Abstract
Objective
Verbal memory difficulties are common among individuals with late-life depression (LLD), though there is limited knowledge about disruptions to underlying cerebral circuitry. The purpose of this study is to examine aberrations to cerebral networks implicated in encoding novel verbal semantic material among older adults with LLD.
Methods
Twenty-four older adults with early-onset LLD and 23 non-depressed comparisons (NDC) participated in the study. Participants completed a word list-learning task while undergoing fMRI.
Results
In the context of equivalent recall and recognition of words following scanning and similar hippocampal volumes, patients with LLD exhibited less activation in structures known to be relevant for new learning and memory, including hippocampus, parahippocampal gyrus, insula, and cingulate, relative to non-ill comparisons. An important region in which the LLD group displayed greater activation than the NDC group was in inferior frontal gyrus (IFG), an area involved in cognitive control and controlled semantic/phonological retrieval and analysis; this region may be critical for LLD patients to consolidate encoded words into memory.
Conclusions
Functional irregularities found in LLD patients may reflect different modes of processing to-be-remembered information and/or early changes predictive of incipient cognitive decline. Future studies might consider mechanisms that could contribute to these functional differences, including HPA-axis functioning and vascular integrity, and utilize longitudinal designs in order to understand whether functional changes are predictive of incipient cognitive decline.
Keywords: depression, memory, hippocampus, aging
Introduction
Neuropsychological impairment has been documented in older adults with Major Depressive Disorder (MDD) across domains, and especially in the areas of episodic memory, processing speed, and executive function (Dybedal et al., 2013; Elderkin-Thompson et al., 2007; Lamar et al. 2012; Yen et al, 2011). Although neuroimaging research has primarily focused on the role of executive functioning in late-life depression (LLD; e.g., Aizenstein et al., 2009; Alexopoulos et al., 2012), individuals with LLD also frequently exhibit poor memory on objective neuropsychological measures (Dillon et al., 2011).
There are multiple hypotheses for why individuals with LLD would experience memory loss. Structural MRI studies have demonstrated lower hippocampal volume among older patients with MDD (Steffens et al., 2011; Zhao et al, 2008). Depressed individuals with and without objective memory loss demonstrated increased left hippocampal resting state fMRI connectivity with the bilateral posterior cingulate and right dorsal medial prefrontal cortex, and decreased anticorrelation with the left dorsal lateral prefrontal cortex relative to non-depressed elderly. Only LLD with comorbid memory loss demonstrated increased right hippocampal connectivity with bilateral ventromedial prefrontal cortex and middle occipital gyrus, as well as decreased right hippocampal connectivity with bilateral dorsal anterior cingulate and right dorsal lateral prefrontal cortex (Xie et al., 2013). These were functional abnormalities in cerebral structures involved in memory encoding and consolidation in LLD, greater in those presenting objective memory impairment relative to those without impairment. This is significant as early memory deficits in LLD may be a warning sign of impending cognitive decline, as LLD is associated with an increased risk of developing dementia (Diniz et al., 2013; Ownby et al., 2006). As the aforementioned cognitive and neuroimaging studies were not longitudinal, it is not clear which patients could have continued to decline and/or develop dementia. Data suggests that cerebral functional changes often appear before demonstrable behavioral changes (Forsberg et al., 2008; Fujishiro et al., 2013; Park et al., 2012; see Risacher et al., 2013), and may serve as a marker for continuing cognitive decline or functional impairment, making development of sensitive functional probes imperative.
To this end, task-based functional MRI methodology, primarily in executive functioning abilities, has been applied in order to better understand cerebral abnormalities among older patients with MDD (e.g., Aizenstein et al., 2009; Alexopoulos et al., 2012; Dybedal et al., 2013). Executive functioning can play a significant role in supporting memory processes, and measures of memory often include significant contributions from executive functioning components. For example, list-learning tasks such as the California Verbal Learning Test-2 (CVLT; Delis et al., 2000) uses words from distinct semantic categories. If detected by the participant, the semantic categories offer a clustering strategy for increased encoding (Winocur & Moscovitch, 2011). Executive functioning drives discernment to the lists’ semantic organization and utilization of the clustering strategy; as a result, variance in executive functioning skill impacts how much is remembered. As memory and executive functioning processes are often intertwined, it is difficult to parse out the relative contributions of executive functioning and memory processes. Memory tasks that exclude strong executive functioning contributions may be sensitive for identifying those early in the course of memory decline or in pinpointing specific functional impairments. For example, a study of LLD adults reported that impairment in semantic organization mediates performance on the CVLT and is absent in non-depressed older adults (Elderkin-Thompson et al, 2007).
One known study of subsyndromal depressive symptoms in late life was conducted using a memory paradigm intended to specifically engage hippocampal circuitry. It utilized a face-name associative memory encoding task and found that depressive symptom severity was positively associated with deactivation in the dorsal posterior cingulate cortex (using a region-of-interest approach) during encoding of novel (minus familiar) word pairs relative to baseline (Woo et al., 2009).
To our knowledge, there have been no functional imaging studies in LLD that use recall-based memory tasks with diminished contributions from executive functioning circuits. In the current study, we use the Semantic List Learning Test (SLLT), in which lists of words are presented with semantic category labels, reducing the necessity for subjects to generate their own organizational strategies for encoding words. It also utilizes a Brown-Peterson paradigm, such that a distractor task is presented immediately following encoding in order to reduce the ability of subjects to use short-term-memory stores (a frontally mediated process) to augment weaker primary memory. The SLLT also allows for objective examination of memory performance, as it includes paper-and-pencil recall and recognition assessments immediately following scanning. We included only patients in this study with first onset of depression < 55 due to possible etiological differences between early and late onset LLD (Murata et al., 2001; Sachs-Ericsson et al., 2013) and to minimize the likelihood of physiological (i.e., cardiovascular, metabolic processes) contribution to disease pathogenesis. We hypothesized that LLD adults would demonstrate poorer performance on the SLLT relative to non-depressed comparison adults (NDC), and that LLD adults would demonstrate BOLD fMRI abnormalities in regions relevant to memory encoding and consolidation (Papez, 1937).
