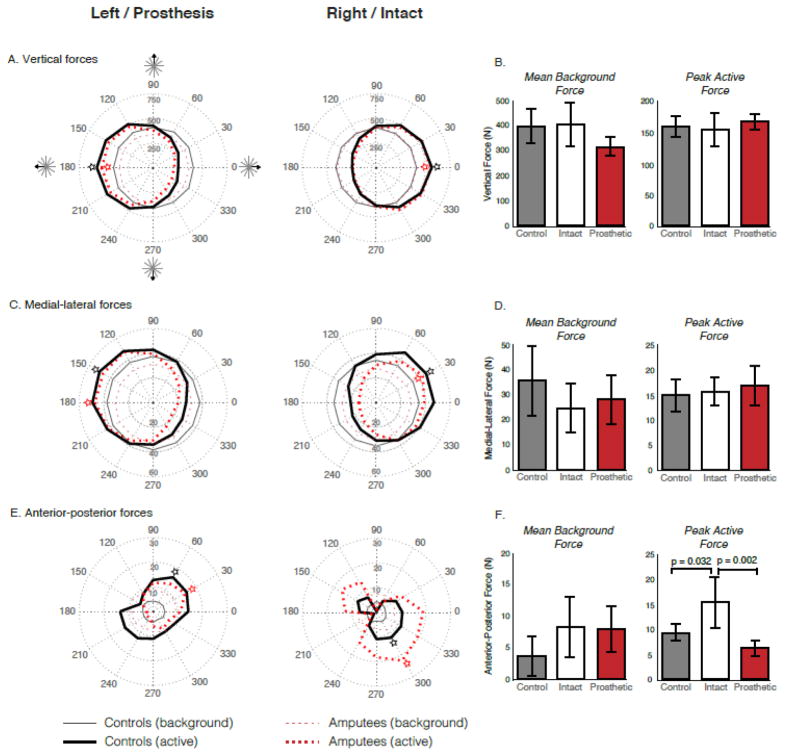
Differences in force magnitude and direction were also observed between the prosthetic, intact and control legs. There were no significant differences in the mean background forces or the peak active forces between the left and right legs of control subjects (P > 0.05). The mean ML, AP, and vertical background forces were not significantly different between the prosthetic, intact or control legs (P > 0.05) (Fig. 4). Neither the ML (P = 0.891), nor the vertical (P = 0.635) peak active forces were significantly different between the prosthetic, intact or control legs, only the AP peak active force was significantly different between leg conditions (leg effect: P = 0.002). Specifically, the intact leg of individuals with TTLL produced a significantly larger peak AP force than the prosthetic leg (P = 0.002) and the control leg (P = 0.032). While the prosthetic leg produced a smaller peak AP force than the control leg, this difference was not statistically significant (P = 0.416) (Fig. 4). Beyond the magnitude of the horizontal forces, inspection of Figure 5 reveals that the direction of the resultant horizontal forces also differed between cohorts, specifically when comparing the prosthetic leg with that of the control leg (Fig. 5B).
Figure 4.
Background and active period ground reaction forces. A, C, E: Polar plots for each leg depicting the mean background (thin lines) and active period (thick lines) vertical (A), medial-lateral (B), and anterior-posterior (C) ground reaction force for controls (solid black lines) and individuals with transtibial limb loss (dashed red lines) across each direction of sway. B, D, F: Mean background force (± 1SD) and peak active force (± 1SD) along the vertical (B), medial-lateral (D), and anterior-posterior (F) axes for the control (grey), intact (white) and prosthetic (red) legs. Polar plots reveal a non-significant difference in vertical (prosthetic vs. control leg), medial-lateral (prosthetic vs. control; intact vs. control), and anterior-posterior (prosthetic vs. control; intact vs. control) background forces. The patterns of active forces were largely comparable between controls and individuals with transtibial limb loss along the vertical and medial-lateral axes, with no significant differences in the peak active forces. The patterns of active forces were vastly different between cohorts along the anterior-posterior axis, with significant differences between the control and intact leg (p = 0.032), as well as the intact and prosthetic leg (p = 0.002). Peak active forces were generally exerted in the same direction by controls and individuals with transtibial limb loss (denoted by star).
Figure 5.
