Abstract
Excessive accumulation of pro-oxidant metals, observed in affected brain regions, has consistently been implicated as a contributor to the brain pathology including neurodegenerative diseases and acute injuries such as stroke. Furthermore, the potential interactions between metal toxicity and other commonly associated etiological factors, such as misfolding/aggregation of amyloidogenic proteins or genomic damage, are poorly understood. Decades of research provide compelling evidence implicating metal overload in neurological diseases and stroke. However, the utility of metal toxicity as a therapeutic target is controversial, possibly due to a lack of comprehensive understanding of metal dyshomeostasis-mediated neuronal pathology. In this article, we discuss the current understanding of metal toxicity and the challenges associated with metal-targeted therapies.
Metal accumulation in the brain, a common phenomenon in the pathophysiology of neurodegenerative diseases and stroke
The optimum levels of trace elements and their homeostasis in individual organs are essential for maintaining vital functions. Nutritional deficiencies and metabolic disorders exhibit potential cause-and-effect relationship in many pathological conditions. Although many metal ions, some at trace levels, are essential for life, excess accumulation can be highly toxic and possibly fatal. Neurodegeneration is believed to be the most common manifestation of metal toxicity. For example, etiological link between abnormal metal accumulation in the brain and aging or various neurological disorders, including Alzheimer’s Disease (AD), Parkinson’s Disease (PD), Amyotrophic Lateral Sclerosis (ALS), Wilson’s Disease (WD), and stroke was observed [1–7]. Acute metal toxicity is implicated in stroke and certain conditions that result from hereditary defects in the regulation of metal homeostasis. For example, the dysregulation of metal ions due to the acute release of free iron (Fe) following hemorrhagic stroke causes massive neuronal injury [3,8]. Furthermore, neurotoxicity from acute increase in the level of zinc (Zn) and other transition metals may play a critical role after ischemic focal brain injury [9].
Environmental/dietary sources versus genetic factors
Fe accumulation in the brain during hemorrhagic stroke is thought to be due to the breakdown of hemoglobin. However, the source and the mechanisms of accumulation of other metals in the brain are unclear, because only in a few cases, this could be linked to dietary or occupational exposures [10]. It is generally believed that both environmental and genetic factors are responsible for abnormal metal accumulation in neurological conditions. Environmental factors include malnutrition, contaminated water, food, and beverages, occupational/hazardous exposures, and medical procedures. Individual metal ions have specific physiological function(s) as co-factors for many essential vitamins and proteins. High incidences of neurodegenerative diseases, such as ALS, AD, and PD, have been observed in employees in the automobile and paint industries and in other metal-utilizing factories [11,12]. Recent studies suggest that various genetic factors predispose neurons to enhanced metal toxicity. These include alterations in metal mobility or uptake across the blood-brain barrier and metal storage proteins in the brain, including lactoferrin and ferritin/transferrin. Friedreich’s ataxia, a genetic disorder of Fe metabolism, is caused by insufficient level of the Fe chaperone, frataxin, which leads to dysregulation of Fe trafficking in mitochondria and to mitochondrial genome damage by Reactive Oxygen Species (ROS) [13–16]. Similarly, abnormal Fe metabolism is responsible for the etiopathogenesis of hereditary ferritinopathy [17]. Another well-known, autosomal recessive disorder, Wilson’s disease, arises from a lethal mutation in the ATP7B gene, which encodes a copper (Cu) transporter, leading to supra-physiological, accumulation of free Cu in the brain and liver [18]. Aging is another contributor to chronic accumulation of brain metals [19]. Fe(III), Cu(II), and Zn(II) ions play critical roles in the gradual progression of AD and PD in an age-dependent manner by stabilizing misfolded amyloid beta sheets [20,21].
Thus, complex interactions between genetic predisposition and environmental/dietary influences appear to induce accumulation of free metal ions in the brain.