Methods
Participants
This study was approved by the Institutional Review Board at the University of Michigan, and all participants gave informed consent prior to participation. Forty-seven participants (24 LLD, 23 NDC) were recruited through geriatric psychiatry and primary care clinics, clinical research volunteer databases, and community advertisements. One additional subject was excluded due to significant atrophy observed on the anatomical scan and a second subject was excluded due to significant dorsal section of the brain missing from functional scans. Exclusionary criteria for all participants included contraindications for MRI, Mini Mental State Exam < 24 (see O’Bryant et al., 2008), uncontrolled hypertension or diabetes, any neurological disorder, head injury with loss of consciousness of > 5 minutes, and major medical conditions that could affect the central nervous system. Participants were also excluded based upon any history of psychotic symptoms, bipolar disorder, schizophrenia, current substance use disorder or history of substance dependence within 5 years of the MRI scan. All LLD participants had age of depression onset < age 55. Individuals were not excluded on the basis of taking psychotropic medications, although those with PRN anxiolytic usage were encouraged to avoid use the day of the scan. NDC participants were free from a personal history of psychiatric illness. All LLD participants were diagnosed according to the Structured Clinical Interview for the DSM-IV criteria (Spitzer et al., 1994). Depression severity was measured with the Hamilton Rating Scale for Depression–Second Edition (Hamilton, 1967). It is relevant to note that participants were not experiencing overt memory deficits at the time of recruitment and testing, and performance on the CVLT was not utilized as part of inclusion/exclusion criteria. CVLT data was available for 21 participants in each group, and the average age-corrected z-scores for long delay recall and recognition in each group were within the normal range. One NDC and four LLD participants achieved a score that was at least 1.5 standard deviations below the age-corrected mean for free recall. Table 1 lists specific demographic, cognitive, and medical characteristics for the sample.
Table 1.
Sample Demographic and Clinical Characteristics
| Variables | LLD (n = 24) M (SD) |
NDC (n = 23) M (SD) |
|---|---|---|
| Age | 65.8 (8.2) | 67.9 (8.1) |
| Education | 15.9 (2.7) | 16.7 (2.1) |
| Sex (female n) | 14 | 10 |
| Hamilton Depression Rating Scale1 | 15.71 (5.2) | .96 (1.0) |
| Charlson Comorbidity Index | 024 (0.49) | 0.1 (0.34) |
| CVLT-2 Delayed Recall Z-Score2 | .10 (1.1) | .52 (1.0) |
| Years of illness3 (MDD only) | 39.8 (16.8) | NA |
| On psychotropic medication4 (%) | 78 | NA |
| Diabetes (n) | 0 | 3 |
| Hypertension (n) | 11 | 6 |
| Sleep apnea (n) | 2 | 1 |
| Heart condition (n) | 5 | 2 |
| Anemia | 2 | 0 |
Note. LLD = Late Life Depression. NDC = Non-Depressed Comparison, CVLT-2 = California Verbal Learning Test-2.
t(45) = −13.64, p < .001.
n = 21 per group.
Years ill missing for one subject.
Medication status missing for one LLD; one NDC subject was taking trazadone for sleep. Of the participants with LLD, 22% (n = 5) were unmedicated, 30% (n = 7) were taking SSRI/SNRI only, 30% (n = 7) were taking a SSRI/SNRI in addition to another psychoactive medication (e.g., bupriopion, trazodone, benzodiazepine), 13% (n = 3) were taking non-SSRI/SNRI antidepressants (i.e., trazodone, bupropion, gabapentin), and one participated was taking a benzodiazepine (PRN) only.
Measures
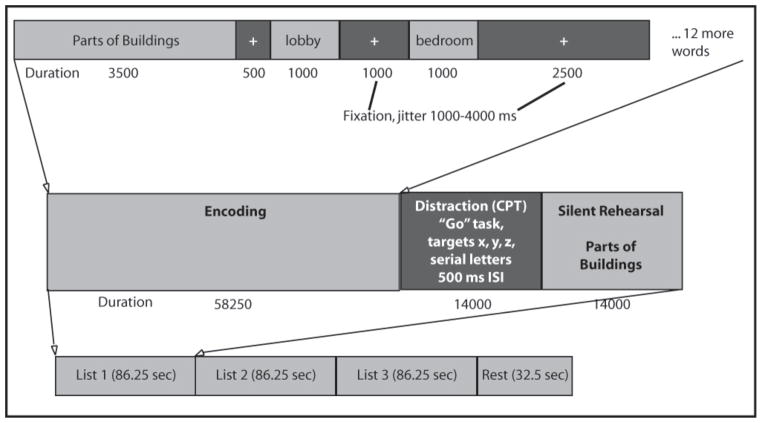
The SLLT, designed to test learning and memory, is composed of three types of blocks that were presented during fMRI scanning: Encoding, Distraction, and Rehearsal (see Figure 1).
Figure 1.
ILLUSTRATION OF SEMANTIC LIST LEARNING TEST
Subjects were presented with 14 words from one of 15 semantic categories during each encoding block. Lists were taken from word category and frequency work by Winograd (1968), with five of each low, medium, and high frequency categories. Lists were respectively matched for average number of syllables, and categories had sufficient items for both within list targets and same list distractors (for the recognition part of the task). In the task, a prompt with the name of the semantic category being studied was displayed for 3.5 seconds. Words from that category were then presented for one second each with a one to four second jittered inter-stimulus interval, during which a fixation cross was presented. The total time for each encoding block was 58.25 seconds. Subjects then completed a “Go” distractor task, intended to reduce recency effects during recall/recognition by preventing rehearsal of list items held in short term memory (Brown, 1958; Peterson & Peterson, 1959). The final portion of each list presentation consisted of a rehearsal block that lasted for 14 seconds. Here, participants saw the category prompt and were asked to silently rehearse words that were just presented during the previous encoding phase. Participants were presented with a total of 15 different word lists over five runs (three lists per run), for a total of 210 words. All participants were presented with the same word lists, but the order in which each list appeared was randomized. At the end of each of the five runs there was a rest period of 32 seconds.
