Abstract
Chemical cross-linking provides an effective avenue to reduce the conformational entropy of polypeptide chains and hence has become a popular method to induce or force structural formation in peptides and proteins. Recently, other types of molecular constraints, especially photoresponsive linkers and functional groups, have also found increased use in a wide variety of applications. Herein, we provide a concise review of using various forms of molecular strategies to constrain proteins, thereby stabilizing their native states, gaining insight into their folding mechanisms, and/or providing a handle to trigger a conformational process of interest with light. The applications discussed here cover a wide range of topics, ranging from delineating the details of the protein folding energy landscape to controlling protein assembly and function.
Keywords: Protein folding, aggregation, self-assembly, cross-linker, phototrigger, light-activation
1 Introduction
The ability to spontaneously self-assemble, which includes intramolecular folding and intermolecular complexation or association, is a hallmark of biological molecules. Research over the past few decades has resulted in tremendous progress in our understanding of the fundamental principles that govern, for example, how proteins fold and aggregate [1-3]. However, the majority of naturally occurring proteins lack a structural element or motif that can be used as a molecular switch or constraint to direct a self-assembly process of interest towards a specific direction, making it difficult or impossible to precisely control the outcome. Thus, a great deal of effort has been made in recent years to develop new strategies to extrinsically modify protein structures, aiming to manipulate and/or control protein/peptide self-assemblies. In particular, the strategies of using a chemical cross-linker to confine the molecule of interest to a specific conformation and employing a photoswitchable cross-linker or functional group to induce a desired conformational transition or function with light have gained popularity. This is due to the fact that these methods are not only useful in controlling the structural framework of proteins, but also in helping understand the molecular mechanism of the self-assembly process. Herein, we present a brief review of the recent advances that utilize molecular constraints in peptides and proteins, with an emphasis on discussing the applications of photoresponsive structural cross-linkers and photolabile motifs to explore protein folding, misfolding, and aggregation.
Below, we will first provide a brief account of the available cross-linking methods for constraining polypeptides to particular structures, and then discuss how well-chosen cross-linkers can be used to gain detailed insights into the folding energy landscape of proteins. Next, we will review recent applications of various light-activated moieties, including photoresponsive cross-linkers and photolabile groups, in protein folding and aggregation studies, as well as in creating light-responsive biomaterials. Finally, we will briefly mention how light can be used to control biological functionality.
2 Stapling to fold
Because folding leads to a large decrease in conformational entropy, stable structures can emerge only when the associated enthalpic gain overcomes this energetic penalty. For relatively small systems, however, this requirement may be difficult to achieve. Therefore, various sidechain-to-sidechain cross-linking strategies have been put forth to reduce the conformational entropy of the unfolded state, thus forcing short peptides to fold into α-helices or β-sheets (Figure 1). Such examples include disulfide bond formation [4-10], ring-closing metathesis [11-14], lactam bridge formation [15-19], hydrocarbon bridges [20-23], and hydrazone [24] and oxime linkages [25-27]. In particular, due to the ease of incorporation and natural abundance of cysteines in biological systems, cysteine alkylation [28-30] has become a popular method for incorporating cross-linkers that stabilize α-helical conformations [31, 32]. For example, Jo et al. have used this strategy to create stable and potent α-helical peptide inhibitors for calpain [32]. Besides these sidechain-to-sidechain covalent constraints, attempts have also been made to cross-link two backbone atoms together [33-35].
Figure 1.

Cartoon representation of the effect of a covalent cross-linker or staple on α-helix formation.
3 Probing the details of the folding energy landscape via cross-linking
The strategy of chemical cross-linking is not only useful for increasing the stability of folded conformations [36], but also is an effective tool to study the mechanisms of protein folding and, in particular, the structure of the folding transition state ensemble [37-42]. For example, Sosnick and coworkers have exploited the metal binding property of histidine and used divalent metal ions (e.g., Zn2+ and Co2+) to create an unconventional linker in protein systems of interest via engineered bihistidine sites [43, 44]. Because the apparent strength of this cross-linker depends on the metal ion concentration as well as the local structure, they showed that it is possible to use this cross-linking approach in combination with thermodynamic and kinetic measurements, to determine whether a specific native structural element is formed in the transition state ensemble. Analogous to mutational Φ-value analysis [45], if the increase in protein stability induced by metal ion binding arises exclusively from the increase in the folding rate, the structural element stabilized by the metal-ion linker is formed in the transition state. Similarly, covalent cross-linkers, such as dichloroacetone structures and disulfide bridges, have also been utilized to elucidate the nature of the folding transition state ensemble [37-39, 41, 42, 46].
