Abstract
Objective
To determine population-based neonatal mortality rates in low and middle income countries and to examine gestational age, birth-weight and timing of death to assess the potentially preventable neonatal deaths.
Methods
A prospective observational study was conducted in communities in five low-income countries (Kenya, Zambia, Guatemala, India, and Pakistan) and one mid-income country (Argentina). Over a two-year period, all pregnant women in the study communities were enrolled by trained study staff and their infants followed to 28 days of age.
Results
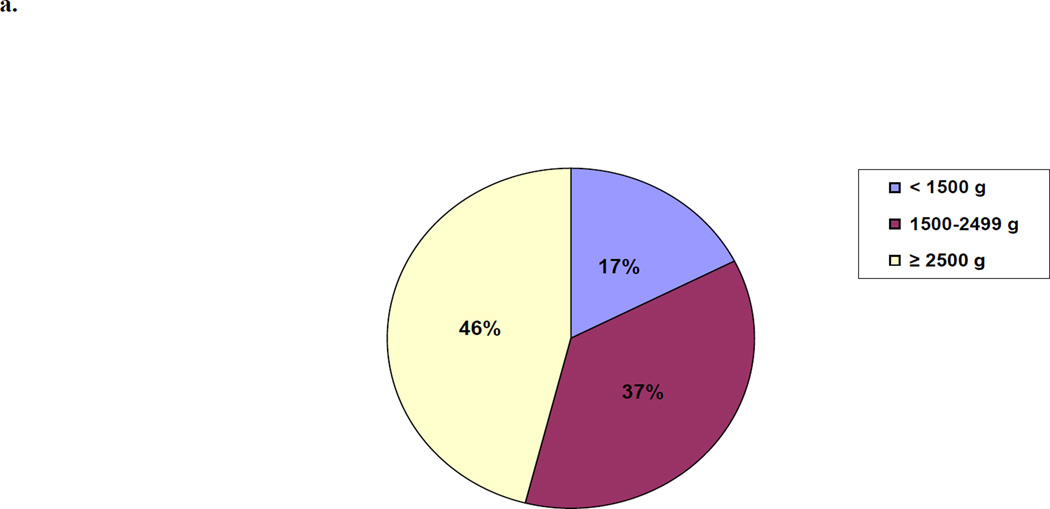
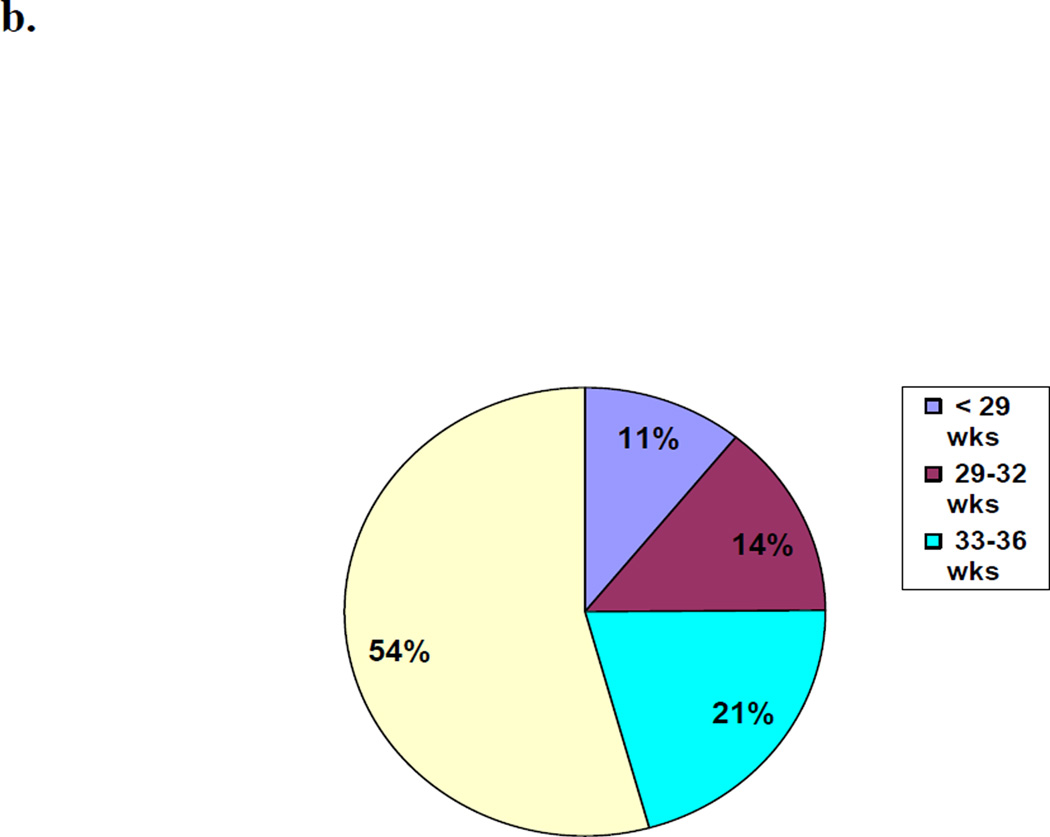
Between October 2009 and March 2011, 153,728 babies were delivered and followed through day 28. Neonatal death rates ranged from 41 per 1000 births in Pakistan to 8 per 1000 in Argentina. 54% of the neonatal deaths were >37 weeks and 46% weighed 2500 grams or more. Half the deaths occurred within 24 hours of delivery.