Procedure
Participants were verbally introduced to the task by the experimenter prior to entering the scanner. They were told they would observe lists of categorically related words presented one at a time, and that they should silently read and remember the words to the best of their ability, utilizing the list category as a semantic encoding strategy. They were informed that a different (distractor) task would then appear for which they had to make a button-press response each time they saw the letters “x,” “y,” or “z” presented in a visual stream (500 ms each, 14 seconds total per block), on which participants were trained prior to scanning. Lastly, they were told that a silent rehearsal phase would occur, during which they would be asked to rehearse the words from the list that appeared just prior to the distraction phase without vocalization or movement of the lips.
After scanning, subjects first completed a recall task in which they wrote down all the words they could remember for each of the semantic categories presented. Category name prompts were provided as recall cues. Subjects then completed a recognition task in which they had to discern words seen inside the scanner from a list of correct words among semantically related and unrelated distractors. Correctly recalled words and those that were not recalled were used as regressors in an event-related analysis of the fMRI data. Correctly recognized words and those that were not recognized were used in the same manner.
fMRI procedures were similar in detail and followed the method used in our previous work (e.g., Langenecker et al., 2007, Langenecker et al., 2011; Weisenbach et al., 2012; see supplementary material). High-resolution T1 SPGR anatomical images (echo time = 3.4 ms, repetition time = 10.5 ms, 27° flip angle, number of excitations = 1, slice thickness = 1.5 mm, field of view = 24 cm, matrix size = 256 × 256) were obtained after SLLT administration and used in voxel-based morphometry analyses. To assure that any between-group differences were not due to atrophy in the LLD group, we utilized voxel based morphometry (VBM). Given the relevance of the hippocampus to memory encoding, we first tested for differences in hippocampal volume by creating a hippocampal ROI using the WakeForest PickAtlas (Maldjian et al., 2003). We conducted additional VBM analyses of functional regions found to be more active in the NDC group relative to the MDD group. VBM analyses were conducted with the VBM8 toolbox in SPM8 (Kurth et al., 2010) with a
Statistical Analyses
Behavioral data were examined using t tests and employed a statistical threshold of p < .05. We investigated group differences in recall hits, recall intrusion errors, recognition hits, recognition false positives, d′ (a sensitivity index that provides the separation between means of the signal and noise distributions, compared against the standard deviation of the noise distribution), and β (a measure of response bias). In calculating d′ and β for recall, total number of possibly recognized items was used to generate the false alarm rate. There was one outlier in the NDC group for recognition false positive errors, and this was winsorized so that it was equivalent to the next most poorly performing person within the NDC group. For VBM analysis, a two-sample t test was performed to assess for group differences in hippocampal volume and functional regions found to be more active in the NDC group relative to the MDD group. To assure that volumetric differences of relevant regions was minimal between the two groups, we employed a liberal threshold of p < .05, minimum threshold cluster of 80mm3 for all VBM analyses. Functional images were normalized to fit a MNI canonical template and were smoothed at a 5 mm FWHM. For fMRI data three contrasts of interest were run. First, word encoding blocks were compared to silent rehearsal blocks. Second, event-related encoding of correctly recalled words were compared to non-recalled words, as well as the inverse (encoding of non-recalled compared to recalled words). Finally, we tested event-related recognized words compared to not recognized words, as well as the inverse (encoding of non-recognized compared to recognized words). Group analyses with t tests were conducted with these contrasts, run in SPM8. AlphaSim correction (1000 iterations) was used for all whole brain analyses, balancing height (p < .003) and extent (264 mm3) thresholds to achieve a whole brain correction of p < .05. For the hippocampal ROI analysis, a threshold of p < .05, 80mm3 was utilized. In a post hoc analysis of activation in the inferor frontal gyrus (IFG), the MarsBaR toolbox (Brett et al., 2002) was used to extract mean signal change in IFG region of interest (ROI) for correlation with performance measures of recall, recognition, d′, and β. All fMRI analyses were performed with and without the inclusion of the five individuals with poor CVLT performance.
Results
Group Comparisons for Cognitive Performance
The LLD and NDC groups did not differ in performance for recall hits, recall intrusion errors, recognition hits, recognition and false positive errors (all ps > .31; see Figure 2). The two groups did not differ in, d′ for recall (LLD M = 1.11, SD = .67; NDC M = 1.12, SD = .62), β for recall (LLD M = .94, SD = .05; NDC M = .93, SD = .06), d′ for recognition (LLD M = 1.39, SD = .56; NDC M = 1.32, SD = .58), nor β for recognition (LLD M = .47, SD = .48; NDC M = .46, SD = .50).
Figure 2.
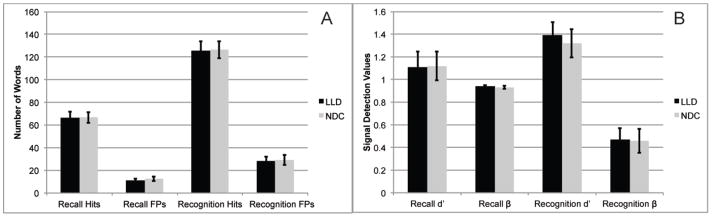
PERFORMANCE DURING RECALL AND RECOGNITION TASKS. Illustrates equivalent performance for recall hits and intrusion errors and recognition hits and (FP) errors (all ps > .31).
Voxel Based Morphometry
Hippocampal volume, corrected for whole brain volume, was not significantly different between LLD and NDC. The volumes of functional regions found to be more active in NDC relative to LLD (below) were also not significantly different between LLD and NDC.
fMRI Activation During Encoding Minus Rehearsal of Words
LLD activation for encoding-rehearsal foci are listed in Table 2 as is NDC activation. Relative to LLD, greater activation was found in NDC in right middle frontal, insula, cuneus, and caudate, left dorsal cingulate, precuneus and putamen, and bilateral globus pallidus. LLD did not activate any region to a significantly greater degree than NDC. Table 2 and Figure 3 display regions activated in each of LLD and NDC separately and in NDC minus LLD contrast, both for whole brain and hippocampal ROI analyses. After removing the five participants with memory impairment, similar between-group differences were detected in frontal and subcortical regions. Differences in insula, cuneus, and globus pallidus were no longer significant, and there were additional activation differences in left inferior frontal gyrus and right claustrum.