Confining a peptide to well-defined conformations enables a folding/unfolding reaction to proceed from a unique position on the folding free energy landscape (see below), potentially allowing for control of the flux of a particular folding pathway or isolation of a specific folding event. In one example, Serrano et al. [47] have taken advantage of this strategy and used a cyclic peptide generated by linking its Lys (lysine) and Asp (aspartic acid) sidechains via a lactam bridge to determine the nucleation time of α-helix formation. In another example, we have shown that it is possible to use a cross-linker to eliminate the free energy gap between the unfolded and transition states, thus allowing for assessment of the folding rate on the downhill side of the free energy barrier [48]. Specifically, we employed a well-studied β-hairpin, Trpzip4, as a model to illustrate the feasibility of this approach. As shown (Figure 2), when a sidechain-to-sidechain disulfide bond was introduced to prevent the unfolding of the turn of this β-hairpin, which has been shown to be formed in the transition state [49], infrared temperature-jump (T-jump) measurements revealed the existence of two folding pathways; one having a relaxation rate that is almost identical to the wild-type and another relaxing an order of magnitude faster (∼500 ns). This faster kinetic event, which was not observed for the uncross-linked β-hairpin [50], was attributed to a barrierless folding process. In other words, the disulfide cross-linked state in Figure 2 mimics the folding transition state of β-hairpins and its folding time likely represents an upper limit for folding of β-hairpins on a barrierless energy landscape. Indeed, this time agrees well with that predicted by end-to-end loop-closure kinetics for unstructured polymer chains.
Figure 2.

Cartoon illustration of the effect of a disulfide cross-linker on the folding free energy surface of a β-hairpin, where the polypeptide backbone is colored in blue, the hydrogen bonds are colored in green, and the disulfide cross-linker is colored in orange. Our study [48] suggests that it is possible to use an appropriately-placed linker to significantly decrease or even abolish the folding free energy barrier.
Furthermore, we have recently hypothesized and demonstrated, in a proof of principle study, that it is possible to use a well-chosen cross-linker to help assess the effect of internal friction on protein folding dynamics [51]. Specifically, we showed that when a m-xylene cross-linker was introduced in such a manner that it spans the central portion of the single α-helix in the miniprotein Trp-cage 10b, both the folding and unfolding rates of the cross-linked variant (referred to as 4-8-CL-Trp-cage) show a similar decrease (Figure 3). Because in this case the m-xylene cross-linker does not alter the protein stability to any appreciable extent, these results were interpreted within the framework of internal friction. In other words, the m-xylene acts to increase the roughness of the folding energy landscape, but has no significant effect on the native interactions. Interestingly, our results suggest that a cross-linker of this size can increase the roughness of the Trp-cage 10b folding energy landscape by 0.4-1.0 kBT, depending on the direction of the underlying conformational motion. Considering the fact that naturally occurring proteins are much larger and encounter interactions between neighboring chains more frequently, our findings suggest that internal friction, arising from the excluded volume effect and/or local steric hindrance between sidechains, will have a significant effect on protein conformational and folding dynamics.
Figure 3.
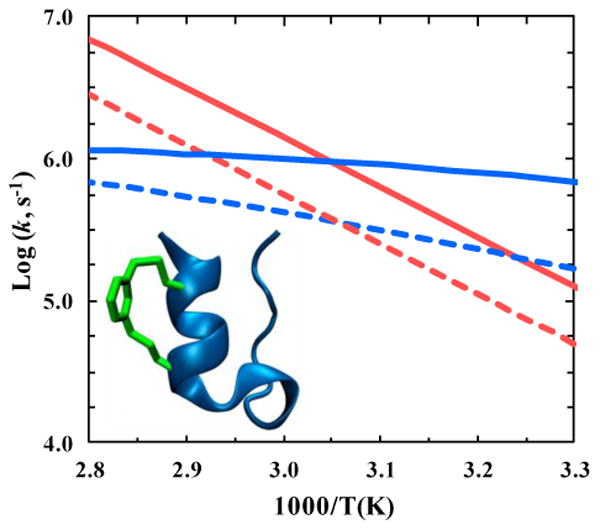
Temperature dependence of the folding (blue) and unfolding (red) rate constants of Trp-cage 10b (solid lines) and 4–8-CL-Trp-cage (dashed lines). As indicated in the inset, the latter contains a m-xylene cross-linker. This figure was generated based on the results of ref. [51].
