Abstract
Chemotherapy is a general treatment option for various cancers, including lung cancer. In order to find compounds with superior bioactivity and less toxicity against lung cancer, novel spin-labeled 5-fluorouracil (5-FU) derivatives (3a–f) were synthesized and evaluated against four human tumor cell lines (A-549, DU-145, KB, and KBvin). Two promising compounds 3d and 3f exhibited IC50 values of 2.76 and 2.38 μM, respectively, against non-small cell lung carcinoma cell line A-549. These compounds were twofold more cytotoxic than 5-FU and less toxic against other tested cell lines. Compound 3f exhibited seven times more selective cytotoxicity against A-549 than 5-FU. Our results suggest that compounds 3d and 3f merit further investigation for development into clinical trial candidates for non-small cell lung cancer.
Keywords: 5-Fluorouracil, Spin-labeled, Nitroxide, Cytotoxicity
Introduction
5-Fluorouracil (5-FU, 1) is a widely used clinical treatment for solid tumors, such as colorectal, breast, liver, gastric, and other cancers. 5-FU is used as a combination chemotherapy for lung cancer (Ghoshal and Jacob, 1997; Huang et al., 2007; Isanbor and O’Hagan, 2006). As an antimetabolite, 5-FU was first synthesized in 1957 (Heidelberger et al., 1957). It is a pyrimidine analogue of DNA and RNA, with a fluorine atom at the C-5 position in place of the hydrogen of uracil (Zhang et al., 2008). 5-FU is an irreversible inhibitor of thymidylate synthetase and consequently interferes with the formation of thymidylic acid from deoxyuridylic acid to inhibit DNA synthesis. As a uracil analogue, 5-FU may be incorporated into RNA to give an aberrant form of RNA, leading to cytotoxicity and cell death (Bosch et al., 1958; Cohen et al., 1958; Goldberg et al., 1966; Madoc-Jones and Bruce, 1968; Kufe and Major, 1981). However, 5-FU is poorly selective toward tumors, and it causes some undesirable side effects, such as gastrointestinal tract reaction, bone marrow depression, dermatologic changes, alopecia, leucopenia, thrombocytopenia, neurotoxicity, cardiotoxicity, and even death (Kennedy, 1999). These side effects have interfered significantly with 5-FU’s clinical application. Accordingly, it is necessary to continuously design and synthesize 5-FU analogues that have broad-spectrum or selective antineoplastic activities and can overcome the clinical limitations.
Furthermore, the introduction of a stable nitroxyl radical into a pharmaceutical molecule can, to some extent, reduce toxicity and augment antineoplastic activities (Gadjeva, 2002; Jin et al., 2006; Zheleva and Gadjeva, 2001). In our previous studies, we successfully prepared several series of spin-labeled antineoplastic drugs by modifying different bioactive molecules. When compared with the parent compounds, many of the spin-labeled compounds showed significant antineoplastic activity with markedly decreased toxicity (Liu et al., 2007, 2012; Liu and Tian, 2005; Tian et al., 2002; Wang et al., 1993). In addition, L-amino acids are actively transplanted into mammalian tissue, have good water solubility, and are often used as carrier vehicles for some drugs. Based on the above facts, we introduced the nitroxyl radical moiety into 5-FU via a hydrophilic amino acid spacer, in an attempt to simultaneously circumvent 5-FU’s limitations and develop superior pharmacological profiles. Herein, we report the synthesis and structure–cytotoxicity relationships of a series of novel spin-labeled derivatives of 5-FU as potential antitumor agents.