Table 2.
A–C. Foci of significant activation for (A) Encoding Minus Rehearsal, (B) Recalled versus Not Recalled Words, and (C) Recognized versus Not Recognized Words
| MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Group | Lobe | Region | BA | x | y | z | Z | mm3 |
| A. Encoding Minus Rehearsal | ||||||||
| LLD | Frontal | Dorsal Anterior Cingulate** | 32 | 10 | 22 | 27 | 5.28 | 23352 |
| Middle Frontal ** | 6 | −31 | −3 | 40 | 4.4 | 6464 | ||
| Anterior Cingulate ** | 24 | 24 | −3 | 44 | 4.1 | 2392 | ||
| Temporal | Insula** | 13 | 40 | 30 | 19 | 3.8 | 1960 | |
| 13 | 45 | −40 | 31 | 3.2 | 320 | |||
| Occipital | Inferior Occipital | 19 | −46 | −70 | −2 | 4.0 | 1248 | |
| Subcortical | Cerebellum-Declive** | -- | 10 | −75 | −13 | 7.2 | 167200 | |
| Thalamus** | -- | −17 | −19 | 15 | 5.3 | 20128 | ||
| Putamen** | -- | −25 | 22 | 3 | 3.6 | 2344 | ||
| NDC | Frontal | Inferior Frontal | 47 | 17 | 28 | −13 | 3.8 | 1272 |
| Dorsal Cingulate** | 31 | −10 | −21 | 43 | 3.2 | 456 | ||
| Temporal | Superior Temporal | 38 | −36 | 8 | −24 | 4.1 | 600 | |
| 38 | −31 | 12 | −33 | 3.8 | 512 | |||
| 38 | 36 | 12 | −26 | 4.1 | 400 | |||
| Parietal | Posterior Cingulate | 31 | 24 | −17 | 48 | 4.9 | 3128 | |
| 31 | 15 | −38 | 49 | 3.9 | 1496 | |||
| Postcentral** | 3 | −32 | −25 | 41 | 3.6 | 1384 | ||
| Precuneus | 7 | −13 | −38 | 56 | 3.4 | 952 | ||
| Occipital | Fusiform | 19 | 45 | −67 | −9 | 5.0 | 3128 | |
| 19 | −38 | −71 | −7 | 4.8 | 1632 | |||
| 18 | −25 | −91 | −16 | 4.7 | 1392 | |||
| Subcortical | Putamen** | -- | −15 | 8 | −10 | 3.5 | 608 | |
| -- | 36 | −1 | 8 | 3.9 | 480 | |||
| Caudate Head** | -- | 4 | 8 | 5 | 3.7 | 312 | ||
| Hippocampus* | -- | 26 | −17 | −17 | 2.2 | 144 | ||
| NDC-LLD | Frontal | Middle Frontal | 6 | 26 | −6 | 60 | 4.0 | 1680 |
| Dorsal Cingulate | 24 | −20 | −14 | 50 | 3.7 | 1120 | ||
| Temporal | Insula** | 13 | 38 | 16 | −8 | 3.5 | 744 | |
| 13 | 44 | 14 | 2 | 3.3 | 376 | |||
| Parietal | Precuneus** | 7 | −16 | −38 | 54 | 3.2 | 304 | |
| Occipital | Cuneus** | 18 | 24 | −92 | 26 | 3.7 | 344 | |
| Subcortical | Caudate | -- | 16 | −4 | 20 | 3.9 | 1608 | |
| Lateral Globus Pallidus** | -- | −22 | 8 | 6 | 3.1 | 360 | ||
| -- | 26 | −12 | 8 | 3.9 | 312 | |||
| Putamen | -- | −20 | 6 | 12 | 3.3 | 360 | ||
| B. Recalled versus Not Recalled Words | ||||||||
| LLD | Frontal | Dorsal Anterior Cingulate | 32 | −2 | 14 | 50 | 3.8 | 584 |
| NDC | Frontal | Medial Frontal** | 11 | −8 | 56 | −14 | 3.6 | 480 |
| NDC-LLD | Frontal | Medial Frontal | 10 | −6 | 56 | −12 | 3.8 | 272 |
| Subcortical | Caudate** | -- | −40 | −22 | −2 | 3.3 | 272 | |
| C. Recognized versus Not Recognized Words | ||||||||
| LLD | Frontal | Dorsal Anterior Cingulate** | 32 | −2 | 14 | 50 | 4.9 | 6680 |
| Inferior Frontal | 45 | −48 | 22 | 14 | 4.5 | 3632 | ||
| 47 | 40 | 20 | −6 | 3.3 | 360 | |||
| Middle Frontal | 46 | 44 | 22 | 26 | 4.6 | 2624 | ||
| Precentral** | 6 | −32 | 8 | 28 | 4.6 | 2584 | ||
| 6 | 38 | −4 | 38 | 4.1 | 344 | |||
| Temporal | Fusiform** | 37 | −48 | −50 | −14 | 3.9 | 528 | |
| Subcortical | Thalamus/Mammillary Body** | -- | 12 | −16 | 2 | 3.3 | 800 | |
| Putamen** | -- | 32 | −2 | 12 | 4.1 | 584 | ||
| -- | 22 | 2 | 14 | 4.2 | 304 | |||
| Subcortical | Parahippocampal Gyrus*, ** | 36 | −32 | −14 | −22 | 2.2 | 168 | |
| 28 | 26 | −12 | −22 | 2.5 | 104 | |||
| Cerebellum | Uvula** | -- | 10 | −74 | −32 | 3.7 | 400 | |
| NDC | Temporal | Fusiform | 20 | 56 | −30 | −22 | 3.3 | 360 |
| Subcortical | Hippocampus* | -- | 30 | −14 | −16 | 2.7 | 136 | |
| Parahippocampal Gyrus* | 36 | −28 | −40 | −2 | 2.2 | 128 | ||
| LLD-NDC | Frontal | Inferior Frontal | 45 | −48 | 22 | 14 | 3.7 | 440 |
| NDC-LLD | Temporal | Superior Temporal** | 39 | −55 | −60 | 9 | 3.3 | 544 |
| Occipital | Middle Occipital | 19 | 47 | −73 | 8 | 3.4 | 280 | |
| Subcortical | Hippocampus*, ** | -- | 32 | −16 | −14 | 2.6 | 120 | |
Note:
indicates hippocampal ROI analysis at p < .05
Figure 3.