4 Triggering protein conformational events with light
Due to the ease and convenience of manipulating light, phototriggering is becoming a more widely used method to initiate protein conformational changes. In comparison to other initiation methods, phototriggering can potentially offer several advantages, since light-triggered reactions, such as isomerization and cleavage, (1) can take place on ultrafast timescales, (2) can lead to significant changes in backbone geometry, (3) and can provide precise conformational control between equilibrium and non-equilibrium states. While there are a large number of molecules that strongly interact with light, only a small fraction have found use as a phototrigger in protein conformational studies. This is because the photoreaction of an effective trigger must meet several criteria, some of which depend on the nature of the question being investigated: (1) it should be selectively initiated by light that has a wavelength higher than 310 nm, as lower-wavelength light could potentially excite protein backbone and sidechain electronic transitions, (2) it should occur on a timescale that is faster than that of the kinetic event of interest, (3) it should have a sufficiently high quantum yield, and (4) it should ideally produce relatively inert byproducts. In practice, other considerations include the reversibility of the photoreaction, as well as the rate of the reversible reaction [52], and the ease of incorporation into the protein system of interest.
Over the past few decades, a substantial number of molecules have been examined as potential phototriggers. Because of its easy incorporation into peptides and proteins, early attempts have explored the utility of using a disulfide as a phototrigger [53]. While these studies showed that disulfides can be cleaved with ultraviolet light (∼270 nm) [54, 55], the free radicals formed upon photoexcitation often undergo ultrafast geminate recombination and are also very reactive, making it a less-than-ideal trigger. Recently, several studies have shown that it is possible to remediate these pitfalls. For instance, Kolano et al. [54] have shown that it is possible to reduce the degree of geminate recombination after photocleavage by placing a disulfide in a strained geometry. Another alternative, which is currently being pursued in our lab [56], is to break the disulfide bond irreversibly using an electron transfer mechanism [57-60].
Other examples of irreversible phototriggers include tetrazines, hydrazines, and dimethoxybenzoins [61]. In particular, Tucker et al. [62] have shown that tetrazines are ideal irreversible phototriggers due to their relative ease of incorporation, their fast timescales of reaction (picoseconds), relatively high photochemical yields (∼22%), their excitation wavelengths (355 nm), and their inert byproducts (N2 and nitriles). As shown (Figure 4), using S,S-tetrazine as an ultrafast phototrigger, which creates a ‘kink’ in the α-helix investigated, and isotopically labeled residues [63] as structural probes in a two-dimensional infrared spectroscopy (2D IR) experiment, they were able to obtain snapshots along the time course of the photo-induced conformational relaxation process [64].
Figure 4.

Top: Cartoon illustration of the principle of using light to remove a kink that is introduced by a tetrazine cross-linker in a model α-helix. Bottom: Representative molecular snapshots along the time course of helix reorganization initiated by photoexcitation of the tetrazine chromophore with a 2 ps laser pulse centered at 355 nm. This figure is adapted from ref. [64], with permission from the National Academy of Sciences.
Photolabile molecules (i.e., photocages) constitute another type of irreversible phototrigger [65-67], and have also been used to trigger protein conformational events [68]. The main idea is to use light to remove a moiety that disrupts native structure formation from the protein of interest, thus initiating folding. The advantage of using photocages, in comparison to photoresponsive cross-linkers, is that they can be localized to a single amino acid sidechain, thus offering greater flexibility. The disadvantage, on the other hand, is that typically more sample is needed and, in some cases, the diffusion of the photoproduct out of the protein matrix can significantly decrease the effective time-resolution of the method. Examples of photocages that have been used to study protein folding include 4,5-dimethoxy-2-nitrobenzene [69] and 4-(bromomethyl)-6,7-dimethoxycoumarin [70].