Molecular basis of metal toxicity
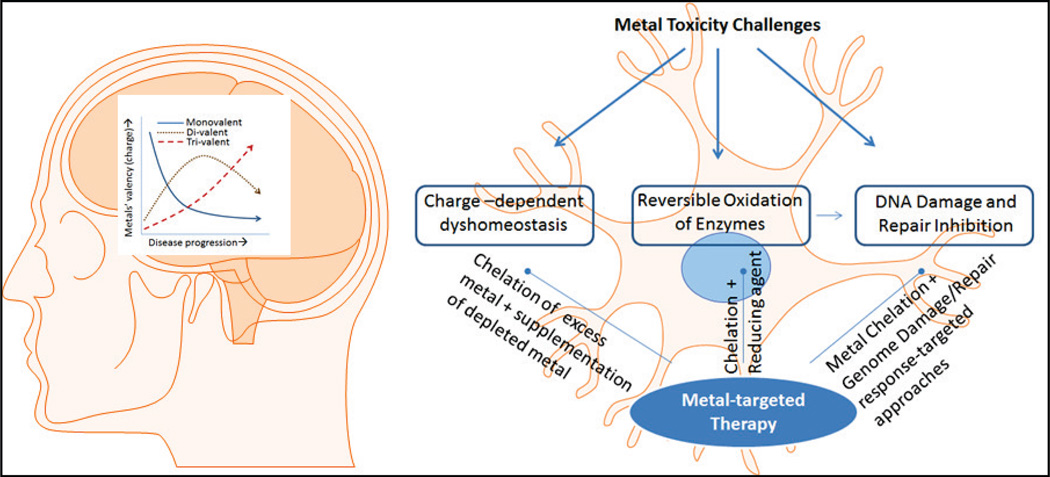
As schematically represented in Figure 1, metal dyshomeostasis is deleterious to human cells. The intracellular and extracellular levels of metals are tightly regulated through a complex network. Excessive concentration of non-sequestered metal salts could cause cellular toxicity and pathological damage. In addition to altering the membrane potential, particularly in neurons, metal ions can bind to and affect the activity of proteins/enzymes and nucleic acid, which may cause cytotoxicity. In addition, the major cause of oxidative toxicity from transition metals is the generation of ROS, the most pervasive oxidant in cells [22–25]. In addition, many heavy metals, such as cadmium and lead, are also pro-oxidant and highly toxic. These could cause membrane depolarization by blocking calcium-ion influx and cell death [26–28].
Figure 1.
Molecular basis for metal neurotoxicity and its potential as a therapeutic target. Studies suggest a charge-based dyshomeostasis of metals in neurons affected by degenerative diseases. Typically, trivalent metals increase in late-stage AD, whereas divalent metal ions increase in early AD. The increase in metal ions could reversibly inhibit DNA repair enzymes, inducing genomic damage. Metal chelation therapy should address these challenges based on recent molecular understanding of the phenomenon.
Individual metal accumulation versus metal homeostatic imbalance
As already stated, metal content is tightly regulated under physiological conditions in the normal brain through sequestration by storage proteins (e.g., ferritin, transferrin, and ceruloplasmin). The stored metals are released only in response to metabolic needs. Abnormal sequestration leading to metal overload is a common feature of neuronal pathologies; remarkably, studies have revealed unique charge-dependent changes in brain metal homeostasis with the progression of disease severity in AD and PD [29]. For example, the level of divalent Fe(II) or Cu(II) increases in the brain during the early phase of AD [30]. Interestingly, in AD and PD cases with no evident dietary metal exposure, the overall brain metal burden was found unaltered; instead, there is a charge-dependent redistribution of specific metals in the affected brain regions. For example, increase in Fe in PD patients, is simultaneously associated with decrease in Zn [5]. This may imply that the impact of an increase or decrease of an individual metal is not restricted to that metal alone, but causes a more dramatic overall homeostatic imbalance of metals, presumably due to loss of regulation of metals across cell membranes. This may be important for formulating metal chelation therapy, which should include supplementation of the depleted metal, in addition to chelating the increased metal ions.
It is noteworthy that in contrast to AD and PD, for other neurological disorders and stroke, there are limited data regarding trace metal homeostasis or inter-elemental relationships in the brain. A comprehensive database for the pathological dyshomeostasis of metals is critical for early diagnosis and for improving our understanding of the role of metals in neurotoxicity.
