Abstract
A complex partnership between the host and the vast intestinal microbial ecosystem serves numerous biological activities including nutrition, immunity, and barrier function. In this issue of Immunity, Singh et al. (2014) demonstrate that microbial-derived butyrate mediated its protective activity against inflammation and colorectal cancer through GPR109a signaling.
From birth to adulthood, humans progressively acquire and maintain a complex microbial ecosystem at various body sites and cavities. Early on, these microorganisms establish a complex relationship with the host, resulting in the modulation of several biological functions. A prime example of such a relationship is the intestine, where trillions of microorganisms influence nutritional and immunological functions. Understanding and deciphering the dialog occurring between the microbiota and the intestine at homeostasis as well as during disease states has been the subject of intense investigation. From a metabolic standpoint, the microbiome operates at an organ level, extracting energy and nutrients and generating numerous and important by-products from the diet, all of which benefit bacterial communities (adaptation, growth, maintenance), but most importantly assure essential host homeostatic function (Nicholson et al., 2012). For example, dietary fibers and nondigestible carbohydrates (starch, cellulose, fructans, xylans, inulin) are processed by various microbial enzymatic systems to produce diverse beneficial compounds such as essential vitamins (vitamin K, biotin, cobalamin, riboflavin, and niacin) and short chain fatty acids (SCFA) such as propionate, acetate, and butyrate (Nicholson et al., 2012). However, microbial disturbances as identified in patients with inflammatory bowel diseases (IBD) and colorectal cancer (CRC) bring changes to the “functional” aspect of the microbiome, among them the depletion of butyrate-producing bacteria (Guzman et al., 2013). Butyrate not only represents a primary source of energy for intestinal epithelial cells (IECs) but also modulates host immune response, thereby acting as a key microbial metabolite for intestinal homeostasis. Various G protein-coupled receptors (Gpr) such as Gpr41, Gpr43, and Grp109a mediate SCFA activities, but the molecular and cellular events responsible for butyrate-mediated beneficial effects in the intestine are still unclear. In this issue, Singh et al. (2014) provide key evidence that bacterial-derived butyrate and dietary fibers attenuate intestinal inflammation and CRC development through Gpr109a-mediated T regulatory (Treg) cell differentiation (Figure 1). The study shows how diet, microbiota, and immune cells form an intricate communication network essential for the maintenance of intestinal homeostasis.
Figure 1. Interplay between Diet, Microbiome, and Gpr109a in the Intestine.
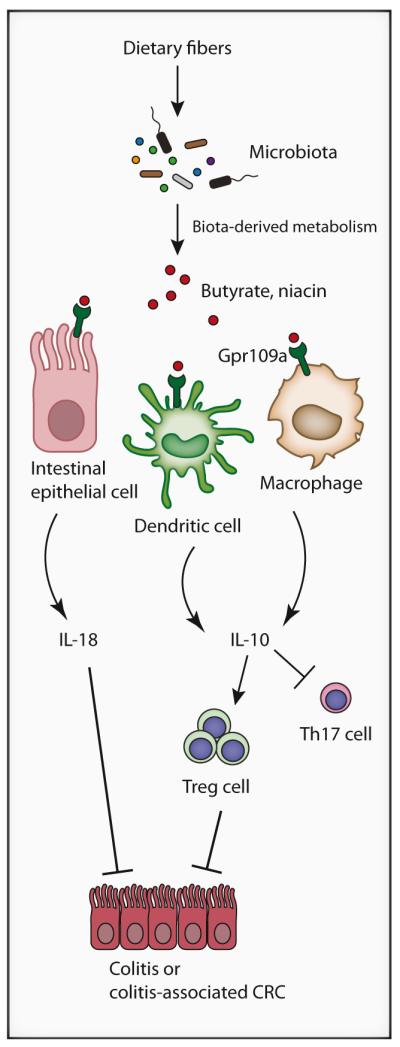
Microbiota-derived enzymatic activities generate metabolites such as butyrate and niacin from dietary fibers. Butyrate and niacin engage Gpr109a presents on intestinal epithelial cells, dendritic cells, and macrophages triggering production of cytoprotective IL-18, immunoregulatory IL-10, and class 1A aldehyde dehydrogenase (Aldh1a). This environment (IL-10, Aldh1a) favors the development of T regulatory cells (Treg) from naive T cells, while repressing generation of proinflammatory Th17 cells, thereby preventing conditions leading to the development of colitis and colitisassociated colorectal cancer.