Reversible phototriggers have the advantage of using less material and, thus, have received more attention. In particular, azobenzene and its derivatives [71-77] have become the most popular choice. Similar to stilbene [78-80], azobenzene undergoes ultrafast isomerization at the excited electronic state [81, 82]; it is this process (Figure 5), which can effectively change the distance between the two ends of the azobenzene linker, that is used to modulate protein structures in practice. When choosing a specific azobenzene linker, it is important to note that at equilibrium in the dark the moiety is predominantly (>90%) in the trans configuration, and the cis to trans conversion at the ground electronic state occurs on a seconds to minutes timescale, depending on temperature and the functionalization of the azobenzene cross-linker [83]. In protein conformational studies, di-iodoacetamide azobenzene, which can be inserted into proteins via cysteine alkylation [83, 84], is a widely used reversible phototrigger. Upon excitation with 355 nm light, this phototrigger can achieve an isomerization rate of (1 ps)-1 and a yield of ∼50% [85], making it ideally suited for studying ultrafast conformational rearrangements of small peptides and/or secondary structural elements. For example, Hamm and coworkers [84] have utilized this strategy to study the mechanism of α-helix formation and found that the coil-to-helix transition is inherently heterogeneous. Despite many successful applications of azobenzene-based cross-linkers, we note that developing reversible phototriggers is still a very active research area. For example, efforts to expand the excitation wavelength of azobenzene derivatives to the visible regime [86] and to identify/design other reversible phototriggers, such as spiropyrans [87] and thioamides [88-90], still continue.
Figure 5.

Top: Isomerization of azobenzene. Bottom: A cartoon representation of a β-hairpin peptide containing an azobenzene phototrigger in the trans (left) or cis (right) conformation. Adapted from ref. [71], with permission from John Wiley and Sons, Inc.
Finally, another type of widely used phototriggering strategy, which has been recently reviewed [91], uses light to induce a change in the solution conditions, such as temperature [92, 93], pH [94-96], or electronic property of the protein [97-99], which consequently alters the folding/unfolding equilibrium of interest and thus induces a conformation relaxation.
5 Manipulating biological assemblies with light
It is now a widely accepted notion that intermolecular self-assembly of polypeptides, which typically leads to formation of highly ordered fibrillar structures that are rich in β-sheet content [100], further decreases the free energy of the system. However, in vivo formation of such insoluble amyloid fibrils has been shown to be associated with several degenerative diseases such as Alzheimer's, Parkinson's, and Type II Diabetes [101, 102]. On the other hand, the same process has been actively pursued as a way to generate biological architectures and scaffolds, such as peptide hydro-gels, that are posited to yield many new applications, ranging from regenerative medicine to the delivery of therapeutics [103, 104]. Because of their apparent importance, a wide variety of studies have been conducted to understand the mechanisms of amyloid formation and to find ways to combat this pathological condition. In particular, a recently pursued direction is to use light and phototriggers to manipulate and control the self-assembly process of interest, and also, to reverse the higher order structures thus formed.
For example, azobenzene has been introduced into various aggregation-prone systems to serve as a photoswitch between different aggregation or molecular states. These include the studies of Waldauer et al. [105] and Deeg et al. [106], which demonstrated reversible switching between aggregated and non-aggregated states of azobenzene-linked amyloid-like peptides, and the study of Doran et al. [107], which used an azobenzene-containing Aβ(1-42) peptide to investigate whether turn nucleation is the rate-limiting step in fibril self-assembly. In addition, Hamil et al. [108] have shown that an azobenzene-containing and light-responsive surfactant can inhibit Aβ(1-40) fibril formation when the azobenzene linkage is in the trans form.
Similar to applications in protein folding studies, light-induced decaging also finds novel use in studying the assembly of amyloid structures. For instance, Taniguchi et al. [109] applied a coumarin-derived photocage to trigger an intramolecular acyl migration, which converts a nonnative O-acyl group to an N-acyl group within the backbone of Aβ(1-42), restoring the native backbone composition and thus initiating aggregation upon illumination Recently, we demonstrated the feasibility of using Ly-sine(4,5-dimethoxy-2-nitro-benzyloxycarbonyl) (Lys(nvoc)), which produces a native Lys sidechain upon decaging (Figure 6), to disassemble amyloid fibrils [110]. The working principle is that generation of a charged moiety (i.e., Lys) in a predominantly hydrophobic environment, which places the system in an energetically unfavorable position, will cause fibrils to dissociate. In a proof of principle study [110], we showed that this approach is effective in disassembling fibrils formed by a small amyloidogenic peptide wherein one of the native Phe (phenylalanine) residues was replaced by Lys(nvoc). As shown in the AFM images in Figure 6, the modified peptide self-assembles into well-ordered fibrils that can be destroyed by light. Using similar principles, a nitrobenzyl derivative serving as a photolabile linker between a hydrophobic alkyl chain and a small peptide was also efficient in disassembling well-formed fibrils [111, 112].
Figure 6.