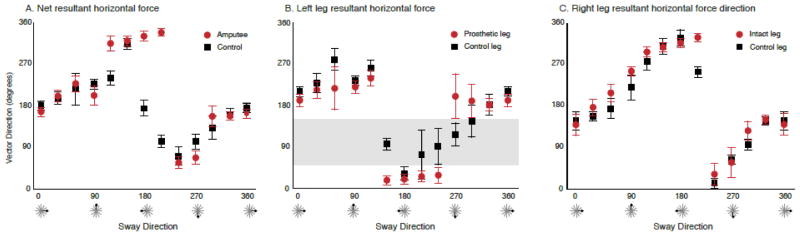
Direction of resultant horizontal force vectors. A: Direction (mean ± 1SD) of the net resultant horizontal force vectors for each of the 12 sway directions. The direction of the net resultant horizontal force vectors was similar between controls (black squares) and individuals with transtibial limb loss (red circles) across sway directions, the main exception being sway in the direction of the prosthesis (leftward). B: Direction (mean ± 1SD) of the resultant horizontal force vector exerted by the prosthetic (red circles) and left leg of controls (black squares). Controls exerted forces across a wider range of directions than individuals with transtibial limb loss who tended to constrain the forces they exerted with their prosthetic leg to two principle directions, approximately 180 and 0 degrees. Note that individuals with transtibial limb loss did not exert forces with their prosthetic leg across a range of directions, namely 45 to 160 degrees (shaded area). C: Direction (mean ± 1SD) of the resultant horizontal force vector exerted by the intact leg of individuals with transtibial limb loss (red circles) and right leg of controls (black squares). Note that the direction of the resultant horizontal force vectors was virtually equivalent across all sway directions.
4.0 Discussion
Individuals with TTLL employ different interlimb kinetic strategies to achieve surprisingly similar dynamic balance performance compared to controls in most directions. Specifically, during lateral perturbations that loaded the prosthetic leg, similar CoM but greater net CoP displacement (Fig. 2A) was found, resulting in a higher, not lower stability margin in the direction considered the least stable. Differences in interlimb coordination of individual leg CoP and ground reaction forces were also observed across directions suggesting more reliance on the intact leg for balance. Limited directions of force were identified in the prosthetic leg compared to the intact and control legs, however it is unclear whether this reflects a compensatory strategy or a functional limitation related to the design of prostheses. The exaggerated net CoP response that was observed among individuals with TTLL may impose additional unintended balance challenges and result from a fear of falling and/or sensorimotor limitations of the prosthesis.
The exaggerated net CoP response may have developed as a result of a history of falls. Repeated falls could create the desire to maintain a greater margin for error to reduce the likelihood of a loss of balance, particularly when using the prosthetic leg [39]. Four of the five individuals with TTLL in this study reported at least one fall in the past 12 months, suggesting that their control strategy for maintaining balance could have been influenced by their falls history. Another explanation could be the inability to accurately perceive the perturbation, or to generate appropriately scaled forces with the prosthetic leg. It is possible that the prosthetic leg was used passively as a strut rather than actively to generate forces. Regardless of its cause, this exaggerated response may impose unforeseen challenges to maintaining balance control. If individuals with unilateral TTLL use motor responses that are larger than necessary, and that lie at the limits of their capabilities, this may constrain their ability to respond to continued or larger perturbation(s). If so, a disproportionate response may leave them with little in reserve to cope with continued or subsequent perturbations, ultimately resulting in a loss of balance and potential for serious injury. Testing this hypothesis would require identifying the maximal capacity of subjects during reactive balance, comparing it to responses across other perturbation magnitudes, and assessing whether the use of an exaggerated response across perturbation magnitudes does in fact limit their ability to respond effectively to continued perturbations. This would likely require an examination of stepping [9,27,40–42] and non-stepping responses.
The differences in balance between individuals with TTLL was likely not due to their initial posture, but rather the execution of the motor response following a perturbation. While visually distinct, we found that the background forces were not significantly different between controls and individuals with TTLL (Fig. 4), consistent with the results of Nederhand et al., (2012). This does not exclude the possibility that adjustment(s) to background posture or force patterns would not improve reactive balance among individuals with LLL [43,44].