Conclusions
In our population-based low and middle income country registries, the majority of neonatal deaths occurred in babies >37 weeks gestation and almost half weighed at least 2500 grams. Most deaths occurred shortly after birth. With access to better medical care and hospitalization, especially in the intrapartum and early neonatal period, many of these neonatal deaths might be prevented.
Keywords: Neonatal mortality, low-income countries, preterm birth
Introduction
An estimated 3.6 million neonatal deaths occur worldwide each year, almost 99% of these in low and middle-income countries. (1, 2) About half of the deaths occur at home.(2, 3) Neonatal mortality rates range as high as 40 to 50 per 1,000 live births in low-income countries, especially in areas of sub-Saharan Africa and south Asia, while neonatal mortality rates as low as 2 to 4 per 1,000 live births are reported in many high-income countries.(1, 2)
In high income countries, the majority of neonatal deaths occur in infants born weighing < 1500 grams. (4–6) To survive, these infants often need highly technical and expensive newborn intensive care which is often not available in low income countries. In contrast, data from Africa suggest that less than10% of all neonatal deaths occur in <1500 gram infants with much of the mortality occurring in larger infants.(4–6) A better understanding of these differences should enable health policy makers to initiate more effective programs aimed at lowering neonatal mortality rates.(1, 7)
Neonatal mortality rates are not reported routinely in the vital statistics in many low-income countries and therefore, little is known about the gestational age, birth weight and timing associated with these neonatal deaths, especially since about half of all deliveries occur at home.(1, 3) We sought to determine population-based neonatal mortality rates and to characterize these deaths by gestational age, birth weight and time of death in prospective, well-defined birth cohorts in low and middle income countries.
Methods
The study was conducted by the Global Network for Women’s and Children’s Health Research (Global Network), a National Institutes of Health-funded multi-country research network. The study was conducted in seven sites in six countries: Argentina, Guatemala, Kenya and Zambia, India (sites in Belgaum and Nagpur), and Pakistan. Prospective data registries were initially created to establish baseline delivery and pregnancy outcome rates for a trial of neonatal resuscitation training to improve birth outcomes.(8–11) The registries then were maintained and expanded to additional sites. The data presented here were collected between October 2009 and March 2011. This study was reviewed and approved by the institutional ethics review committees of all participating study sites, the partner institutions in the US and the data center, Research Triangle Institute. Consent was obtained at the community level from government and health officials and by local ethics committees; women provided informed consent to collect data regarding their pregnancies and outcomes.
Each Global Network site included 6 to 24 distinct geographic communities with approximately 300 to 500 annual births per community. The goal of the registry was to obtain pregnancy outcomes of all deliveries occurring in the communities. Each site went to great lengths to insure that all pregnant women who were included in the registry and that all outcomes of the mothers and babies were obtained. As examples, data collection was overseen by trained study coordinators (health workers, nurses or physicians) and monitored on a monthly basis to reduce missing data, correct data errors and ensure consistency in reporting over time. Each community had one or more paid registry administrators to collect data and maintain contact with community birth attendants to ascertain data on pregnancies and subsequent deliveries. These registry administrators and all birth attendants were trained to distinguish stillbirths from live births. In sites where some or all women delivered in hospitals, hospital logs were routinely checked to verify births among women living in the study clusters. Sites also utilized culturally appropriate methods to ensure completeness of reporting. In one country, for example, village elders were provided with a cell phone to notify the study team of deliveries in the elder’s village. Finally, sites with available vital records periodically cross – referenced government logs with registry delivery data as an additional surveillance method. We believe these multiple methods of ascertainment increased the likelihood that our data reflect close to 100% identification of all pregnancies and delivery outcomes. Across all sites, of the pregnant women identified, 98.8% provided consent to obtain relevant delivery and follow-up data with rates ranging from 95% in Argentina to 99.5% in Kenya and the 2 Indian sites. Of the pregnancies registered in the system, we were unable to obtain delivery data for 1.6.% of the women and 28 day follow up data for 2.9%. No site had > than 5% loss to follow-up.