Representative AFM images showing the amyloid fibrils (left panel) formed by a mutant of Aβ(16-22) (sequence KLVF*FAE, where F* represents Lys(nvoc)) and the effectiveness of using light to disassemble these fibrils (right panel). Adapted from ref. [110], with permission from the American Chemical Society.
In addition, we and others have applied such photoswitching strategies to control the integrity of peptide hydrogels since their molecular packing and architectures are similar to those of amyloid fibrils [113]. Because of their ability to swell in water and, perhaps more importantly, their biological compatibility, peptide hydrogels hold potential for numerous biomedical applications such as drug delivery, tissue engineering scaffolds, and cell growth [104]. Since light is an external stimulus that offers precise spatial and temporal control, light-responsive hydrogels are expected to find great use in applications where such controls are required.
In one application, a photoswitch either an azobenzene [114-116] or a spiropyran [117], which links hydrogel-forming dipeptides together, was used to control π-π stacking between sidechains and hydrogen bonding patterns and thus the morphology of the hydrogel with light. Nilsson and coworkers [118] have shown that this strategy is also effective for longer peptides. In another application, He et al. [119] designed photodegradable hydrogels by linking a biaryl-substituted tetrazole to a small peptide, which undergoes a rapid intramolecular photoclick reaction that leads to a rearrangement within the ring system and disruption of the hydrogel matrix.
Moreover, Schneider and coworkers [120] have shown that the light-activated release of a nitrobenzyl-cage from a cysteine residue can trigger the self-assembly of the MAX1 amphiphilic β-hairpin peptide, which spontaneously self-assembles into hydrogels in its folded conformation. To demonstrate photoactivity in the opposite direction, we recently showed that modification of two Phe residues in the peptide hydrogel sequence (FKFE)2 with Lys(nvoc) preserves the ability of the peptide to form a macroscopic hydrogel as shown in the AFM image in Figure 7A. However, these mutations render the formed hydrogel photodegradable in response to light, as indicated in Figure 7B [121]. In a slightly different treatment, Griffin et al. [122] utilized an ortho-nitrobenzyl cage as a photodegradable linker to tether a cell-adhesive peptide to a macromer-based hydrogel, thus allowing light-induced release. Furthermore, by incorporating photocages to the termini of cell-adhesive peptides derived from fibronectin (RGDS), which are typically covalently secured to a polymeric 3D hydrogel, photorelease of the cage prompts cell patterning and cell migration [123-126].
Figure 7.

(A) AFM image of the hydrogel formed by a Lys(nvoc) containing peptide (KFE8-C). (B) Infrared spectra of KFE8-C in the amide I′ region before and after irradiation with 355 nm light for 90 min, showing the effectiveness of using light to disassemble the underlying β-sheet network of the hydrogel. The amide I′ band reports on the carbonyl stretching vibrations of the peptide backbone and is an established reporter of secondary structures. The blue spectrum indicates that KFE8-C assembles into well-ordered anti-parallel β-sheets indicated by the bands centered at 1620 and 1685 cm-1. After irradiation, the secondary structure undergoes a β-sheet to disordered conformational transition, evidenced by the appearance of a single, broad band centered at ∼1650 cm-1. Adapted from ref. [121], with permission from Elsevier.
Indirect approaches, where the light-absorbing molecule is not directly linked to the hydrogel-forming peptide, have also been explored. For example, Raeburn et al. [127] employed photoacid generators, molecules that release an acid byproduct upon irradiation, to induce hydrogelation of low pH assembling dipeptide units. Similarly, Messersmith and coworkers [128] have shown that a 16-residue ionic complementary peptide, which gels at high salt concentrations, can be triggered by light when suspending the peptide in a solution of near-IR degradable liposomes containing CaCl2.
6 Controlling biological function with light
Another highly important and actively pursued area of research is to develop methods that would allow us to regulate or control a biological process of interest, in vivo or in vitro, with light. While an in-depth summary [67, 129-132] of the recent advances in this field is beyond the scope of this Review, we would like to highlight several examples to underscore the rapid growth of this exciting research field. For instance, photoremovable protecting groups have been used to trigger acquisition of protein functions [133-135], to investigate enzyme binding and inhibition [136-138], protein translocation [139], chemotactic activity [140], peptide mediated lipid degradation [141], phosphopeptide-binding for signaling pathways [142, 143], and to regulate or control the function or activity of oligonucleotides [144, 145], neurotransmitters [146, 147], hormones [148], and signaling molecules [149, 150].