Results and discussion
Design and synthesis of novel 5-FU analogues
The synthetic route to the N-(1-oxyl-2,2,6,6-tetramethyl-4-piperidinyloxycarbonyl)-amino acids 9a–f is depicted in Scheme 1. 4-Hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (5) was synthesized in 83 % yield by catalytic oxidation of 4-hydroxy-2,2,6,6-tetramethylpiperidine (4) with sodium tungstate–hydrogen peroxide–EDTA (Rozantsev, 1964). The reaction of 5 with N,N-carbonyldiimidazole gave N-(1-oxyl-2,2,6,6-tetramethylpiperidinyloxycarbonyl)-imidazole (6) (Staab and Mannschreck, 1962). Without purification, this intermediate (6) was reacted with p-toluenesulfonic acid monohydrate to give a highly reactive tosylate (7). When dissolved in an aqueous solution of sodium azide, 7 was converted instantaneously into the alkoxycarbonyl azide (8). Compounds 9a–f were obtained in good yields by reaction of 8 with the appropriate amino acids in the presence of MgO (Hankovszky et al., 1979).
Scheme 1.
Synthesis of compounds 9a–f. Reagents and conditions: i Na2WO4/H2O2/EDTA; ii N,N′-carbonyl-diimidazole/THF; iii p-toluenesulfonic acid monohydrate; iv NaN3/H2O; v amino acids/MgO, 24 h
Subsequently, commercially available 5-FU (1) was heated with 37 % aqueous formaldehyde for about 50 min at 60 °C to yield 1-hydroxymethyl-5-fluorouracil (2) (Ahmad et al., 1987; Ouyang et al., 2011). The target esters 3a–f were obtained in excellent yields by the straightforward acylation of intermediate 2 with the corresponding N-(1-oxyl-2,2,6,6-tetramethyl-4-piperidinyloxycarbonyl)-amino acids 9a–f in the presence of N,N-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) (Scheme 2). The presence of the nitroxide group in the target compounds 3a–f was confirmed from ESR and IR spectra. In the ESR spectra, the nitroxide group showed a triplet with hyperfine coupling constants (aN) in the range of 15.90–16.28 G due to interaction between an unpaired electron and nitrogen nucleus of the aminoxyl radical (g value = 2.006). In the IR spectra, bands characteristic of the nitroxyl moiety appeared at 1,370 ± 7 cm−1 as shown in Table 1. Furthermore, melting point and high-resolution mass spectrometry (ESI) data also characterized the target compounds 3a–f (Table 1).
Scheme 2.
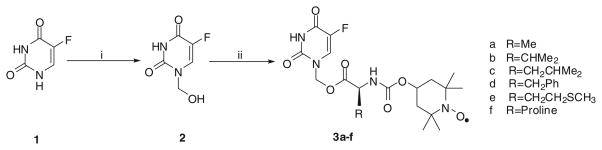
Synthesis of target compounds 3a–f. Reagents and conditions: i HCHO; ii 9a–f/DCC/DMAP, 2 h
Table 1.
Physical and spectroscopic data of compounds 3a–f
| Cmpd. | Molecular formula | mp (°C) | HRMS ([M + NH4]+)
|
IR (cm−1) | ESR
|
||
|---|---|---|---|---|---|---|---|
| Calcd. | Found | aN (G) | g | ||||
| 3a | C18H26FN4O7 | 85–87 | 447.1792 | 447.1786 | 3,330 (N–H), 1,698 (C=O), 1,364 (N–O), 1,244 (C–F) | 16.16 | 2.0060 |
| 3b | C20H30FN4O7 | 64–66 | 475.2437 | 475.2440 | 3,341 (N–H), 1,717 (C=O), 1,367 (N–O), 1,241 (C–F) | 15.81 | 2.0058 |
| 3c | C21H32FN4O7 | 87–89 | 489.2593 | 489.2603 | 3,338 (N–H), 1,717 (C=O), 1368 (N–O), 1,263 (C–F) | 16.04 | 2.0058 |
| 3d | C24H30FN4O7 | 73–75 | 523.2437 | 523.2442 | 3,341 (N–H), 1,715 (C=O), 1,365 (N–O), 1,259 (C–F) | 16.10 | 2.0061 |
| 3e | C20H30FN4O7S | 69–71 | 507.2157 | 507.2162 | 3,333 (N–H), 1,716 (C=O), 1,365 (N–O), 1,246 (C–F) | 15.88 | 2.0060 |
| 3f | C20H28FN4O7 | 83–85 | 473.2280 | 473.2275 | 3,440 (N–H), 1,715 (C=O), 1,370 (N–O), 1,245 (C–F) | 16.02 | 2.0060 |
Effects of novel 5-FU analogues on tumor cell growth
Target compounds 3a–f were evaluated for in vitro cyto-toxicity against four tumor cell lines, human alveolar adenocarcinoma (A-549), human prostate carcinoma (DU-145), human nasopharyngeal carcinoma (KB), and human vincristine-resistant nasopharyngeal carcinoma (KBvin). The parent compound 5-FU (1) was included as a positive control, and the obtained IC50 values are shown in Table 2. The selectivity index (SI) against A-549 was calculated as mean IC50 against DU-145, KB, and KBvin divided by IC50 against A-549. Our results demonstrated that 3f showed the best SI (7.5) against A-549.