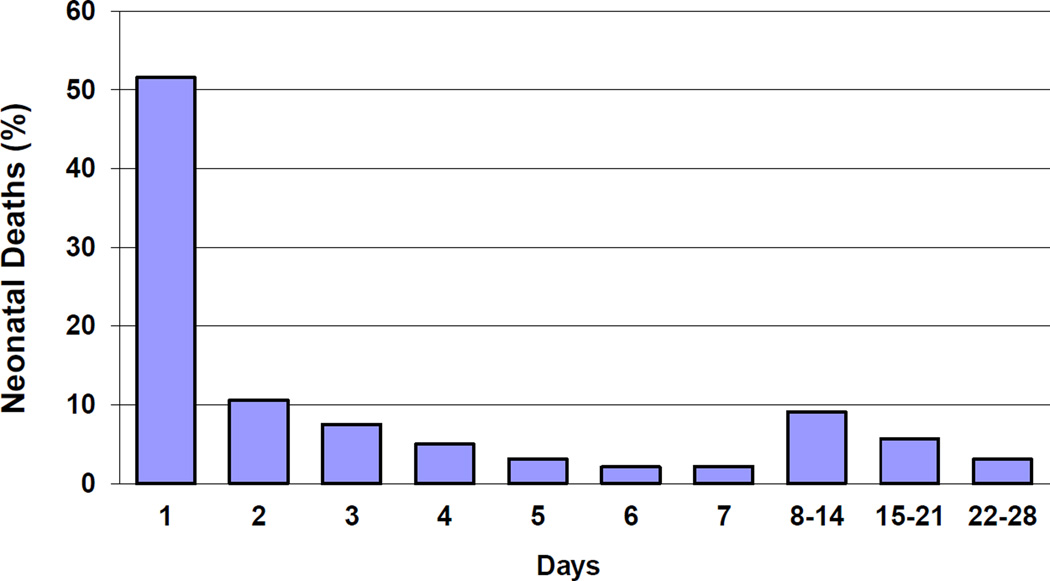
Women were generally identified and registered between 24 weeks of pregnancy and delivery. After delivery, the study coordinator collected the details of the delivery and the status of the newborn data, as recorded by the birth attendant. Data included maternal demographics, and neonatal and maternal outcomes at delivery. Gestational age was determined using last menstrual period (LMP), or best obstetric estimate, where LMP was unknown. The birth weight was generally measured within 48 hours of delivery (85% of the babies were weighed within 48 hours and 93% within the first 7 days) using study scales. When birth weight could not be obtained by scale, weight was estimated to distinguish infants less than and greater than 1500 g and 2500 g. Health workers were trained using pictures and models to make this determination. Women were followed at 42 days post-delivery to assess the neonatal and maternal status. For this study, we present neonatal mortality rates at 28 days for all live births. The timing of neonatal death was defined by the day of death. For Figure 4, deaths which occurred on the day of delivery or on the next day were considered deaths on day one. Deaths on the second day after delivery were considered to have occurred on day 2, etc.
Figure 4.
Timing of Neonatal deaths
The type of delivery attendant was categorized as physician, nurse, nurse midwife or equivalent, traditional birth attendant (TBA), family or unattended. Location of delivery included hospital, health center or home (including the TBA’s home or in transit). Finally, antenatal care was defined as having at least one visit with a skilled health provider.
All data were entered at each study site and transmitted for central analyses; data edits, including inter and intra-form consistency checks, were performed at entry with additional edits performed by the data center. The data were analyzed using SAS- version 9.2 (Cary NC, US). Because of similarities in results, and for ease of display, we elected to group the Indian sites and the sub-Saharan African sites (Kenya and Zambia). We elected not to group Pakistan, or the Latin American sites (Argentina and Guatemala) since there were large differences in location of births and type of caregiver as well as neonatal mortality rates.
Results
From October 2009 to March 2011, 153,728 births with 28-day outcomes were recorded in the 106 communities of the participating sites. A total of 3,882 neonatal deaths occurred during the study period. The mean neonatal mortality rate across all births in all sites was 27.3 per 1000 deliveries, ranging from 8 per 1000 in Argentina to 41 per 1000 deliveries in Pakistan. (Table 1) Measured birth weights were available for 74% of neonatal deaths and 91% of all births. When a measured weight was unavailable, an estimated weight was obtained. Altogether, birth weight was available for 99% of neonatal deaths and 99% of all births. Gestational age by LMP was available for 95% of all births. Follow-up results of the mother and child(ren) were obtained at 28–45 days after birth for 97.1% of study participants.
Table 1.