Metals cause genomic damage directly and via generation of ROS
Oxidative genomic damage is the most common type of damage caused by pro-oxidant metals due to ROS generated via Fenton and Haber-Weiss reactions. In addition, some heavy metals including essential metals produce DNA damage via direct binding and cause strand breaks [31,32]. Both ROS and metals induce a multitude of oxidative modifications in DNA bases and sugar moieties, including DNA strand breaks. Persistent accumulation of this damage could lead to secondary double-strand breaks, which are the most toxic genomic damage [33,34].
Inhibition of genome repair machinery by metals
While marked increase in genomic damage is observed in a majority of neurodegenerative diseases, the neurons also show decreased ability to repair the damage [35,36]. Moreover, the lack of a direct correlation between repair deficiencies and expression of repair enzymes suggests the involvement of additional mechanisms. Repair defects induced by heavy metals have been commonly attributed to their direct binding to DNA, which interferes with the repair machinery’s ability for genome damage scanning/recognition and repair [37–39]. We recently showed that physiological levels of iron and copper salts inhibited the NEIL1/2 enzymes, two key components of the oxidized DNA base repair machinery (via base excision repair or BER) [6,37,40–43]. Inhibition of these enzymes is due to metal binding to themselves rather than to DNA, which involves reversible oxidation of cysteine residues in the enzymes. Furthermore, these metal ions disrupt protein-protein interactions during BER, which is critical for coordinating the complete repair at the chromatin level. Thus our studies, in contrast to previous observations, suggest that metal-induced defects in genome repair is caused by direct binding of redox metals to specific repair proteins, which affects their redox state and structure/function. It is likely that persistent accumulation of genomic damage could elicit inflammatory responses (i.e., microglia activation), further contributing to neuronal dysfunctions [6].
Metal-targeted therapies: Past and current challenges
Metal chelation therapy has been explored as a strategy to eliminate excess metal ions from the body. This treatment has had mixed success due to challenges intrinsically associated with chelation and the inherent complexity of metal dynamics in the body.
Some metal chelators successfully reduce metal build-up in animals and in in vitro models. Although chelators were shown to reduce metal accumulation in the humans in clinical trials, several challenges prevented their broad application. First, most available chelating compounds fail to cross the Blood-Brain Barrier (BBB). Desferrioxamine B (DB), an iron chelator, was one of the first metal chelators used in AD patients, where it caused a significant decline in amyloid plaque levels and decreased the cognition deterioration rate [44]. However, DB caused anemia over time [45]. Clioquinol (CQ), another metal chelator, was reported to restore metal homoestasis in several animal models of neurodegeneration and in AD patients [29,45,46]. CQ efficiently crosses the BBB, preferentially binds Cu(II) and Zn(II), and inhibits amyloid-β deposition [47]. However, a CQ derivative, 8-hydroxyquinoline (8-OHQ; also named PBT2; Prana Biotechnology), failed in a phase III trial due to non-significant clearance of β-amyloid plaques in patients with mild AD (Product News, J Gerontol. Nurs. 40, 5–6, 2004).
Magnetic resonance imaging data from phase II clinical trials indicated that deferiprone (DFP), a metal chelator used to treat thalassemia patients, significantly decreases Fe levels in the Substantia Nigra (SN) in patients with early or late PD [48]. However, after 24-month treatment, the chelation benefits of DFP disappeared, and Fe deposition reappeared in the SN. These data suggest that a readjusted treatment time should be considered for long-term benefits [48]. In contrast to other chemical chelators, DFP alleviates Fe accumulation by donating chelated Fe to unsaturated transferrin, and allows balanced retention and chemical redistribution of Fe in the body [49].
In an investigation using a mouse stroke model, the ferrous chelator 2,2'-bipyridine was shown to be effective in reducing brain injury following Intra Cerebral Hemorrhage [ICH] and ischemia [50,51]. Although this chelator appears to be promising for preventing brain injury after stroke, recent reports suggest that bipyridine does not prevent iron-induced damage in three ICH rat models [52]. Thus, its efficacy as a therapeutic agent remains unclear.