Consistent with prior studies in perturbed [25,27] and unperturbed standing [45,46], compensations in the intact leg were required to preserve anterior-posterior balance. If the individuals with TTLL in our study had not increased the CoP displacement under their intact leg during forward sway (Fig. 3), the peak CoM displacement would have exceeded the net CoP response, resulting in a loss of balance. This asymmetric strategy was effective in maintaining a similar stability margin as controls (Figure 2A, D), and was achievable due the relatively high mobility level of the amputee subjects in this study. However, older adults with TTLL, or those with additional co-morbidities such as neuropathy, may lack adequate contralateral sensorimotor function to effectively compensate for the reduced biomechanical contributions of the prosthetic leg to anterior-posterior balance. Additional research is required to determine how these compensations are affected by aging and additional impairment. The differences in interlimb contributions to balance also highlights the need for interventions and innovations in prosthetic technology to reduce the need for contralateral compensations.
Consistent with previous work [25,26], force-generating asymmetries between legs were observed in the anterior-posterior direction, with the intact leg exerting significantly greater peak AP forces than the prosthetic or control legs. Deficits in force magnitude were limited to the AP direction, as individuals with unilateral TTLL are able to use their prosthetic leg to produce ML and vertical forces of equivalent magnitude to those of controls and their own intact leg (Fig. 4A, C, E). The large difference in the AP force magnitude may reflect limitations in the ability to actively generate plantarflexion torque with the prosthetic leg. Further, this exaggerated force response may characterize a preferred strategy used by individuals with TTLL to simplify the control of reactive balance. If a single exaggerated yet safe response is consistently used, it may remove the need to scale the response according to the perturbation and/or need for contralateral compensation, thereby simplifying control.
Individuals with TTLL demonstrated limited directions of force with their prosthetic leg, altering interlimb force coordination for directional control of the CoM. Individuals with unilateral TTLL exerted active resultant horizontal GRFs with their prosthetic leg that clustered around the medial-lateral directions, while forces generated by control and intact legs were distributed across all directions in the horizontal plane (Fig. 5B–C). By restricting forces to a set of two direction-invariant vectors, the challenge of maintaining balance in response to horizontal plane perturbations may be simplified [47]. Such a restriction of force direction has been observed previously for non-preferred stances in both humans (i.e. narrow stance) and animals [32,48]. It is possible that the force constraint strategy simplifies the control of balance under novel, or challenging balance conditions. We only observed the constraint in the prosthetic leg suggesting that the intact leg regulates multi-directional control of the CoM among individuals with unilateral TTLL. However, the medial-lateral force constraint may also be due to limitations in sagittal plane ankle range of motion or plantarflexor torque generation in the prosthetic ankle.
4.4 Study limitations
We studied only five individuals with TTLL, limited to those of traumatic etiology. Given the increasing age of the general population [49], and the rising incidence of diabetes [50], there is an unmistakable need to study individuals with dysvascular limb loss as well as older adults with lower limb loss. However, for this initial study the traumatic population was specifically selected to avoid additional confounds that may influence reactive balance control. Despite a limited sample size we were able to identify significant differences between cohorts. Nevertheless, a study including a larger cohort of subjects is certainly warranted to confirm these results. The perturbation parameters selected in this study were selected based upon previous work [32,34] and were sufficient to examine the initial active response period. However, the short time interval between peak platform acceleration and deceleration precluded us from looking at a longer time window. Here we examined whole-body kinematics and endpoint forces to describe differences in reactive postural balance control between individuals with unilateral TTLL and unimpaired adults. An analysis of the muscle coordination patterns that underlie the differences in control strategies used to stabilize the same CoM displacement [36,51,52], may provide additional insight into their production and the design of interventions to improve balance control among individuals with LLL.
5.0 Conclusion
Surprisingly, we found that individuals with non-dysvascular unilateral TTLL were equally capable of maintaining dynamic standing balance in response to external perturbations as age-matched unimpaired controls. Individuals with TTLL used significantly different interlimb contributions to stabilize the same CoM displacement, revealing functional asymmetries in CoP and ground reaction forces between the prosthetic, intact and control legs. These functional asymmetries between legs suggest that individuals with unilateral TTLL may be limited in their ability to exert forces with their prosthetic leg that are equal in magnitude and direction to those of unimpaired adults. These deficits in force production may underlie many of the postural and walking balance impairments attributed to individuals with lower limb loss.