Characteristics of study area
| Deliveries (n) |
< 1 Prenatal visit (%) |
Home births (%) |
Deliveries by community birth attendant (%) |
Cesarean delivery (%) |
Neonatal deaths (Rate/1000) |
|
|---|---|---|---|---|---|---|
| Africa | ||||||
| Kenya | 18,406 | 4.9 | 64.6 | 50.4 | 1.2 | 16.2 |
| Zambia | 15,903 | 1.9 | 53.0 | 33.7 | 1.0 | 21.9 |
| India | ||||||
| Belgaum | 55,175 | 0.8 | 16.6 | 7.9 | 8.2 | 25.4 |
| Nagpur | 14,532 | 0.1 | 14.2 | 8.7 | 15.6 | 24.9 |
| Pakistan | 32,665 | 22.7 | 56.9 | 53.9 | 5.8 | 44.6 |
| Guatemala | 10,387 | 4.1 | 71.1 | 70.5 | 11.2 | 26.5 |
| Argentina | 6,660 | 6.1 | 1.0 | 0.0 | 28.5 | 8.7 |
| Total | 153,728 | 6.4 | 37.5 | 29.4 | 7.9 | 27.3 |
Overall, 6.5% of women received no antenatal care visits. Pakistan had the highest proportion (22.7%) of women with no antenatal care, while the remaining sites had 6% or less with no antenatal care. Overall, about one-third of the births occurred outside a health facility. In Guatemala, Kenya, Pakistan and Zambia more than half of the deliveries occurred at home. Similarly, about one-third of the births were delivered by a traditional (non-skilled) birth attendant. About 8% of the births were Cesarean deliveries; 28.5% and 15.6% of the births at Argentina and Nagpur, India were by cesarean deliveries, respectively, while Kenya, Zambia and Pakistan had cesarean delivery rates of 5% or less.
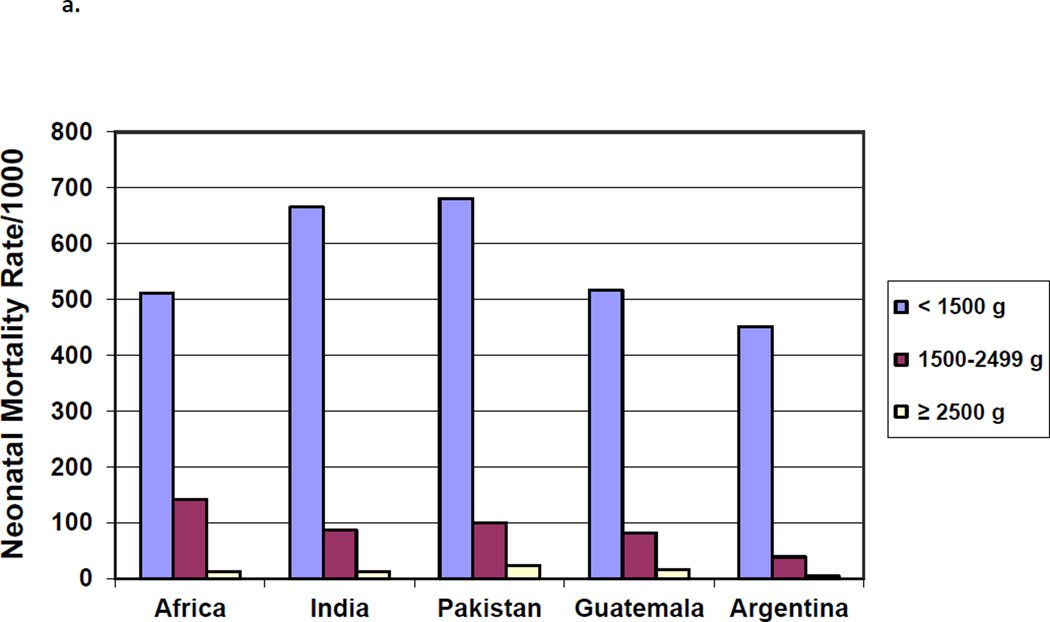
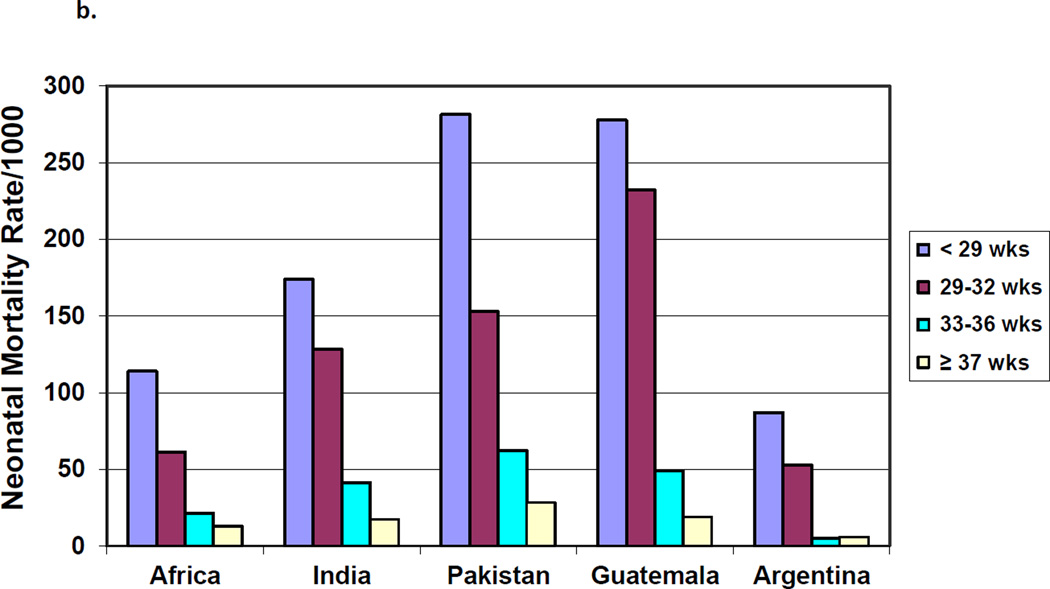
The highest mortality rates were for infants born weighing < 1500 grams, with rates for <1500 g infants ranging from approximately 700/1000 births for India and Pakistan to about 450/1000 in Argentina. (Figure 1a) Among infants ≥2500 grams at birth, mortality rates ranged from 23 per 1000 in Pakistan to 5 per 1000 in Argentina. As with low birth weight, in each geographic area, neonatal mortality rates for infants born at the lowest gestational ages were significantly higher than for infants born at term (≥37 weeks) (Figure 1b).