How could the microbiota and associated metabolites exert a protective role in the intestine? Singh et al. (2014) first observed that Foxp3+ (Treg) cell number and frequency in the lamina propria of Grp109a−/− mice were significantly lower than in WT mice, a profile matching impaired immunosuppressive IL-10 secretion and enhanced production of proinflammatory IL-17. This inflammatory intestinal phenotype may be the consequence of defective tolerogenic instruction provided by mononuclear cells. Indeed, when Singh et al. (2014) coincubated colonic macrophages and DCs from Grp109a−/− mice with naive CD4+ T cells obtained from OT-II TCR transgenic mice, they noticed that these mononuclear cells were unable to induce Treg cell differentiation compared to their WT counterpart. The fact that butyrate-treated splenic DCs and macrophages from Grp109a−/− mice failed to release both IL-10 and class 1A aldehyde dehydrogenase (Aldh1a) and to promote a Treg cell phenotype (high IL-10, low IL-17) in a coculture assay clearly shows the key role of the receptor in mediating butyrate immune response. Moreover, niacin, which is also a Gpr109a ligand and bacterial-derived product, reproduces butyrate effect on DC, macrophage, and Treg cell activities. This highlights the central role of Gpr109a in capturing and processing signaling generated by microbial-derived metabolites. Antibiotic treatment causes massive perturbation in microbial ecosystems, and loss of microbial entities with concurrent decreased biochemical activities (metabolites) are bound to influence intestinal immune regulation. Singh et al. (2014) observed that niacin supplementation restores the number of Treg cells depleted by antibiotic treatment of WT mice, an effect nullified in Gpr109a−/− mice. These findings demonstrate that Gpr109a is essential for mucosal immunoregulatory functions afforded by the microbial metabolite butyrate.
Disrupting the dialog between microbiota and the host, especially at the level of mucosal immunology, is expected to have profound effects on disease development. By using the AOM+DSS model of colitis-associated CRC, Singh et al. (2014) showed that development of inflammation and CRC are exacerbated in Grp109a−/− mice compared to WT mice, a phenomenon associated with defective production of colonic IL-10 and cytoprotective IL-18 and heightened level of IL-17. The authors also observed a similar increased tumorigenesis when ApcMin/+ mice were crossed to Gpr109a−/− mice, showing that the impact of Gpr109a on cancer is not restricted to a chronic wounding model (AOM+DSS). Interestingly, by using bone-marrow transfer studies, Singh et al. (2014) showed that the contribution of radio-resistant cells to Gpr109a-mediated anticarcinogenic effect is greater than the one afforded by immune cells. The fact that IL-18 administration decreased tumorigenesis in Grp109a−/− mice suggests that a complex network of immunoregulatory molecules (IL-10, Aldh1, IL-18) from various cellular sources (immune cells, epithelial cells?) participate in intestinal homeostasis (Figure 1).
The importance of the microbiota in Gpr109a-mediated protective effect is clearly demonstrated in antibiotic-treated WT mice, which displayed enhanced AOM+DSS-induced tumorigenesis compared to untreated mice. Administration of niacin to antibiotic-treated mice was able to decrease tumor development, an effect not observed in Grp109a−/− mice. Remarkably, the antibiotic experiment also indicates that the microbiota not only protects against tumorigenesis as seen in WT mice, but also promotes CRC in Grp109a−/− mice. This deleterious effect was associated with expansion of bacteria belonging to the Prevotellaceae family and TM7 phylum, although the events leading to these microbial compositional changes were not established.