Highlights.
Balance control deficits limit the mobility of individuals with lower limb loss
We examined reactive balance responses to postural perturbations in amputees
Amputees and controls demonstrated similar whole-body responses to perturbations
Amputees used asymmetric interlimb force coordination strategies to retain balance
Force production deficits may underlie balance impairments in lower limb amputees
Acknowledgments
The authors would like to thank Stacie Chvatal, PhD for her contributions to the design of the experiment and her help with data collection.
Funding: NIH NS053822, NSF EFRI 1137229
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jayakaran P, Johnson GM, Sullivan SJ, Nitz JC. Instrumented measurement of balance and postural control in individuals with lower limb amputation. Int J of Rehabil Res. 2012;35:187–196. doi: 10.1097/MRR.0b013e3283550ff9. [DOI] [PubMed] [Google Scholar]
- 2.Miller WC, Speechley M, Deathe B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch Phys Med Rehab. 2001;82:1031–1037. doi: 10.1053/apmr.2001.24295. [DOI] [PubMed] [Google Scholar]
- 3.Ülger Ö, Topuz S, Bayramlar K. Risk factors, frequency, and causes of falling in geriatric persons who has had a limb removed by amputation. Top Geriatr Rehabil. 2010;26:156–163. [Google Scholar]
- 4.van Velzen JM, van Bennekom CA, Polomski W, Slootman JR, van der Woude LH, Houdijk H. Physical capacity and walking ability after lower limb amputation: a systematic review. Clin Rehabil. 2006;20:999–1016. doi: 10.1177/0269215506070700. [DOI] [PubMed] [Google Scholar]
- 5.McWhinnie DL, Gordon AC, Collin J. Rehabilitation outcome 5 years after 100 lower-limb amputations. Br J Surg. 1994;81:1596–1599. doi: 10.1002/bjs.1800811110. [DOI] [PubMed] [Google Scholar]
- 6.Pezzin LE, Dillingham TR, MacKenzie EJ. Rehabilitation and the long-term outcomes of persons with trauma-related amputations. Arch Phys Med Rehabil. 2000;81:292–300. doi: 10.1016/s0003-9993(00)90074-1. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Pauley T, Devlin M, Heslin K. Falls sustained during inpatient rehabilitation after lower limb amputation: prevalence and predictors. Am J Phys Med Rehabil. 2006;85:521–532. doi: 10.1097/01.phm.0000219119.58965.8c. [DOI] [PubMed] [Google Scholar]
- 9.Curtze C, Hof AL, Otten B, Postema K. Balance recovery after an evoked forward fall in unilateral transtibial amputees. Gait Posture. 2010;32:336–341. doi: 10.1016/j.gaitpost.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Ku PX, Abu Osman NA, Wan Abas WAB. Balance control in lower extremity amputees during quiet standing: a systematic review. Gait Posture. 2014;39:672–682. doi: 10.1016/j.gaitpost.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Fernie GR, Holliday PJ. Postural sway in amputees and normal subject. J Bone Joint Surg Am. 1978;60:895–898. [PubMed] [Google Scholar]
- 12.Isakov E, Mizrahi J, Ring H, Susak Z, Hakim N. Standing sway and weight-bearing distribution in people with below-knee amputations. Arch Phys Med Rehabil. 1992;73:174–178. [PubMed] [Google Scholar]
- 13.Buckley JG, O’Driscoll D, Bennett SJ. Postural sway and active balance performance in highly active lower-limb amputees. Am J Phys Med Rehabil. 2002;81:13–20. doi: 10.1097/00002060-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Hermodsson Y, Ekdahl C, Persson BM, Roxendal G. Standing balance in trans-tibial amputees following vascular disease or trauma: a comparative study with healthy subjects. Prosthet Orthot Int. 1994;18:150–158. doi: 10.3109/03093649409164400. [DOI] [PubMed] [Google Scholar]
- 15.Rougier PR, Bergeau J. Biomechanical analysis of postural control of persons with transtibial or transfemoral amputation. Am J Phys Med Rehabil. 2009;88:896–903. doi: 10.1097/PHM.0b013e3181b331af. [DOI] [PubMed] [Google Scholar]