Table 2.
Cytotoxic activity of 3a–f against four human cancer cell lines
| Compounds | IC50 (μM)
|
SIa | |||
|---|---|---|---|---|---|
| A-549 | DU-145 | KB | KBvin | ||
| 3a | 12.39 ± 0.91 | 19.58 ± 0.64 | > 20 | > 20 | 1.7 |
| 3b | 13.10 ± 1.81 | > 20 | > 20 | > 20 | 1.5 |
| 3c | 11.14 ± 0.77 | 12.87 ± 0.32 | 14.30 ± 0.23 | 17.08 ± 1.66 | 1.3 |
| 3d | 2.76 ± 0.02 | 13.15 ± 0.34 | 17.62 ± 0.75 | 19.09 ± 0.78 | 6.0 |
| 3e | 10.28 ± 0.62 | 11.36 ± 0.71 | 11.60 ± 0.57 | 11.71 ± 0.12 | 1.1 |
| 3f | 2.38 ± 0.12 | 15.53 ± 0.97 | 18.29 ± 0.21 | > 20 | 7.5 |
| 5-FU | 5.09 ± 0.46 | 10.97 ± 0.45 | 12.79 ± 0.22 | 13.70 ± 0.46 | 2.4 |
Selectivity index (fold selective against A-549): (mean IC50 against DU-145, KB, and KBvin)/IC50 against A-549
5-FU and its spin-labeled derivatives showed the same order of cell line sensitivity: A-549 > DU-145 > KB > KBvin (decreasing potency of test compound). Against the A-549 cell line, compounds 3d and 3f, with IC50 values of 2.762 and 2.38 μM, respectively, were twofold more potent than 5-FU, with an IC50 value of 5.09 μM. Furthermore, these compounds exhibited good selectivity against A-549, suggesting less toxicity for normal cells. Against the DU-145, KB, and KBvin cell lines, compound 3e, with IC50 values of 11.36, 11.60, and 11.71 μM, respectively, was as or slightly more potent than 5-FU, with IC50 values of 10.97, 12.79, and 13.70, respectively. Against the indicated cell lines, the rank orders of activity based on the different amino acid linkages were as follows: for A-549, L-proline > L-phenylalanine > L-methionine > L-leucine > L-alanine > L-valine; for DU-145, L-methionine > L-leucine > L-phenylalanine > L-proline > L-alanine > L-valine; for KB L-methionine > L-leucine > L-phenylalanine > L-proline > L-valine ≥ L-alanine; and for KBvin, L-methionine > L-leucine > L-phenylalanine > L-valine ≥ L-proline ≥ L-alanine. These results showed that the structures of the L-amino acids can have potential effects on the bioactivity of these compounds.