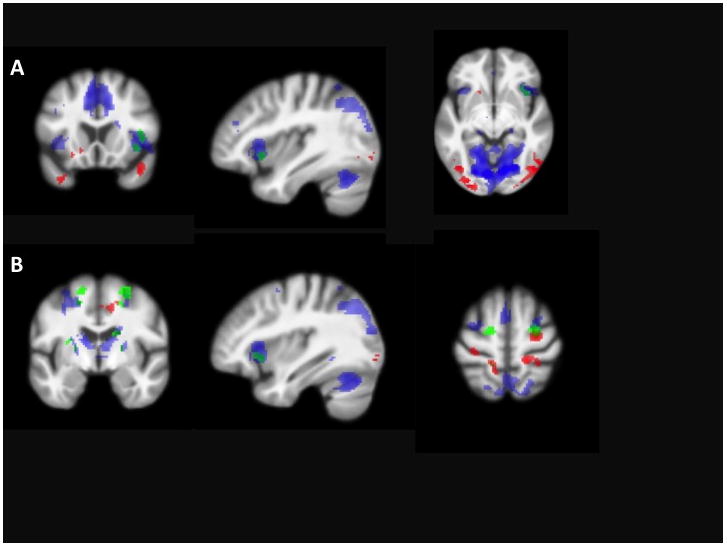
ACTIVATION DURING ENCODING VERSUS REHEARSAL. Panels A (38 16 −8) and B (36 −6 60) illustrate statistically significant activation in areas for the LLD group (blue), the NDC group (red), and the NDC minus LLD contrast (green).
Activation During Encoding of Recalled Versus Not Recalled Words
During encoding of correctly recalled versus not recalled words, LLD activated left dorsal anterior cingulate, while NDC activated left medial frontal gyrus. NDC activated left caudate and left medial frontal gyrus to a greater degree than did LLD, who did not activate any area to a significantly greater degree than did NDC (see Table 2, Figure 4). When the five memory impaired individuals were removed, LLD displayed additional activation in left inferior frontal gyrus, and group-differences in activation disappeared.
Figure 4.
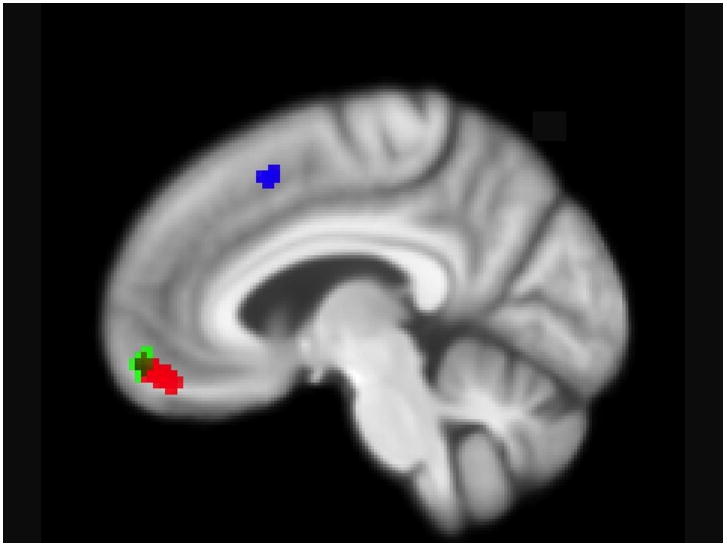
ACTIVATION DURING ENCODING OF RECALLED VERSUS NOT RECALLED WORDS. Illustrates medial frontal regions that are statistically significant in the LLD group (blue), the NDC group (red,), and NDC greater than LLD (green; −6 56 −12).
Activation During Encoding of Recognized Versus Not Recognized Words
During encoding of correctly recognized versus not recognized words, LLD activated a number of frontal regions (left medial and right middle frontal and bilateral IFG and precentral gyri), as well as left fusiform gyrus, right thalamus/mammillary body, putamen, and uvula, and bilateral parahippocampal gyrus, while NDC activated only right fusiform gyrus and hippocampus and left parahippocampal gyrus. In group comparisons, LLD activated left IFG to a significantly greater degree than did NDC, while NDC activated left superior temporal and right middle occipital gyri and hippocampus to a greater degree than did LLD (see Table 2, Figure 5). When the five memory impaired individuals were removed from analysis, the LLD groups demonstrated some notable differences in patterns of activation, as listed in Table 2. In group comparisons, LLD still demonstrated greater left IFG activation than NDC, though NDC demonstrated greater activation only in right middle occipital gyrus (see Table 2).
Figure 5.
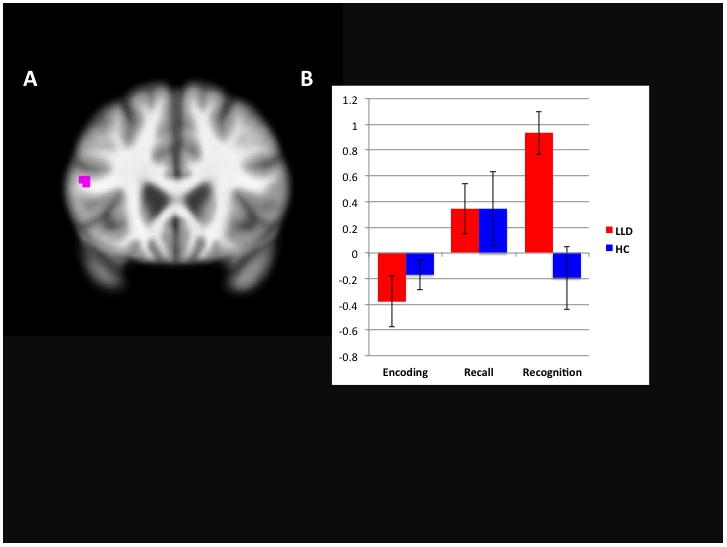
GREATER ACTIVATION IN INFERIOR FRONTAL GYRUS IN LLD DURING ENCODING OF RECOGNIZED VERSUS NOT RECOGNIZED WORDS. Fig. 5A. illustrates activation of IFG greater in LLD than NDC (−68 22 14). Figure 5B illustrates mean extracted activation values from the IFG in each group during encoding minus rehearsal, encoding of recalled versus not recalled words, and encoding of recognized versus not recognized words, respectively.