Figure 1.
a. Neonatal Mortality Rates by Birth Weight
b. Neonatal Mortality Rates by Gestational Age
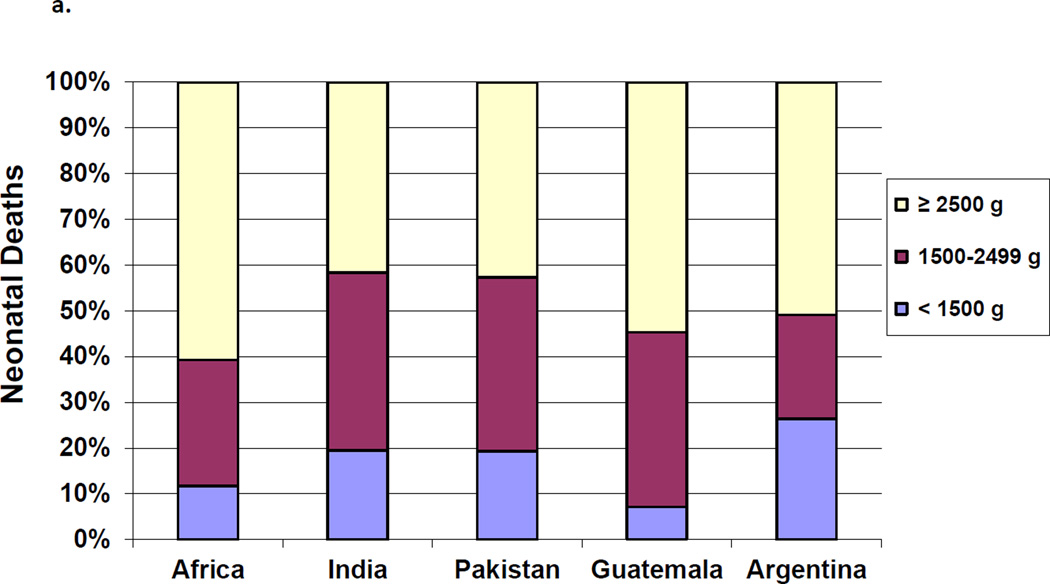
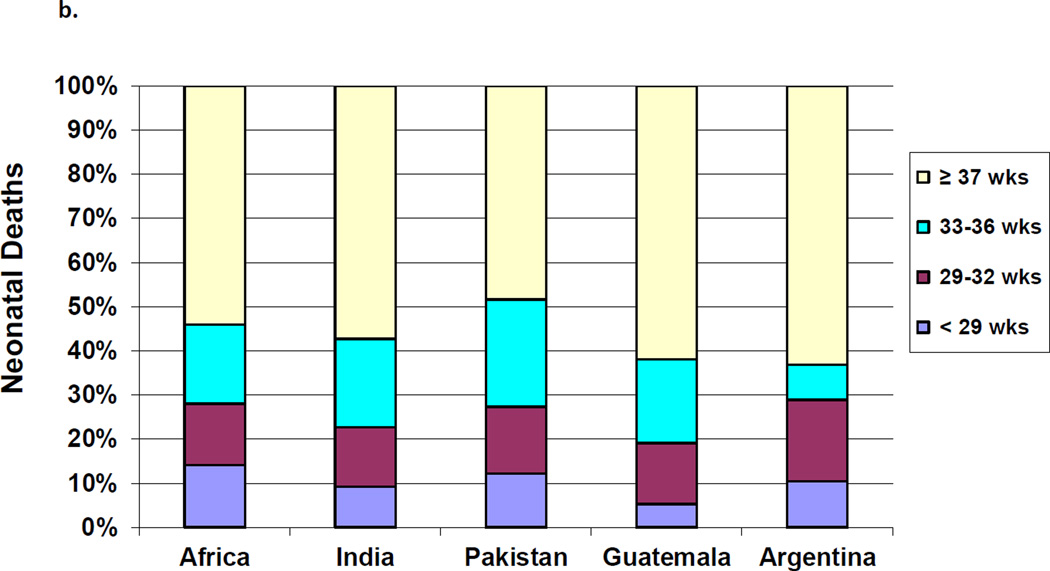
The proportion of neonatal mortality attributed to each birth weight and gestational classification varied by geographic area. The proportion of deaths attributed to neonates < 1500 g ranged from 26% in Argentina to about 13% in the African sites. Across all regions, the majority of neonatal deaths occurred in infants born weighing ≥2500 g, with results ranging from 55% in the African sites to 43% in Pakistan (Figure 2a). By gestational age, those infants born at <29 weeks contributed to 5.3% of the deaths in Guatemala compared to nearly 11% in Argentina, while the those born at ≥37 weeks contributed to almost half of the deaths across all sites.(Figure 2b)
Figure 2.
a. Proportion of Neonatal Deaths by Birth Weight by Region
b. Proportion of Neonatal Deaths by Gestational Age by Region
For all sites combined, we examined the proportion of all neonatal mortality which was attributed to the birth weight and gestational age groups. (Figures 3a and 3b) Almost half (46%) of all neonatal deaths occurred in infants born weighing ≥2500 grams and over half the deaths (54%) were in term neonates (≥37 weeks).
Figure 3.
a. Proportion of Neonatal Deaths by Birth Weight
b. Proportion of Neonatal Deaths by Gestational Age