Natural metal chelators, including curcuminoids and catechins, are predominant components of the rural Asian diet and are believed to be highly beneficial for combating neurotoxicity [43]. Curcumin is the most popular curcuminoid, present in turmeric and known for its unique flavor, has a broad spectrum of pharmacological properties. In recent years, this traditional Indian spice has gained attention for its ability to bind metals and protect neurons. For example, curcumin protects hippocampal neurons against Pb- and Cd-induced lipid peroxidation [53].
Catechins comprise another class of potential metal chelators commonly found in green tea, berries, cocoa, and onions. Epigallo Catechingallate (EGCG) is a common catechin explored for its chemo-protective and neuroprotective functions. Chemically, EGCG possesses iron-chelating properties, by possibly neutralization of ferric iron and formation of redox-inactive iron in neuronal cells [1]. Although the therapeutic potential of natural compounds for acute metal toxicity needs further investigation, their inclusion in a balanced diet could provide a cost-effective strategy for reducing the oxidative burden in patients with neurodegenerative disorders or stroke.
Challenges and future perspectives
There is compelling evidence linking metal toxicity to neuronal dysfunction. In addition, there has been an enormous increase in our understanding of the molecular basis of metal neurotoxicity. Nonetheless, current metal-targeted therapeutic approaches remain to be proven effective. Further, antioxidant therapies have not been as effective as expected. This underscores the need to explore new approaches to unraveling the bases for neuronal pathobiology. Current molecular studies have not effectively improved our ability to rationally apply metal-targeting-based therapeutic approaches. We suggest that recent basic discoveries on metal biochemistry may help develop new approaches for enhancing efficacy of metal chelation therapy. For instance, intracellular metal dyshomeostasis involving auto-depletion of specific metal ions is a common occurrence after individual metal overload; thus, metal chelation strategies should include supplementation of depleted metals. Furthermore, because of reversible oxidation of cysteine residues in various proteins, including the key genome repair enzymes NEIL1 and NEIL2 by pro-oxidant metals [37,40], metal chelation could be combined with specific reducing factors [6,29,43]. Thus, the recent advances discussed here underscore the need to revisit the role of metal toxicity in neurological diseases and stroke in order to develop new therapeutic strategy.
Acknowledgement
The research in the authors’ laboratories is supported by grants from the Muscular Dystrophy Association (MDA 294842; MLH), ALS Association (ALSA 15-IIP-204; MLH), Alzheimer’s Association (NIRG-12-242135; MLH), Melo Brain Funds (M.L.H and K.S.R.), USPHS grants R01 CA158910 (S.M. and M.L.H.) and US NIEHS RO1 ES018948 (I.B.). Velmarini Vasquez is supported by a Doctoral Scholarship granted by the Institute for Training and Development of Human Resources of Panama (IFARHU) and the National Secretariat for Science, Technology, and Innovation of Panama (SENACYT). We thank Hegde lab members Dr. H. Wang and Ms. E. Guerrero for their help.