Note. All images displayed on a mean anatomical brain of the entire sample.
Relationships of Inferior Frontal Gyrus Activation to Performance (see Figure 5)
LLD activated only one region (IFG) to a greater extent than did NDC, in the context of preserved performance. Values in this region were extracted (MarsBaR) during each of the three aforementioned contrasts, in order to understand whether there were any relationships with performance (compensation/interference). Bivariate Pearson correlations were conducted in each group separately, and the entire sample; IFG activation, recall hits and intrusion errors, recall d′, and recall β as well as recognition hits and false positive errors, recognition d′, and recognition β. One significant relationship was observed in LLD only, with a negative relationship between recall intrusion errors and activation in IFG (r = −.44, p = .03). One LLD individual had a large number of recall intrusion errors and after truncation of that outlier, the correlation was no longer significant (r = −.12, p = .57). Results were the same after individuals with poor memory (n = 5) were removed from analysis.
Discussion
This study considers the impact of LLD upon memory and supportive neural circuits. Patients with LLD exhibit less activation in structures known to be relevant for new learning and memory, relative to non-depressed comparisons, despite performing at similar levels on a word-list learning and memory task and having equivalent hippocampal volumes. This phenomenon was observed on a task specifically designed to minimize individual differences in the contribution of executive functioning to memory performance and is present in individuals with LLD who, as a whole, do not have objective memory difficulties on standard clinical measures. Findings suggest that these neuroimaging measures potentially provide more sensitive markers of dysfunction, present before they are detected in standard neuropsychological batteries.
This older NDC sample exhibited activation during encoding (relative to rehearsal) of novel verbal items in regions known to be important in verbal learning and memory, including prefrontal cortex and fusiform gyrus (content processing), hippocampus (storage), and parietal regions (attention; see Kim, 2011). The largest areas of activation for LLD were in dorsal cingulate, middle frontal gyrus, thalamus, and cerebellum, potentially suggesting inefficiencies in loops that are most relevant for verbal memory, as well as the activation of networks thought to support executive functioning.
Given the functional differences observed in LLD relative to NDC, as well as the fact that the LLD group had experienced relatively large “doses” of depression over their lifetimes, it is surprising that performance differences (nor differences in hippocampal volume) were found between the two groups. A first possibility for this may be that functional changes often appear before structural and behavioral changes (see Forsberg et al., 2008; Fujishiro et al., 2013; Park et al., 2012; Risacher et al., 2013). The sample included in this study was, by and large, functioning at a high capacity, without memory deficits, and were, by and large, still in the earlier years of older age. If we were to follow the LLD group longitudinally, however, those that display the greatest functional changes may also experience the most subsequent memory decline. A second possibility, and one that is supported by our findings (though not in conflict with the latter hypothesis proposed), is that LLD arrive at successful performance differently than do NDC. For example, the IFG appears to be critical for LLD patients to consolidate encoded words into memory, as reflected by correct recognition of words subsequent to scanning, but less so for NDC. The IFG is an area involved in cognitive control (Tops & Boksem, 2011) and controlled semantic/phonological retrieval and analysis (Badre & Wagner, 2007; Dobbins & Wagner, 2005). While both groups utilized this region to a similar degree during encoding of subsequent correctly recalled words, LLD continue to do so to an even greater degree during encoding of correctly recognized words. LLD may be utilizing this as a compensatory region during encoding, potentially part of a process that is crucial for consolidation, or the subsequent inhibitory process of correctly rejecting words during the recognition trial. Moreover, the LLD group activated a far greater number of regions during encoding of correctly recognized words than did the NDC group, potentially reflecting the necessity of recruiting additional regions to assist with cognitive control processes. Compensatory processes observed during fMRI may signal the beginning stages of a neurodegenerative process, as has been observed in individuals in the early stages of Mild Cognitive Impairment (e.g., Clément & Belleville, 2012). Alternatively, given that the IFG includes significant language processing regions, it is possible that greater activation in the LLD group is a reflection of greater subvocal rehearsal during the encoding phase. A third possibility is that the SLLT is too easy to reflect performance differences in an older depressed group. Literature suggests that actively depressed individuals tend to have the most difficulty with tasks that are more effortful (Hammar & Ardal, 2012). The SLLT, while not a passive task, is less complex and cognitively demanding than most typical verbal neuropsychological measures used in behavioral paradigms, such as the CVLT, which require examinees to generate their own encoding strategy during the learning phase. Finally,
During encoding of correctly recalled words, both groups demonstrated activation of left medial frontal gyrus, albeit in different anatomical areas. Whereas LLD activated a dorsal region bordering on the anterior cingulate cortex, which is relevant to error detection (Orr & Hester, 2012), NDC displayed activation in a more ventral region thought to be crucial to self-referential processing (Yoshimura et al., 2009). These findings again highlight the different processes by which LLD and NDC arrive at successful retrieval, with LLD perhaps utilizing a cognitive control strategy during encoding, and NDC possibly contextualizing to-be-remembered material to personal experiences. NDC also activated caudate and parahippocampal gyrus to a greater degree than did LLD during encoding of correctly recalled (versus not recalled) words. Recent evidence suggests that the caudate is crucial to goal-directed action selection (Ness & Beste, 2013), and has demonstrated greater activity following semantic encoding strategy training in older adults (Kirchhoff et al., 2012). This may suggest that NDC in our sample utilized a more active encoding strategy than did LLD.