7 Conclusions
In this brief review, we highlighted recent advances in applications of inert and/or photoresponsive molecular constraints to better understand how proteins fold and self-assemble, to control the structural integrity and morphology of high-order biological assemblies, such as fibrils and hydrogels, and to regulate biological activity. While significant progress has been made recently in each and every one of these areas, we believe that further efforts are needed to (1) optimize the photochemical and photophysical properties of existing photoactivatable molecules, including wavelength of excitation, photochemical yield, timescale of photoswitching or photocleavage, and photoproducts, (2) increase the pool of useful photoresponsive cross-linkers and photocages, (3) improve existing incorporation methods and develop in vivo incorporation techniques, and (4) explore new biological and biophysical applications and utilities. For instance, one new application we are currently testing is to take advantage of the mechanical force associated with isomerization of an azobenzene cross-linker to force the folding process of interest to rapidly populate the folding transition state.
Acknowledgments
This work was supported by the National Institutes of Health (GM-065978, AG-039253).
References
- 1.Bryngelson JD, Onuchic JN, Socci ND, Wolynes PG. Funnels, pathways, and the energy landscape of protein folding: A synthesis. Proteins: Struct, Fund, Bioinf. 1995;21:167–195. doi: 10.1002/prot.340210302. [DOI] [PubMed] [Google Scholar]
- 2.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 3.Dill KA, Ozkan SB, Shell MS, Weikl TR. The protein folding problem. Annu Rev Biophys. 2008:289–316. doi: 10.1146/annurev.biophys.37.092707.153558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. General approach to the synthesis of short α-helical peptides. J Am Chem Soc. 1991;113:9391–9392. [Google Scholar]
- 5.Leduc AM, Trent JO, Wittliff JL, Bramlett KS, Briggs SL, Chirgadze NY, Wang Y, Burris TP, Spatola AF. Helix-stabilized cyclic peptides as selective inhibitors of steroid receptor-coactivator interactions. Proc Natl Acad Sci USA. 2003;100:11273–11278. doi: 10.1073/pnas.1934759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell SJ, Blandl T, Skelton NJ, Cochran AG. Stability of cyclic beta-hairpins: Asymmetric contributions from side chains of a hydrogen-bonded cross-strand residue pair. J Am Chem Soc. 2003;125:388–395. doi: 10.1021/ja028075l. [DOI] [PubMed] [Google Scholar]
- 7.Mirassou Y, Santiveri CM, Pérez de Vega MJ, González-Muñiz R, Jimenez MA. Disulfide bonds versus trp⋯trp pairs in irregular β-hairpins: NMR structure of vammin loop 3-derived peptides as a case study. ChemBioChem. 2009;10:902–910. doi: 10.1002/cbic.200800834. [DOI] [PubMed] [Google Scholar]
- 8.Santiveri CM, Leon E, Rico M, Jimenez MA. Context-dependence of the contiribution of disulfide bonds to beta-hairpin stability. Chem-Eur J. 2008;14:488–499. doi: 10.1002/chem.200700845. [DOI] [PubMed] [Google Scholar]
- 9.Almeida AM, Li R, Gellman SH. Parallel β-sheet secondary structure is stabilized and terminated by interstrand disulfide cross-linking. J Am Chem Soc. 2011;134:75–78. doi: 10.1021/ja208856c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan DL, Ramirez M, Shao L, Jacobsen D, Barrera I, Lutkenhaus J, Chen ZL. Two-component protein hydrogels assembled using an engineered disulfide-forming protein-ligand pair. Biomacromolecules. 2013;14:2909–2916. doi: 10.1021/bm400814u. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell HE, Grubbs RH. Highly efficient synthesis of covalently cross-linked peptide helices by ring-closing metathesis. Angew Chem, Int Ed. 1998;37:3281–3284. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 12.Schafmeister CE, Po J, Verdine GL. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J Am Chem Soc. 2000;122:5891–5892. [Google Scholar]