- 16.Kolarova B, Janura M, Svoboda Z, Elfmark M. Limits of stability in persons with transtibial amputation with respect to prosthetic alignment alterations. Arch Phys Med Rehabil. 2013;94:2234–2240. doi: 10.1016/j.apmr.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Vittas D, Larsen TK, Jansen EC. Body sway in below-knee amputees. Prosthet Orthot Int. 1986;10:139–141. doi: 10.3109/03093648609164518. [DOI] [PubMed] [Google Scholar]
- 18.Lenka P, Tiberwala DN. Effect of stump length on postural steadiness during quiet stance in unilateral trans-tibial amputee. Al Ameen J Med Sci. 2010;3:50–57. [Google Scholar]
- 19.Mayer Á, Tihanyi J, Bretz K, Csende Z, Bretz É, Horváth M. Adaptation to altered balance conditions in unilateral amputees due to atherosclerosis: a randomized controlled study. BMC Musculoskelet Disord. 2011;12:118. doi: 10.1186/1471-2474-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isakov E, Mizrahi J, Susak Z, Ona I, Hakim N. Influence of prosthesis alignment on the standing balance of below-knee amputees. Clin Biomech. 1994;9:258–262. doi: 10.1016/0268-0033(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 21.Hlavackova P, Franco C, Diot B, Vuillerme N. Contribution of each leg to the control of unperturbed bipedal stance in lower limb amputees: New insights using entropy. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Visser JE, Carpenter MG, van der Kooij H, Bloem BR. The clinical utility of posturography. Clin Neurophysiol. 2008;119:2424–2436. doi: 10.1016/j.clinph.2008.07.220. [DOI] [PubMed] [Google Scholar]
- 23.Aruin AS, Nicholas JJ, Latash ML. Anticipatory postural adjustments during standing in below-the-knee amputees. Clin Biomech. 1997;12:52–59. doi: 10.1016/s0268-0033(96)00053-8. [DOI] [PubMed] [Google Scholar]
- 24.Rusaw D, Hagberg K, Nolan L, Ramstrand N. Bilateral electromyogram response latency following platform perturbation in unilateral transtibial prosthesis users: Influence of weight distribution and limb position. J Rehabil Res Dev. 2013;50:531–544. doi: 10.1682/jrrd.2012.01.0017. [DOI] [PubMed] [Google Scholar]
- 25.Vrieling AH, van Keeken HG, Schoppen T, Otten E, Hof AL, Halbertsma JPK, et al. Balance control on a moving platform in unilateral lower limb amputees. Gait Posture. 2008;28:222–228. doi: 10.1016/j.gaitpost.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Nederhand MJ, Van Asseldonk EHF, van der Kooij H, Rietman HS. Dynamic Balance Control (DBC) in lower leg amputee subjects; contribution of the regulatory activity of the prosthesis side. Clin Biomech. 2012;27:40–45. doi: 10.1016/j.clinbiomech.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Curtze C, Hof AL, Postema K, Otten B. The relative contributions of the prosthetic and sound limb to balance control in unilateral transtibial amputees. Gait Posture. 2012;36:276–281. doi: 10.1016/j.gaitpost.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–84. doi: 10.1093/geronj/49.2.m72. [DOI] [PubMed] [Google Scholar]
- 29.Topper AK, Maki BE, Holliday PJ. Are activity-based assessments of balance and gait in the elderly predictive of risk of falling and/or type of fall? J Am Geriatr Soc. 1993;41:479–487. doi: 10.1111/j.1532-5415.1993.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter MG, Allum JHJ, Honegger F. Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes. Exp Brain Res. 1999;129:93–113. doi: 10.1007/s002210050940. [DOI] [PubMed] [Google Scholar]
- 31.Arifin N, Abu Osman NA, Ali S, Gholizadeh H, Wan Abas WAB. Postural stability characteristics of transtibial amputees wearing different prosthetic foot types when standing on various support surfaces. Biomed Eng Online. 2014;13:1–6. doi: 10.1155/2014/856279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry SM, Fung J, Horak FB. Effect of stance width on multidirectional postural responses. J Neurophysiol. 2001;85:559–570. doi: 10.1152/jn.2001.85.2.559. [DOI] [PubMed] [Google Scholar]