Thus, we have successfully introduced a stable nitroxyl radical into 5-FU via an L-amino acid linkage. Based on the cytotoxicity results, this modification might result in synergistic action against certain tumor cell lines. Further biological evaluation is in progress to better define the antineoplastic activity of these compounds and to clarify whether spin-labeled 5-FU analogues might display decreased side effects compared with 5-FU.
Conclusion
We have synthesized novel spin-labeled derivatives of 5-FU and evaluated their cytotoxic effects against four tumor cell lines by the SRB method. Among all tested compounds, compounds 3d and 3f were more cytotoxic than 5-FU against the A-549 lung cancer cell line and merit further investigation for development into clinical trial candidates against non-small cell lung cancer.
Experimental
Chemistry
Melting points were taken on a Kofler melting point apparatus and uncorrected. IR spectra were obtained on NIC-5DX spectra photometer, mass spectral analysis was taken on ZAB-HS and Bruker Daltonics APEXII49e instruments, and ESR spectra were obtained with a Bruker ER-200D-SRC X-band spectrometer. The synthetic compounds were purified by flash chromatography on Merck silica gel (70–230 mesh). Thin-layer chromatography (TLC) was performed on silica gel plates with a fluorescent indicator (Merck Silica Gel 60 F2540.25 mm thick). The N-(1-oxyl-2,2,6,6-tetramethyl-4-piperidinyloxycarbonyl)-amino acids (9a–f) (Hankovszky et al., 1979) and 1-hydroxymethyl-5-fluorouracil used for the experiments were prepared by modifications of previous procedures (Ahmad et al., 1987; Ouyang et al., 2011).
General procedure for the synthesis of target compounds (3a–f)
A mixture of a N-(1-oxyl-2,2,6,6-tetramethyl-4-piperidinyloxycarbonyl)-amino acid (0.001 M), 1-hydroxymethyl-5-fluorouracil (0.001 M), and dimethylaminopyridine (DMAP, 0.1 g) was stirred in dichloromethane (10 mL) for 5 min at room temperature under nitrogen. N,N-Dicyclohexylcarbodiimide (DCC, 0.21 g, 0.001 M) was added, and the reaction mixture was stirred for 2 h. The reaction mixture was filtered, and the filtrate was evaporated under reduced pressure. The residue was separated by flash column chromatography (gradient elution with mixtures of dichloromethane–acetone) on silica gel and monitored by TLC. Synthesized target compounds 3a–f were characterized by m.p., ESR, IR, and HRMS spectroscopic analyses.
Cell culture and determination of cytotoxic activity
A-549 (non-small cell lung carcinoma), DU145 (androgen-independent prostate cancer), and KB (nasopharyngeal carcinoma) cell lines were obtained from the Lineberger Comprehensive Cancer Center (UNC-CH). KBvin (vincristine-resistant KB) cell line was a generous gift of Professor Y.-C. Cheng, Yale University. Cells were cultured in RPMI 1640 medium containing 25 mM HEPES and 2 mM L-glutamine (Mediatech), supplemented with 10 % heat-inactivated fetal bovine serum (Hyclone), 100 IU penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (Mediatech) in 5 % CO2 and 95 % air at 37 °C.
Antiproliferative activity was determined by the sulfo-rhodamine B (SRB) colorimetric assay as previously described (Nakagawa-Goto et al., 2011). In brief, cells (3–5 × 103 cells/well) were seeded in 96-well plates filled with culture medium containing various concentrations of sample and incubated for 72 h. At the end of the exposure period, cells were fixed with cold 50 % trichloroacetic acid followed by staining with 0.04 % SRB (Sigma Chemical Co.). The absorbance of solubilized SRB was measured at 515 nm on a Microplate Reader ELx800 (Bio-Tek Instruments, Winooski, VT) with Gen5 software. All results are representative of three or more experiments.
Acknowledgments
This study was supported financially by the National Natural Science Foundation of China (30800720); the Young Scholars Science Foundation of Lanzhou Jiaotong University (2011011); and the Fundamental Research Funds for the Central Universities (lzujbky-2013-69). This study was also supported in part by the Cancer Research Center of Excellence, Taiwan (DOH-100-TD-C-111-005), as well as NIH Grant CA177584 from the National Cancer Institute awarded to K.H. Lee.