Finally, we examined the day of neonatal death overall (Figure 4) and by birth weight and gestational age categories. (Table 2) Overall, more than half of all deaths occurred within the first day of life and 81% within the first week. For those neonates that were born weighing ≥ 2500 grams, 51% of the deaths occurred by day 1 and an additional 30% occurred on day 2–7. For infants born at 1500 to 2499 grams, 48% of the deaths occurred by the first day and an additional 33% from days 2–7. In neonates born at ≥ 37 weeks gestation, 49% of deaths occurred on the first day of life and an additional 32% on days 2–7, and for infants born at 33 to 36 weeks, 46% of the deaths occurred on the first day and 34% from days 2–7. For the lowest gestational age and birth weight categories, over 60% of the neonatal deaths occurred by day 1.
Table 2.
Timing of neonatal mortality by birth weight and gestational age
| Day 1 | Day 2 – 7 | Day 8 – 28 | |
|---|---|---|---|
| Total, N (%) | 2,002 (51.6) | 1,100 (28.3) | 780 (20.1) |
| Birth Weight | |||
| ≥ 2500 g | 50.7* | 27.7 | 21.5 |
| 1500–2499 g | 47.7 | 30.9 | 21.4 |
| < 1500 g | 60.7 | 24.8 | 14.5 |
| Gestational Age | |||
| ≥ 37 wks | 48.6** | 29.8 | 21.6 |
| 33–36 wks | 46.1 | 30.7 | 23.2 |
| < 33 wks | 63.0 | 24.3 | 12.7 |
Percentage of deaths in this birth weight category
Percentage of deaths in this gestational age category
Discussion
We evaluated birth outcomes for more than 150,000 births in distinct, geographically-defined communities in six countries, representing one of the largest studies of this nature to date. The overall neonatal mortality rate was 27.3 per 1000 births, with individual country rates ranging from 8 per 1000 in the Argentinean communities to 41 per 1000 in the Pakistani communities. Neonates weighing 2500 grams or more contributed to 46% of neonatal deaths overall (38% in India to 60% in Africa) and in these neonates, 51% of the deaths occurred in the first day of life. Similarly, term neonates contributed to 54% of deaths overall with 48% of these deaths occurring on the first day of life.