- 33.Oude Nijhuis LB, Allum JHJ, Nanhoe-Mahabier W, Bloem BR. Influence of perturbation velocity on balance control in Parkinson’s disease. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0086650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horak FB, Dimitrova D, Nutt JG. Direction-specific postural instability in subjects with Parkinson’s disease. Exp Neurol. 2005;193:504–521. doi: 10.1016/j.expneurol.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- 36.Torres-Oviedo G, Ting LH. Muscle Synergies Characterizing Human Postural Responses. J Neurophysiol. 2007;98:2144–2156. doi: 10.1152/jn.01360.2006. [DOI] [PubMed] [Google Scholar]
- 37.Winter DA, Patla AE, Prince F, Ishac M, Gielo-Perczak K. Stiffness control of balance in quiet standing. J Neurophysiol. 1998;80:1211–1221. doi: 10.1152/jn.1998.80.3.1211. [DOI] [PubMed] [Google Scholar]
- 38.Winter DA, Prince F, Stergiou P. Medial-lateral and anterior-posterior motor-responses associated with center of pressure changes in quiet standing. Neurosci Res Commun. 1993;12:141–148. [Google Scholar]
- 39.Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture. 2007;25:250–258. doi: 10.1016/j.gaitpost.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Rogers MW, Hedman LD, Johnson ME, Martinez KM, Mille ML. Triggering of protective stepping for the control of human balance: age and contextual dependence. Brain Res Cogn Brain Res. 2003;16:192–198. doi: 10.1016/s0926-6410(02)00273-2. [DOI] [PubMed] [Google Scholar]
- 41.Mille ML, Rogers MW, Martinez K, Hedman LD, Johnson ME, Lord SR, et al. Thresholds for inducing protective stepping responses to external perturbations of human standing. J Neurophysiol. 2003;90:666–674. doi: 10.1152/jn.00974.2002. [DOI] [PubMed] [Google Scholar]
- 42.Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil and Neural Repair. 2013;27:526–533. doi: 10.1177/1545968313478486. [DOI] [PubMed] [Google Scholar]
- 43.Anker LC, Weerdesteyn V, van Nes IJW, Nienhuis B, Straatman H, Geurts ACH. The relation between postural stability and weight distribution in healthy subjects. Gait Posture. 2008;27:471–477. doi: 10.1016/j.gaitpost.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Genthon N, Rougier P. Influence of an asymmetrical body weight distribution on the control of undisturbed upright stance. J Biomech. 2005;38:2037–2049. doi: 10.1016/j.jbiomech.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Nadollek H, Brauer S, Isles R. Outcomes after trans-tibial amputation: the relationship between quiet stance ability, strength of hip abductor muscles and gait. Physiother Res Int. 2002;7:203–214. doi: 10.1002/pri.260. [DOI] [PubMed] [Google Scholar]
- 46.Quai TM, Brauer SG, Nitz JC. Somatosensation, circulation and stance balance in elderly dysvascular transtibial amputees. Clinical Rehabil. 2005;19:668–676. doi: 10.1191/0269215505cr857oa. [DOI] [PubMed] [Google Scholar]
- 47.Macpherson JM. Strategies that simplify the control of quadruped stance. I. Forces at the ground. J Neurophysiol. 1988;60:204–217. doi: 10.1152/jn.1988.60.1.204. [DOI] [PubMed] [Google Scholar]
- 48.Macpherson JM. Changes in a postural strategy with inter-paw distance. J Neurophysiol. 1994;71:931–940. doi: 10.1152/jn.1994.71.3.931. [DOI] [PubMed] [Google Scholar]
- 49.Wiener JM, Tilly J. Population ageing in the United States of America: implications for public programmes. Int J Epidemiol. 2002;31:776–781. doi: 10.1093/ije/31.4.776. [DOI] [PubMed] [Google Scholar]
- 50.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chvatal SA, Ting LH. Common muscle synergies for balance and walking. Front Comput Neurosci. 2013;7:48. doi: 10.3389/fncom.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chvatal SA, Torres-Oviedo G, Safavynia SA, Ting LH. Common muscle synergies for control of center of mass and force in nonstepping and stepping postural behaviors. J Neurophysiol. 2011;106:999–1015. doi: 10.1152/jn.00549.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]