References
- 1.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 2.Justicia C, Ramos-Cabrer P, Hoehn M. Mri detection of secondary damage after stroke: Chronic iron accumulation in the thalamus of the rat brain. Stroke. 2008;39:1541–1547. doi: 10.1161/STROKEAHA.107.503565. [DOI] [PubMed] [Google Scholar]
- 3.Li YV, Zhang JH. Metal ion in stroke (eds) Springer; New York, NY: 2012. [Google Scholar]
- 4.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 5.Hegde ML, Shanmugavelu P, Vengamma B, Rao TS, Menon RB, Rao RV, Rao KS. Serum trace element levels and the complexity of inter-element relations in patients with parkinson's disease. J Trace Elem Med Biol. 2004;18:163–171. doi: 10.1016/j.jtemb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Mitra J, Guerrero EN, Hegde PM, Wang H, Boldogh I, Rao KS, Mitra S, Hegde ML. New perspectives on oxidized genome damage and repair inhibition by pro-oxidant metals in neurological diseases. Biomolecules. 2014;4:678–703. doi: 10.3390/biom4030678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zatta P, Lucchini R, van Rensburg SJ, Taylor A. The role of metals in neurodegenerative processes: Aluminum, manganese, and zinc. Brain Res Bullet. 2003;62:15–28. doi: 10.1016/s0361-9230(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 8.Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab. 2014;34:1070–1075. doi: 10.1038/jcbfm.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galasso SL, Dyck RH. The role of zinc in cerebral ischemia. Mol Med. 2007;13:380–387. doi: 10.2119/2007-00044.Galasso. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hengstler JG, Bolm-Audorff U, Faldum A, Janssen K, Reifenrath M, Gotte W, Jung D, Mayer-Popken O, Fuchs J, Gebhard S, et al. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis. 2003;24:63–73. doi: 10.1093/carcin/24.1.63. [DOI] [PubMed] [Google Scholar]
- 11.Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: An overview of environmental risk factors. Environ Health Perspect. 2005;113:1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pira E, Gianluigi D, Herrero Hernandez E. Occupational exposures and neurodegenerative diseases. Epidemiology. 2004;15:253–2544. doi: 10.1097/01.ede.0000112216.75693.96. [DOI] [PubMed] [Google Scholar]
- 13.Anzovino A, Lane DJ, Huang ML, Richardson DR. Fixing frataxin: 'Ironing out' the metabolic defect in friedreich's ataxia. Br J Pharmacol. 2014;171:2174–2190. doi: 10.1111/bph.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Cabo P, Palau F. Mitochondrial pathophysiology in friedreich's ataxia. J Neurochem. 2013;126(Suppl 1):53–64. doi: 10.1111/jnc.12303. [DOI] [PubMed] [Google Scholar]
- 15.Huang ML, Becker EM, Whitnall M, Suryo Rahmanto Y, Ponka P, Richardson DR. Elucidation of the mechanism of mitochondrial iron loading in friedreich's ataxia by analysis of a mouse mutant. Proc Natl Acad Sci, USA. 2009;106:16381–16386. doi: 10.1073/pnas.0906784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandolfo M. Iron and friedreich ataxia. J Neural Transm Suppl. 2006:143–146. doi: 10.1007/978-3-211-45295-0_22. [DOI] [PubMed] [Google Scholar]
- 17.Vidal R, Delisle MB, Ghetti B. Neurodegeneration caused by proteins with an aberrant carboxyl-terminus. J Neuropathol Exp Neurol. 2004;63:787–800. doi: 10.1093/jnen/63.8.787. [DOI] [PubMed] [Google Scholar]
- 18.Fatemi N, Sarkar B. Molecular mechanism of copper transport in wilson disease. Environ Health Persp. 2002;110(Suppl 5):695–698. doi: 10.1289/ehp.02110s5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagemeier J, Geurts JJ, Zivadinov R. Brain iron accumulation in aging and neurodegenerative disorders. Expert Rev Neurother. 2012;12:1467–1480. doi: 10.1586/ern.12.128. [DOI] [PubMed] [Google Scholar]
- 20.Ayton S, Lei P, Bush AI. Metallostasis in alzheimer's disease. Free Rrad Biol Med. 2013;62:76–89. doi: 10.1016/j.freeradbiomed.2012.10.558. [DOI] [PubMed] [Google Scholar]