This study had several strengths. First, it is one of the largest prospective studies to register women during pregnancy and to capture birth outcomes for a representative population-based sample. Because the study was conducted in geographic areas where a large proportion of the births occurred at home, data from this study are likely to be more representative of these areas than many hospital-based studies. In addition, we used trained coordinators to supervise and evaluate the quality of the data collected. Few low-income country studies of neonatal deaths in communities have captured birth weight or gestational age, often because of cultural or other barriers.(1) Follow-up until 28 days postpartum was very high (97%) implying that very few neonatal deaths were missed
A potential weakness of the study relates to the accuracy of the birth weight and gestational age data. For neonatal deaths, 27% of birth weights were not obtained in contrast to 7% of these measurements for the entire sample. Since neonates that died were weighed less frequently than live births, a greater percent of their birth weights were estimated. Without ultrasound confirmation of gestational age, which rarely occurred in this study, LMP-generated gestational age will be inaccurate in an unknown percent of the cases. Also, despite the fact that 95% of women in this study provided information about their last menstrual period, we were not be able to determine the accuracy of such dating. Thus both the birth weight and gestational age data are subject to various potential biases. Another potential limitation of the study was our inability to ensure that all births were captured. However, in each site, our staff used several methods to determine who in each community was pregnant and who had delivered. In addition, the pregnancy outcomes obtained by our staff were compared to available health records. Finally, techniques such as the evaluation of delivery numbers and neonatal death rates over time and inter-cluster variations were carefully monitored on an ongoing basis to ensure that large unexplained variations in both the number of births and neonatal death were not occurring. Another potential limitation is the quality of the data collected for unattended births. To address this issue, our study coordinators interviewed each mother and other family members. Obtaining information on these home births is critical as they represent a substantial proportion of the live births in these geographic areas. Another potential weakness of this study is the lack of data related to cause of death. While we did attempt to collect cause of death data, we did not feel that the causes were determined with sufficient accuracy to present here, since there were no pathologic autopsies performed and we did not have the resources to perform a formal verbal autopsy on over 4000 neonatal deaths. In most low income studies of neonatal deaths, the most important causes are infection, asphyxia, and prematurity, with most deaths estimated to be equally distributed among these three causes.(1) Finally, while the data were population based, the results may still not be representative of the country as a whole.
A primary gap in neonatal death research and policy planning has been the lack of population-based data including birth weight and gestational age estimates, especially since the majority of the neonatal deaths worldwide occur in home settings, are never registered, and thus no vital statistics are available.(1, 3) The mean neonatal death rate of more than 27.3 per 1000 births represents more than a five-fold increase compared to high-income country rates. An important observation is that in the less developed communities, where nearly all deliveries occurred in home settings without trained health providers, rates were as high as 40 per 1000, compared to the neonatal death rates in Argentina of 8 per 1000, where nearly all deliveries occurred in hospital settings.
Of particular note, the majority of the neonatal deaths occurred at term and almost half were ≥ 2500 grams. Thus, most infants currently dying, if born in reasonable condition, should not suffer the adverse effects of preterm birth and would likely survive. In most high-income countries, the majority of neonatal mortality now is due to those infants born <1500 g and almost all infants born >2500 grams survive.(5) Our results suggest that there is an opportunity to prevent the majority of neonatal deaths by improving access to appropriate obstetrical and neonatal care. While our data suggest that higher quality of health care at delivery is associated with lower neonatal mortality rates, more research on the specific causes of these neonatal death and the circumstances under which they are occurring would assist in defining appropriate interventions.
Conclusions
Low-and middle income countries account for the vast majority of maternal, fetal and neonatal deaths in the world.(1, 6) Comprehensive analyses of maternal, neonatal and fetal deaths have shown that quality obstetric care provided during delivery including access to cesarean delivery could significantly reduce such deaths. (12–15) In our population-based low and middle income country registries, the majority of neonatal deaths occurred in babies ≥37 weeks gestation and almost half weighed at least 2500 grams. Most deaths occurred shortly after birth. With access to better medical care and hospitalization, especially in the intrapartum and early neonatal period, many of these neonatal deaths might be prevented. Our findings support recommendations to improve care around the time of birth.
Acknowledgments
Global Network investigators: Argentina - Fernando Althabe and Jose M. Belizan, (Institute for Clinical Effectiveness and Health Policy); Guatemala - Ana Garces and ManoloMazariegos(San Carlos University); India - Shivaprasad S. Goudar and Bhalchandra S. Kodkany, (Jawaharlal Nehru Medical College) Archana Patel (Lata Medical Research Foundation); Kenya – Fabian Esamai, Hillary Mabaye, Constance Tenge (Moi University); Pakistan – Neelofar Sami, Sarah Saleem, Omrana Pasha (Aga Khan University); Zambia – Elwyn Chomba (University of Zambia); U.S.- Waldemar A. Carlo, (University of Alabama at Birmingham); Nancy Krebs and Michael Hambidge, (University of Colorado); Robert L. Goldenberg, (Drexel University); Richard J. Derman, (University of Missouri, Kansas City); Pierre Buekens, (Tulane School of Public Health and Tropical Medicine); Patricia Hibberd (Massachusetts General); Edward Liechty (Indiana University); Linda L. Wright, Marion Koso-Thomas (Eunice Kennedy Shriver National Institute of Child Health and Human Development); Janet Moore, Elizabeth M. McClure, Tracey Grant, and Dennis Wallace (Research Triangle Institute).