To clearly link microbial-derived metabolites (byturate and niacin) with Gpr109a-mediated protective function, the authors fed ApcMin/+ mice with either a normal chow containing fiber or a fiber-free diet. As opposed to wide-spectrum antibiotic treatment, which eliminates virtually all bacteria, dietary fiber manipulation has a more targeted effect on the biota, essentially regulating the capacity of bacteria to generate SCFA. Remarkably, fiber-free diet enhanced tumorigenesis in ApcMin/+ mice, a phenomenon strongly attenuated by niacin supplementation. Grp109a−/− mice failed to show a similar response to dietary fiber manipulation or niacin supplementation, providing strong evidence that the protective effect is mediated by this receptor. A recent report showed that butyrylated diet also promotes Treg cell development and prevented T-cell-adoptive-transfer-mediated chronic colitis (Furusawa et al., 2013), lending support to the key role of bacteria in promoting an homeostatic phenotype regardless of the initial insult (chemical or naive T cell transfer).
The current study adds an important piece to the complex jigsaw puzzle formed by diet, microbiota, and immune cells interaction in the intestine and brings new understanding regarding the intricate communication network necessary for the maintenance of intestinal homeostasis. Although compelling and informative, the study also raised questions. It is striking to note that other SCFA receptors such as GPR41 and GPR43 are not able to compensate for the lack of Gpr109a, although butyrate binds both receptor types and elicits a Treg-cell-associated anti-inflammatory response (Smith et al., 2013). Comparing inflammation and CRC susceptibility between GPR43-deficient and Gpr109a−/− mice would help understand the potentially divergent role of these receptors in mediating intestinal homeostasis. Furthermore, the cell type (epithelial cells, immune cells) responding to SCFA exposure may also dictate the extent of the protective response. This is clearly demonstrated in immune-cell-derived GPR43 signaling, which mediates an anti-inflammatory response (Maslowski et al., 2009), whereas IEC-derived GPR43 signaling appears to promote an inflammatory phenotype in the DSS model of colitis (Kim et al., 2013). Noteworthy, a recent report showed that butyrate-mediated expansion of Treg cells is Gpr109a independent, suggesting a complex impact of this SCFA on immune cell behaviors (Arpaia et al., 2013). A careful genetic dissection of the cellular compartment (DCs, myeloid cells, epithelial cells) responsible for Gpr109a-mediated protective effect would be necessary to fully capture the essence of the cellular network responding to microbial cues in the intestine.
Antibiotic treatment suggests that cancer-promoting bacteria arise from the biome of Grp109a−/− mice. How does the lack of Gpr109a signaling lead to expansion of potentially cancer-promoting bacteria? Is this phenomenon related to improper development of intestinal Treg cells? Biome with cancer-promoting and cancer-protecting function seems to coexist and factors (host genetics, diet, etc.) altering this balance would probably have functional consequence for the host (Schwabe and Jobin, 2013). Future studies would be required to address these issues.
The intestinal mucosa is bombarded by signals derived from the magma of bacteria and bacterial products present in the intestinal environment. This disparate collection of microbial cues is unlikely to elicit a uniform response from the gut. For example, the microbial structural component polysaccharide A present on Bacteroides fragilis contributes to intestinal homeostasis by promoting differentiation of Foxp3+ Treg cells (Round and Mazmanian, 2010), whereas segmented filamentous bacterium (SFB) favors the differentiation of CD4+ T helper cells into Th17 cells, a more proinflammatory phenotype (Ivanov et al., 2009). Similarly, as reported here and in other studies, microbial-derived metabolites (SCFA) also trigger an immune response from the host. How are these numerous and disparate microbial cues (structural products, metabolites, etc.) integrated into a cohesive and coordinate response by the host? This dichotomy between the deleterious and protecting function of the microbiota and associated microbial products clearly illustrate challenges facing researchers working on this complex system. Regardless, manipulating host immune response by altering microbial composition and activities through dietary intervention and/or specific host receptors could represent a powerful mean to promote and/or maintain intestinal homeostasis. Understanding the various elements implicated in the complex dialog between bacteria and the host holds much promise for pathologies such as IBD and CRC.
REFERENCES
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Guzman JR, Conlin VS, Jobin C. Biomed Res Int. 2013:425146. doi: 10.1155/2013/425146. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Gastroenterology. 2013;145:396–406. pe1–e10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Proc. Natl. Acad. Sci. USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Jobin C. Nat. Rev. Cancer. 2013;13:800–812. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al. Immunity. 2014;40(this issue):128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]