- 13.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Liao W, Arora PS. Enhanced metabolic stability and protein-binding properties of artificial a helices derived from a hydrogen-bond surrogate: Application to Bcl-xL. Angew Chem, Int Ed. 2005;44:6525–6529. doi: 10.1002/anie.200501603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osapay G, Taylor JW. Multicyclic polypeptide model compounds. 2. Synthesis and conformational properties of a highly α-helical uncosapeptide constrained by three side-chain to side-chain lactam bridges. J Am Chem Soc. 1992;114:6966–6973. [Google Scholar]
- 16.Houston ME, Gannon CL, Kay CM, Hodges RS. Lactam bridge stabilization of α-helical peptides: Ring size, orientation and positional effects. J Pept Sci. 1995;1:274–282. doi: 10.1002/psc.310010408. [DOI] [PubMed] [Google Scholar]
- 17.Phelan JC, Skelton NJ, Braisted AC, McDowell RS. A general method for constraining short peptides to an α-helical conformation. J Am Chem Soc. 1997;119:455–460. [Google Scholar]
- 18.Geistlinger TR, Guy RK. Novel selective inhibitors of the interaction of individual nuclear hormone receptors with a mutually shared steroid receptor coactivator 2. J Am Chem Soc. 2003;125:6852–6853. doi: 10.1021/ja0348391. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto K, Kajino M, Inouye M. Development of a series of cross-linking agents that effectively stabilize α-helical structures in various short peptides. Chem-Eur J. 2008;14:857–863. doi: 10.1002/chem.200700843. [DOI] [PubMed] [Google Scholar]
- 20.Verdine GL, Hilinski GJ. Stapled Peptides for Intracellular Drug Targets. Methods Enzymol: Protein engineering for therapeutics. 2012;503:3–33. doi: 10.1016/B978-0-12-396962-0.00001-X. [DOI] [PubMed] [Google Scholar]
- 21.Shim SY, Kim YW, Verdine GL. A new i, i+3 peptide stapling system for alpha-helix stabilization. Chem Biol Drug Des. 2013;82:635–642. doi: 10.1111/cbdd.12231. [DOI] [PubMed] [Google Scholar]
- 22.Rao T, Ruiz-Gomez G, Hill TA, Hoang HN, Fairlie DP, Mason JM. Truncated and helix-constrained peptides with high affinity and specificity for the cFos coiled-coil of AP-1. PLoS One. 2013;8:e59415. doi: 10.1371/journal.pone.0059415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank AO, Vangamudi B, Feldkamp MD, Souza-Fagundes EM, Luzwick JW, Cortez D, Olejniczak ET, Waterson AG, Rossanese OW, Chazin WJ, Fesik SW. Discovery of a potent stapled helix peptide that binds to the 70N domain of replication protein a. J Med Chem. 2014;57:2455–2461. doi: 10.1021/jm401730y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabezas E, Satterthwait AC. The hydrogen bond mimic approach: Solid-phase synthesis of a peptide stabilized as an α-helix with a hydrazone link. J Am Chem Soc. 1999;121:3862–3875. [Google Scholar]
- 25.Haney CM, Horne WS. Dynamic covalent side-chain cross-links via intermolecular oxime or hydrazone formation from bifunctional peptides and simple organic linkers. J Pept Sci. 2014;20:108–114. doi: 10.1002/psc.2596. [DOI] [PubMed] [Google Scholar]
- 26.Haney CM, Loch MT, Horne WS. Promoting peptide [small alpha]-helix formation with dynamic covalent oxime side-chain cross-links. Chem Commun. 2011;47:10915–10917. doi: 10.1039/c1cc12010g. [DOI] [PubMed] [Google Scholar]
- 27.Haney CM, Home WS. Oxime side-chain cross-links in an -helical coiled-coil protein: Structure, thermodynamics, and folding-templated synthesis of bicyclic species. Chem-Eur J. 2013;19:11342–11351. doi: 10.1002/chem.201300506. [DOI] [PubMed] [Google Scholar]
- 28.Szewczuk Z, Rebholz KL, Rich DH. Synthesis and biological-activity of new conformationally restricted analogs of pepstatin. Int J Pept Protein Res. 1992;40:233–242. doi: 10.1111/j.1399-3011.1992.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 29.Walker MA, Johnson T. General method for the synthesis of cyclic peptidomimetic compounds. Tetrahedron Lett. 2001;42:5801–5804. [Google Scholar]
- 30.Timmerman P, Beld J, Puijk WC, Meloen RH. Rapid and quantitative cyclization of multiple peptide loops onto synthetic scaffolds for structural mimicry of protein surfaces. ChemBioChem. 2005;6:821–824. doi: 10.1002/cbic.200400374. [DOI] [PubMed] [Google Scholar]