- 21.Grasso G, Pietropaolo A, Spoto G, Pappalardo G, Tundo GR, Ciaccio C, Coletta M, Rizzarelli E. Copper(i) and copper(ii) inhibit abeta peptides proteolysis by insulin-degrading enzyme differently: Implications for metallostasis alteration in alzheimer's disease. Chemistry. 2011;17:2752–2762. doi: 10.1002/chem.201002809. [DOI] [PubMed] [Google Scholar]
- 22.Balamurugan K, Egli D, Selvaraj A, Zhang B, Georgiev O, Schaffner W. Metal-responsive transcription factor (mtf-1) and heavy metal stress response in drosophila and mammalian cells: A functional comparison. Biol Chem. 2004;385:597–603. doi: 10.1515/BC.2004.074. [DOI] [PubMed] [Google Scholar]
- 23.Chang KC, Hsu CC, Liu SH, Su CC, Yen CC, Lee MJ, Chen KL, Ho TJ, Hung DZ, Wu CC, et al. Cadmium induces apoptosis in pancreatic beta-cells through a mitochondria-dependent pathway: The role of oxidative stress-mediated c-jun n-terminal kinase activation. PloS one. 2013;8:e54374. doi: 10.1371/journal.pone.0054374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondoh M, Araragi S, Sato K, Higashimoto M, Takiguchi M, Sato M. Cadmium induces apoptosis partly via caspase-9 activation in hl-60 cells. Toxicology. 2002;170:111–117. doi: 10.1016/s0300-483x(01)00536-4. [DOI] [PubMed] [Google Scholar]
- 25.Zipfel GJ, Babcock DJ, Lee JM, Choi DW. Neuronal apoptosis after cns injury: The roles of glutamate and calcium. J Neurotrauma. 2000;17:857–869. doi: 10.1089/neu.2000.17.857. [DOI] [PubMed] [Google Scholar]
- 26.Hinkle PM, Kinsella PA, Osterhoudt KC. Cadmium uptake and toxicity via voltage-sensitive calcium channels. J biol Chem. 1987;262:16333–16337. [PubMed] [Google Scholar]
- 27.Marchetti C. Role of calcium channels in heavy metal toxicity. ISRN Toxicol. 2013;2013:184360. doi: 10.1155/2013/184360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pentyala S, Ruggeri J, Veerraju A, Yu Z, Bhatia A, Desaiah D, Vig P. Microsomal ca2+ flux modulation as an indicator of heavy metal toxicity. Indian J Exp Biol. 2010;48:737–743. [PubMed] [Google Scholar]
- 29.Hegde ML, Bharathi P, Suram A, Venugopal C, Jagannathan R, Poddar P, Srinivas P, Sambamurti K, Rao KJ, Scancar J, et al. Challenges associated with metal chelation therapy in alzheimer's disease. J Alzheimers Dis. 2009;17:457–468. doi: 10.3233/JAD-2009-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao KSJ, Rao RV, Shanmugavelu P, Menon RB. Trace elements in alzheimer's disease brain: A new hypothesis. Alz Rep. 1999;2:241–246. [Google Scholar]
- 31.Oliveira SC, Corduneanu O, Oliveira-Brett AM. In situ evaluation of heavy metal-DNA interactions using an electrochemical DNA biosensor. Bioelectrochemistry. 2008;72:53–58. doi: 10.1016/j.bioelechem.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Sudhamani CN, Bhojya Naik HS, Girija D, Sangeetha Gowda KR, Giridhar M, Arvinda T. Novel complexes of co(iii) and ni(ii) containing peptide ligands: Synthesis, DNA binding and photonuclease activity. Spectrochim Acta A Mol Biomol Spectrosc. 2014;118:271–278. doi: 10.1016/j.saa.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 33.Khanna KK, Jackson SP. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 34.Tung EW, Philbrook NA, Macdonald KD, Winn LM. DNA double-strand breaks and DNA recombination in benzene metabolite-induced genotoxicity. Toxicol Sci. 2012;126:569–577. doi: 10.1093/toxsci/kfs001. [DOI] [PubMed] [Google Scholar]
- 35.de Sousa J, de Boni U, Cinader B. Age-related decrease in ultraviolet induced DNA repair in neurons but not in lymph node cells of inbred mice. Mech Ageing Dvelop. 1986;36:1–12. doi: 10.1016/0047-6374(86)90134-x. [DOI] [PubMed] [Google Scholar]
- 36.Rao KS, Annapurna VV, Raji NS. DNA polymerase-beta may be the main player for defective DNA repair in aging rat neurons. Ann N Y Acad Sci. 2001;928:113–120. doi: 10.1111/j.1749-6632.2001.tb05641.x. [DOI] [PubMed] [Google Scholar]