Footnotes
Conflict of interest The authors report no conflict of interests.
Contributor Information
Liu Yang, Environmental and Municipal Engineering School, Lanzhou Jiaotong University, Lanzhou 730000, People’s Republic of China.
Mei-Juan Wang, School of Pharmacy, Lanzhou University, Lanzhou 730000, People’s Republic of China.
Zhi-Jun Zhang, School of Pharmacy, Lanzhou University, Lanzhou 730000, People’s Republic of China.
Susan L. Morris-Natschke, Natural Products Research Laboratories, UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC 27599-7568, USA
Masuo Goto, Natural Products Research Laboratories, UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC 27599-7568, USA.
Jing Tian, School of Pharmacy, Lanzhou University, Lanzhou 730000, People’s Republic of China.
Ying-Qian Liu, Email: yqliu@lzu.edu.cn, School of Pharmacy, Lanzhou University, Lanzhou 730000, People’s Republic of China. Chinese Medicine Research and Development Center, China Medical University and Hospital, Taichung, Taiwan.
Chih-Ya Wang, Natural Products Research Laboratories, UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC 27599-7568, USA.
Xuan Tian, State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, People’s Republic of China.
Xiao-Ming Yang, Natural Products Research Laboratories, UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC 27599-7568, USA.
Kuo-Hsiung Lee, Email: khlee@unc.edu, Natural Products Research Laboratories, UNC Eshelman School of Pharmacy, University of North Carolina, Chapel Hill, NC 27599-7568, USA. Chinese Medicine Research and Development Center, China Medical University and Hospital, Taichung, Taiwan.
References
- Ahmad S, Ozaki S, Nagase T, Iigo M, Tokuzen R, Hoshi A. A facile method for synthesis of N-acyloxymethyl-5-fluorouracils, as a class of antitumor agents. Chem Pharm Bull. 1987;35:4137–4143. doi: 10.1248/cpb.35.4137. [DOI] [PubMed] [Google Scholar]
- Bosch L, Harbers E, Heidelberger C. Studies on fluorinated pyrimidines. V. Effects on nucleic acid metabolism in vitro. Cancer Res. 1958;18:335–343. [PubMed] [Google Scholar]
- Cohen SS, Flaks JG, Barner HD, Loeb MR, Lichtenstein J. The mode of action of 5-fluorouracil and its derivatives. Proc Natl Acad Sci USA. 1958;44:1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadjeva VG. Two spin labeled triazenes: relationship between biochemical and biological activities. Int J Pharm. 2002;247:39–45. doi: 10.1016/s0378-5173(02)00360-5. [DOI] [PubMed] [Google Scholar]
- Ghoshal K, Jacob ST. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem Pharmacol. 1997;53:1569–1575. doi: 10.1016/s0006-2952(97)00040-3. [DOI] [PubMed] [Google Scholar]
- Goldberg AR, Machledt JH, Jr, Pardee AB. On the action of fluorouracil on leukemia cells. Cancer Res. 1966;26:1611–1615. [PubMed] [Google Scholar]
- Hankovszky HO, Hideg K, Lex L, TIgyi J. Nitroxyls; IV. Synthesis of spin-labeled N-(4-piperidinyloxycarbonyl)imidazoles and 4-piperidinyloxycarbonyl azides and their reaction with amino acid derivatives. Synthesis. 1979;1:530–531. [Google Scholar]
- Heidelberger C, Chaudhuri NK, Danenberg P, Mooren D, Griesbach L, Duschinsky R, Schnitzer RJ, Pleven E, Scheiner J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663–666. doi: 10.1038/179663a0. [DOI] [PubMed] [Google Scholar]