Despite a lack of group differences in performance and equivalent hippocampal volume, LLD demonstrate functional abnormalities during encoding of novel verbal material presented in a semantically organized fashion. It is important to note that all LLD participants in this sample were classified as having early-onset depression (< age 55), thought to be etiologically unique to late onset depression but similar in presentation from a phenomenological perspective (see Grayson & Thomas, 2013). Furthermore, these depressed individuals were carefully screened to rule out any early dementia, rendering the sample a more conservative test of our hypotheses. Future research might consider mechanisms accounting for functional activation abnormalities in individuals with LLD, including HPA-axis functioning and cerebrovascular contributions, both widely researched in the depression literature.
It is important to note that when the five memory-impaired individuals were removed from analysis, there were some changes in the pattern of activation, with generally greater activation being displayed in the LLD group, and fewer between-group differences. This suggests that, while non cognitively-impaired LLD patients still demonstrate abnormal activation patterns in regions relevant to learning and memory, the most abnormal patterns of activation are likely to be displayed in those with the poorest cognitive functioning. While most studies of LLD exclude those with overt dementia, they usually include patients with a range of cognitive functioning. While the size of the sample in the current study precludes us from being able to make any strong conclusions in this regard, future studies might consider the contribution of cognitive status (i.e., those with and without Mild Cognitive Impairment) to patterns of abnormal activation during cognitive challenge.
There are a few limitations that should be considered in interpreting results and generalizing findings to the wider population of individuals with LLD. First, our sample was highly educated and largely without objectively defined memory deficits. Results reflect functional abnormalities in memory processing pathways among non-cognitively impaired older adults with early onset depression, and may not generalize to those with subjective memory complaints, objectively defined memory problems, or onset of depression in late life. In regards to the latter, there is evidence to suggest that individuals with late-onset depression perform more poorly on cognitive measures than those with early-onset depression (Delaloye et al., 2010; Sachs-Ericsson et al., 2013). Thus, memory performance and disruption to pathways relevant for memory may be more apparent in a sample of individuals with late-onset depression. Because all patients were actively depressed, it is also not clear whether functional abnormalities represent state or trait effects of depression. Second, as this is not a longitudinal study, the extent to which findings of functional abnormalities might be indicative of incipient cognitive decline above and beyond LLD is unclear. Future studies might consider regions that were found to differ in functioning between groups as potential markers for investigation of prediction of cognitive decline. Third, the majority of LLD patients were taking antidepressant medications, which may impact imaging findings. The sample is underpowered to consider the impact of medication on activation. Fourth, the SLLT presented items visually, rather than orally, providing participants with an additional encoding cue, relative to verbal memory tasks that have been most widely used in the LLD literature (e.g., CVLT) where items are presented only orally. It is possible that we may have observed between-group performance differences and even greater differences in activation during encoding might have been found between the LLD and NDC groups had stimuli been presented orally, as the LLD group would have had to use more executive resources in order to effectively encode stimuli.
Conclusions
Older adults with early-onset active state MDD demonstrate a broader pattern of hypoactivation during list learning encoding relative to non-depressed comparisons, in regions known to be crucial to successful learning and memory. Functional differences are present despite equivalent performance on paper-and-pencil recall and recognition paradigms and may reflect different modes of processing to-be-remembered information and/or early changes predictive of incipient cognitive decline. Future studies might consider mechanisms for functional differences, including HPA-axis functioning and vascular integrity, and utilize longitudinal designs in order to understand whether functional changes are predictive of incipient cognitive decline. In order to better understand the mechanism behind activation differences (e.g., compensation, de-differentiation) future research might also consider enrolling good and poor performers and assessing performance by activation interactions, as well as incorporating connectivity analyses to better understand the relationships among regions relevant to encoding. Given the importance of the role of the caudate in understanding manifestations of LLD (i.e., goal directed behavior, response inhibition; Alexopoulos et al., 2013; Bobb et al., 2012), future research might also consider the effects of proactive and retroactive interference on memory performance and underlying neural processes during LLD. This might entail including a retrieval phase prior to and following the distractor task, with careful effort toward minimizing movement. Finally, given the frequency of subjective memory complaints among older people with depression, it would be interesting to assess relationships between the extent of subjective memory complaints and patterns of activation during encoding of novel stimuli, as this could assist clinicians in assessing the significance of memory complaints in their patients with LLD.
Supplementary Material
Key Points.
Despite performing at similar levels on a word-list learning and memory task and having equivalent hippocampal volumes, patients with early-onset depression in late life (LLD) exhibit less activation in structures known to be relevant for new learning and memory, including hippocampus, parahippocampal gyrus, insula, and cingulate, relative to non-depressed comparisons (NDC).
An important region in which the LLD group displayed greater activation than the NDC group was in inferior frontal gyrus (IFG), an area involved in cognitive control and controlled semantic/phonological retrieval and analysis; this area may be critical for LLD patients to assist in consolidation of memory.
Functional aberrations found in LLD patients may reflect different modes of processing to-be-remembered information, compensatory processes to assist in memory, and/or early changes predictive of incipient cognitive decline.
Acknowledgments
Source of Funding: This research was supported by funding from the University of Michigan Depression Center (SLW, SAL), University of Michigan fMRI Laboratory (SLW, SAL), the Michigan Alzheimer’s Disease Research Center Pilot Grant Fund (SLW, SAL), and the National Alliance for Research on Schizophrenia and Depression (SAL). We also thank Laura Gabriel, Michael-Paul Schallmo, Brennan Haase, Katie Hazlett, Rachel Kay, Hadia Leon, Alyssa Barba, and Arpita Mohanty for their contributions to this project in data collection and processing.