- 37.Hegde ML, Hegde PM, Rao KS, Mitra S. Oxidative genome damage and its repair in neurodegenerative diseases: Function of transition metals as a double-edged sword. J Alzheimers Dis. 2011;24(Suppl 2):183–198. doi: 10.3233/JAD-2011-110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Swiercz R, Englander EW. Elevated metals compromise repair of oxidative DNA damage via the base excision repair pathway: Implications of pathologic iron overload in the brain on integrity of neuronal DNA. J Neurochem. 2009;110:1774–1783. doi: 10.1111/j.1471-4159.2009.06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder RD. Role of active oxygen species in metal-induced DNA strand breakage in human diploid fibroblasts. Mutat Res. 1988;193:237–246. doi: 10.1016/0167-8817(88)90034-x. [DOI] [PubMed] [Google Scholar]
- 40.Hegde ML, Hegde PM, Holthauzen LM, Hazra TK, Rao KS, Mitra S. Specific inhibition of neil-initiated repair of oxidized base damage in human genome by copper and iron: Potential etiological linkage to neurodegenerative diseases. J Biol Chem. 2010;285:28812–28825. doi: 10.1074/jbc.M110.126664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hegde ML, Izumi T, Mitra S. Oxidized base damage and single-strand break repair in mammalian genomes: Role of disordered regions and posttranslational modifications in early enzymes. Prog Mol Biol Transl Sci. 2012;110:123–153. doi: 10.1016/B978-0-12-387665-2.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegde ML, Mantha AK, Hazra TK, Bhakat KK, Mitra S, Szczesny B. Oxidative genome damage and its repair: Implications in aging and neurodegenerative diseases. Mech Ageing Dvelop. 2012;133:157–168. doi: 10.1016/j.mad.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegde ML. Molecular characterization of neuroprotective activities of plant based products could revive their utilization and lead discovery of new drug candidates for brain diseases. J Pharm Bioallied Sci. 2014;6:63–64. [PMC free article] [PubMed] [Google Scholar]
- 44.Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF. Intramuscular desferrioxamine in patients with alzheimer's disease. Lancet. 1991;337:1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- 45.Budimir A. Metal ions, alzheimer's disease and chelation therapy. Acta Pharm. 2011;61:1–14. doi: 10.2478/v10007-011-0006-6. [DOI] [PubMed] [Google Scholar]
- 46.Barcia E, Salama A, Fernandez-Carballido A, Negro S. Protective effects of clioquinol on human neuronal-like cells: A new formulation of clioquinol-loaded plga microspheres for alzheimer's disease. J Drug Target. 2011;19:637–646. doi: 10.3109/1061186X.2010.523789. [DOI] [PubMed] [Google Scholar]
- 47.Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 48.Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G, Garcon G, Rouaix N, et al. Targeting chelatable iron as a therapeutic modality in parkinson's disease. Antioxid Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sohn YS, Breuer W, Munnich A, Cabantchik ZI. Redistribution of accumulated cell iron: A modality of chelation with therapeutic implications. Blood. 2008;111:1690–1699. doi: 10.1182/blood-2007-07-102335. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Wu T, Li M, Wang J. Efficacy of the lipid-soluble iron chelator 2,2'-dipyridyl against hemorrhagic brain injury. Neurobiol Dis. 2012;45:388–394. doi: 10.1016/j.nbd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Methy D, Bertrand N, Prigent-Tessier A, Mossiat C, Stanimirovic D, Beley A, Marie C. Beneficial effect of dipyridyl, a liposoluble iron chelator against focal cerebral ischemia: In vivo and in vitro evidence of protection of cerebral endothelial cells. Brain Res. 2008;1193:136–142. doi: 10.1016/j.brainres.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 52.Caliaperumal J, Wowk S, Jones S, Ma Y, Colbourne F. Bipyridine, an iron chelator, does not lessen intracerebral iron-induced damage or improve outcome after intracerebral hemorrhagic stroke in rats. Transl Stroke Res. 2013;4:719–728. doi: 10.1007/s12975-013-0272-3. [DOI] [PubMed] [Google Scholar]
- 53.Daniel S, Limson JL, Dairam A, Watkins GM, Daya S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J Inorg Biochem. 2004;98:266–275. doi: 10.1016/j.jinorgbio.2003.10.014. [DOI] [PubMed] [Google Scholar]