- Huang J, Wang JW, Gong T, Zhang ZR. Synthesis and characterization of insulin-5-Fu conjugate, enabling insulin as multi-drug carrier via dendritic approach. Chin Chem Lett. 2007;18:247–250. [Google Scholar]
- Isanbor C, O’Hagan D. Fluorine in medicinal chemistry: a review of anti-cancer agents. J Fluor Chem. 2006;127:303–319. [Google Scholar]
- Jin Y, Chen SW, Tian X. Synthesis and biological evaluation of new spin-labelled derivatives of podophyllotoxin. Bioorg Med Chem. 2006;14:3062–3068. doi: 10.1016/j.bmc.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Kennedy BJ. 5-Fluorouracil toxicity: old or new? Cancer. 1999;86:1099–1100. doi: 10.1002/(sici)1097-0142(19991001)86:7<1099::aid-cncr1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kufe DW, Major PP. 5-Fluorouracil incorporation into human breast carcinoma RNA correlates with cytotoxicity. J Biol Chem. 1981;256:9802–9805. [PubMed] [Google Scholar]
- Liu YQ, Tian X. Synthesis of novel spin-labeled derivatives of podophyllotoxin as potential antineoplastic agents. Synth Commun. 2005;35:2749–2758. [Google Scholar]
- Liu YQ, Yang L, Tian X. Podophyllotoxin: current perspectives. Curr Bioact Compd. 2007;3:37–66. [Google Scholar]
- Liu YQ, Ohkoshi E, Li LH, Yang L, Lee KH. Design, synthesis and cytotoxic activity of novel spin-labeled rotenone derivatives. Bioorg Med Chem Lett. 2012;22:920–923. doi: 10.1016/j.bmcl.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Madoc-Jones H, Bruce WR. On the mechanism of the lethal action of 5-fluorouracil on mouse L cells. Cancer Res. 1968;28:1976–1981. [PubMed] [Google Scholar]
- Nakagawa-Goto K, Wu PC, Lai CY, Hamel E, Zhu H, Zhang L, Kozaka T, Ohkoshi E, Goto M, Bastow KF, Lee KH. Antitumor agents. 284. New desmosdumotin B analogues with bicyclic B-ring as cytotoxic and antitubulin agents. J Med Chem. 2011;54:1244–1255. doi: 10.1021/jm1011947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang L, He DS, Zhang J, He G, Jiang B, Wang Q, Chen ZJ, Pan JZ, Li YH, Guo L. Selective bone targeting 5-fluorouracil prodrugs: synthesis and preliminary biological evaluation. Bio-org Med Chem. 2011;19:3750–3756. doi: 10.1016/j.bmc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Rozantsev EG. Organic free radicals with a hydroxy group. Izv Akad Nauk SSSR Ser Kim. 1964;2:2187–2191. [Google Scholar]
- Staab HA, Mannschreck A. Synthesis of carboxylic acid esters by the imidazolide method. Chem Ber. 1962;95:1284–1297. [Google Scholar]
- Tian X, Zhang FM, Li WG. Antitumor and antioxidant activity of spin labeled derivatives of podophyllotoxin (GP-1) and congeners. Life Sci. 2002;70:2433–2443. doi: 10.1016/s0024-3205(02)01482-0. [DOI] [PubMed] [Google Scholar]
- Wang JZ, Tian X, Tsumura H, Shimura K, Ito H. Antitumor activity of a new low immunosuppressive derivative of podophyllotoxin (GP-11) and its mechanisms. Anticancer Drug Des. 1993;8:193–202. [PubMed] [Google Scholar]
- Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551–1569. doi: 10.3390/molecules13081551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheleva AM, Gadjeva VG. Spin labelled nitrosoureas and triazenes and their non-labelled clinically used analogues—a comparative study on their physicochemical properties and antimelanomic effects. Int J Pharm. 2001;212:257–266. doi: 10.1016/s0378-5173(00)00611-6. [DOI] [PubMed] [Google Scholar]