Funding: Supported by NIH grants from the Global Network for Women’s and Children’s Health Research (U01 HD040477, U01, HD0434475, U01 HD043464, U01 HD040657, U01 HD042372, U01 HD040607, U01 HD040636, U01 HD058322).
Abbreviations
- LMP
last menstrual period
- TBA
traditional birth attendant
- g
grams
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest.
- Jose M Belizan, Elizabeth M McClure, Robert L Goldenberg: Took lead in developing the analyses plan, conceptualizing the manuscript, reviewing the analyses and writing the initial draft the paper and final edits.
- Omrana Pasha, Shivaprasad Goudar, Fernando Althabe, Fabian Esamai, Archana Patel, Neelofar Sami, Albert Manasyan, and Bhala Kodkany: Participated in the study design and protocol development, oversaw the study implementation at a field site, including data quality review/audits and participated in writing the manuscript and reviewed the final version.
- Linda L Wright, Alan H Jobe, Pierre Buekens, Waldemar A Carlo, K Michael Hambidge, Edward A Liechty, Patricia Hibberd, Richard Derman, Marion Koso-Thomas – participated in the study design and ongoing study monitoring, participated in the discussion of development of the manuscript, reviewed and edited the manuscript.
- Janet Moore and Dennis Wallace performed the statistical analyses throughout the trial, performed the analyses for the paper and reviewed the final manuscript
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? The Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.UNICEF. State of the World's Children 2011. New York: UNICEF; 2011. [Google Scholar]
- 3.Darmstadt GL, Lee AC, Cousens S, Sibley L, Bhutta ZA, Donnay F, et al. 60 Million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths? Int J Gynaecol Obstet. 2009;107(Suppl 1):S89–S112. doi: 10.1016/j.ijgo.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander GR, Kogan M, Bader D, Carlo W, Allen M, Mor J. US birth weight/ gestational age-specific neonatal mortality: 1995–1997 rates for whites, hispanics, and blacks. Pediatrics. 2003;111(1):e61–e66. doi: 10.1542/peds.111.1.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Were FN, Bwibo NO. The contribution of very low birth weight deaths to infant mortality. East Afr Med J. 2009;86(8):374–377. doi: 10.4314/eamj.v86i8.54157. [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 7.Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C GAPPS Review Group. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10(Suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenberg RL, McClure EM, Belizán JM. Commentary: reducing the world's stillbirths. BMC Pregnancy Childbirth. 2009;9(Suppl 1):S1.9. doi: 10.1186/1471-2393-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlo WA, Goudar SS, Jehan I, Chomba E, Tshefu A, Garces A, et al. First Breath Study Group. Newborn-care training and perinatal mortality in developing countries. N Engl J Med. 2010;362:614–2310. doi: 10.1056/NEJMsa0806033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlo WA, Goudar SS, Jehan I, Chomba E, Tshefu A, Garces A, et al. First Breath Study Group. High mortality of very-low-birth-weight infants born in communities in developing countries despite birth attendant training. Pediatrics. 2010;126:e1072–e1080. doi: 10.1542/peds.2010-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClure EM, Wright LL, Goldenberg RL, Goudar SS, Parida SN, Jehan I, et al. NICHD FIRST BREATH Study Group. The global network: a prospective study of stillbirths in developing countries. Am J ObstetGynecol. 2007;197:247.e1–247.e5. doi: 10.1016/j.ajog.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L for the Lancet Neonatal Survival Steering Team. Evidence-based, cost-effective interventions: how many newborn babies can we save? The Lancet. 2005;365:977–978. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 13.Jones G, Steketee RW, Black RE, Bhutta Z, Morris SS the Bellagio Child Survival Study Group. How many child deaths can we prevent this year? Lancet. 2003;362:65–71. doi: 10.1016/S0140-6736(03)13811-1. [DOI] [PubMed] [Google Scholar]
- 14.Koblinsky M, Matthews Z, Hussein J, Mavalankar D, Mridha MK, Anwar I, Achadi E, Adjei S, Padmanabhan P, Marchal B, De Brouwere V, van Lerberghe W Lancet Maternal Survival Series steering group. Going to scale with professional skilled care. Lancet. 2006;368(9544):1377–1386. doi: 10.1016/S0140-6736(06)69382-3. [DOI] [PubMed] [Google Scholar]
- 15.Wall SN, Lee AC, Carlo W, Goldenberg R, Niermeyer S, Darmstadt GL, Keenan W, Bhutta ZA, Perlman J, Lawn JE. Reducing intrapartum-related neonatal deaths in low- and middle-income countries-what works? SeminPerinatol. 2010;34:395–407. doi: 10.1053/j.semperi.2010.09.009. [DOI] [PubMed] [Google Scholar]