Footnotes
This work was presented in part at the 2013 Annual Meeting of the International College of Geriatric Psychoneuropharmacology
References
- Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17:30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, et al. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hopton MJ, Yuen G, et al. Functional connectivity in apathy of late-life depression: a preliminary study. J Affect Disord. 2013;149:398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bobb DS, Adinoff B, Laken SJ, et al. Neural correlates of successful response inhibition in unmedicated patients with late-life depression. Am J Geriatr Psychiatry. 2012;20:1057–1069. doi: 10.1097/JGP.0b013e318235b728. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, et al. Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan. 2002. Available on CD-ROM in NeuroImage 16. [Google Scholar]
- Brown JA. Some tests of the decay theory of immediate memory. Quarterly Journal of Experimental Psychology. 1958;10:12–21. [Google Scholar]
- Clément F, Belleville S. Effect of disease severity on neural compensation on neural compensation of item and associative recognition in mild cognitive impairment. J Alzheimers Dis. 2012;29:109–123. doi: 10.3233/JAD-2012-110426. [DOI] [PubMed] [Google Scholar]
- Delaloye C, Moy G, de Bilbao F, et al. Neuroanatomical and neuropsychological features of elderly euthymic depressed patients with early- and late-onset. J Neurol Sci. 2010;299:19–23. doi: 10.1016/j.jns.2010.08.046. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, et al. Calfornia Verbal Learning Test-Second Edition, Adult Version. The Psychological Corporation; San Antonio: 2000. [Google Scholar]
- Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177–187. doi: 10.1016/j.jad.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, et al. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dybedal GS, Tanum L, Sundet K. Neuropsychological functioning in late-life depression. Front Psychol. 2013:381. doi: 10.3389/fpsyg.2013.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Mintz J, Haroon E, et al. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol. 2007;22:261–270. doi: 10.1016/j.acn.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Fujishiro H, Iseki E, Kasanuki K, et al. A follow up study of non-demented patients with primary visual cortical hypometabolism: prodromal dementia with Lewy bodies. J Neurol Sci. 2013;334:48–54. doi: 10.1016/j.jns.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Grayson L, Thomas A. A systematic review comparing clinical features in early age at onset and late age at onset late-life depression. J Affect Disord. 2013;150:161–170. doi: 10.1016/j.jad.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hammar A, Ardal G. Effortful information processing in patients with major depression- a 10-year follow-up study. Psychiatry Res. 2012;198:420–423. doi: 10.1016/j.psychres.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Anderson BA, Barch DM, et al. Cognitive and neural effects of semantic encoding strategy training in older adults. Cereb Cortex. 2012;22:788–799. doi: 10.1093/cercor/bhr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. NeuroImage. 2010;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kurth F, Luders E, Gaser C. VBM8 Toolbox Manual. 2010. [Google Scholar]
- Lamar M, Charlton R, Zhang A, et al. Differential associations between types of verbal memory and prefrontal brain structure in healthy aging and late life depression. Neuropsychologia. 2012;50:1823–1829. doi: 10.1016/j.neuropsychologia.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Briceno EM, Hamid NM, et al. An evaluation of distinct volumetric and functional MRI contributions toward understanding age and task performance: a study in the basal ganglia. Brain Res. 2007;1135:58–68. doi: 10.1016/j.brainres.2006.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Weisenbach SL, Giordani B, et al. Impact of chronic hypercortisolemia on affective processing. Neuropharmacology. 2012;62:217–225. doi: 10.1016/j.neuropharm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, et al. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Murata T, Kimura H, Omori M, et al. MRI white matter hyperintensities, (1)H-MR spectroscopy and cognitive function in geriatric depression: a comparison of early- and late-onset cases. Int J Geriatr Psychiatry. 2001;16:1129–1135. doi: 10.1002/gps.501. [DOI] [PubMed] [Google Scholar]
- Ness V, Beste C. The role of the striatum in goal activation of cascaded actions. Neuropsychologia. 2013;51:2562–2571. doi: 10.1016/j.neuropsychologia.2013.09.032. [DOI] [PubMed] [Google Scholar]
- O’Bryant SE, Humphreys JD, Smith GE, et al. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch Neurol. 2008;65:963–967. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C, Hester R. Error-related anterior cingulate cortex activity and the prediction of conscious error awareness. Front Hum Neurosci. 2012;6:177. doi: 10.3389/fnhum.2012.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez JW. A proposed mechanism of emotion. Archives of Neurology and Psychiatry. 1937;7:103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Park KW, Yoon HJ, Kang DY, et al. Regional cerebral blood flow differences in patients with mild cognitive impairment between those who did and did not develop Alzheimer’s disease. Psychiatry Res. 2012;203:201–206. doi: 10.1016/j.pscychresns.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Peterson LR, Peterson MJ. Short-term retention of individual verbal items. J Exp Psychol. 1959;58:193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ. Neuroimaging and other biomarkers for Alzheimer’s disease: the changing landscape of early detection. Annu Rev Clin Psychol. 2013;9:621–48. doi: 10.1146/annurev-clinpsy-050212-185535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs-Ericsson N, Corsentino E, Moxley J, et al. A longitudinal study of differences in late- and early-onset geriatric depression: depressive symptoms and psychosocial, cognitive, and neurological functioning. Aging Ment Health. 2013;17:1–11. doi: 10.1080/13607863.2012.717253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, et al. Structured Clinical Interview for DSM-IV (SCID-IV) American Psychiatric Press; Washington D.C: 1994. [Google Scholar]
- Steffens DC, McQuoid DR, Payne ME, et al. Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2011;19:4–12. doi: 10.1097/JGP.0b013e3181d6c245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tops M, Boksem MA. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front Psychol. 2011;2:330. doi: 10.3389/fpsyg.2011.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenbach SL, Rapport LJ, Briceno EM, et al. Reduced emotion processing efficiency in healthy males relative to females. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss137. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. Memory transformation and systems consolidation. J Int Neuropsychol Soc. 2011;17:766–80. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- Winograd E. List differentiation as a function of frequency and retention interval. Journal of Experimental Psychology. 1968;76:1–18. doi: 10.1037/h0025380. [DOI] [PubMed] [Google Scholar]
- Woo SL, Prince SE, Petrella JR, et al. Modulation of a human memory circuit by subsyndromal depression in late life: a functional magnetic resonance imaging study. Am J Geriatr Psychiatry. 2009;17:24–29. doi: 10.1097/JGP.0b013e318180056a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Li W, Chen G, et al. Late-life depression, mild cognitive impairment and hippocampal functional network architecture. Neuroimage Clin. 2013;3:311–320. doi: 10.1016/j.nicl.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen YC, Rebok GW, Gallo JJ, et al. Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. Am J Geriatr Psychiatry. 2011;19:142–150. doi: 10.1097/JGP.0b013e3181e89894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S, et al. Self-referential processing of negative stimuli within the ventral anterior cingulate gyrus and right amygdala. Brain Cogn. 2009;69:218–225. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.