- 31.Muppidi A, Wang ZY, Li XL, Chen JD, Lin Q. Achieving cell penetration with distance-matching cysteine cross-linkers: A facile route to cell-permeable peptide dual inhibitors of Mdm2/Mdmx. Chem Commun. 2011;47:9396–9398. doi: 10.1039/c1cc13320a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo H, Meinhardt N, Wu YB, Kulkarni S, Hu XZ, Low KE, Davies PL, DeGrado WF, Greenbaum DC. Development of alpha-helical calpain probes by mimicking a natural protein-protein interaction. J Am Chem Soc. 2012;134:17704–17713. doi: 10.1021/ja307599z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patgiri A, Jochim AL, Arora PS. A hydrogen bond surrogate approach for stabilization of short peptide sequences in alpha-helical conformation. Acc Chem Res. 2008;41:1289–1300. doi: 10.1021/ar700264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, Chen K, Kulp JL, Arora PS. Evaluation of biologically relevant short α-helices stabilized by a main-chain hydrogen-bond surrogate. J Am Chem Soc. 2006;128:9248–9256. doi: 10.1021/ja062710w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimartino G, Wang D, Chapman RN, Arora PS. Solid-phase synthesis of hydrogen-bond surrogate-derived α-helices. Org Lett. 2005;7:2389–2392. doi: 10.1021/ol0506516. [DOI] [PubMed] [Google Scholar]
- 36.Byrne A, Kier BL, Williams DV, Scian M, Andersen NH. Circular permutation of the trp-cage: Fold rescue upon addition of a hydrophobic staple. RSC Adv. 2013;3:19824–19829. doi: 10.1039/C3RA43674H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke J, Fersht AR. Engineered disulfide bonds as probes of the folding pathway of barnase: Increasing the stability of proteins against the rate of denaturation. Biochemistry. 1993;32:4322–4329. doi: 10.1021/bi00067a022. [DOI] [PubMed] [Google Scholar]
- 38.Grantcharova VP, Riddle DS, Baker D. Long-range order in the src SH3 folding transition state. Proc Natl Acad Sci USA. 2000;97:7084–7089. doi: 10.1073/pnas.97.13.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Lau WL, DeGrado WF, Gai F. T-jump infrared study of the folding mechanism of coiled-coil GCN4-p1. Biophys J. 2005;89:4180–4187. doi: 10.1529/biophysj.105.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du DG, Gai F. Understanding the folding mechanism of an alpha-helical hairpin. Biochemistry. 2006;45:13131–13139. doi: 10.1021/bi0615745. [DOI] [PubMed] [Google Scholar]
- 41.Shandiz AT, Capraro BR, Sosnick TR. Intramolecular cross-linking evaluated as a structural probe of the protein folding transition state. Biochemistry. 2007;46:13711–13719. doi: 10.1021/bi701042e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung HS, Shandiz A, Sosnick TR, Tokmakoff A. Probing the folding transition state of ubiquitin mutants by temperature-jump-induced downhill unfolding. Biochemistry. 2008;47:13870–13877. doi: 10.1021/bi801603e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krantz BA, Sosnick TR. Engineered metal binding sites map the heterogeneous folding landscape of a coiled coil. Nat Struct Biol. 2001;8:1042–1047. doi: 10.1038/nsb723. [DOI] [PubMed] [Google Scholar]
- 44.Bosco GL, Baxa M, Sosnick TR. Metal binding kinetics of bi-histidine sites used in Ψ analysis: Evidence of high-energy protein folding intermediates. Biochemistry. 2009;48:2950–2959. doi: 10.1021/bi802072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fersht AR, Sato S. Phi-value analysis and the nature of protein-folding transition states. Proc Natl Acad Sci USA. 2004;101:7976–7981. doi: 10.1073/pnas.0402684101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moran LB, Schneider JP, Kentsis A, Reddy GA, Sosnick TR. Transition state heterogeneity in GCN4 coiled coil folding studied by using multisite mutations and crosslinking. Proc Natl Acad Sci USA. 1999;96:10699–10704. doi: 10.1073/pnas.96.19.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano AL, Tucker MJ, Gai F. Direct assessment of the alpha-helix nucleation time. J Phys Chem B. 2011;115:7472–7478. doi: 10.1021/jp200628b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markiewicz BN, Yang L, Culik RM, Gao YQ, Gai F. How quickly can a beta-hairpin fold from its transition state? J Phys Chem B. 2014;118:3317–3325. doi: 10.1021/jp500774q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du D, Tucker MJ, Gai F. Understanding the mechanism of beta-hairpin folding via phi-value analysis. Biochemistry. 2006;45:2668–2678. doi: 10.1021/bi052039s. [DOI] [PubMed] [Google Scholar]
- 50.Du D, Zhu Y, Huang CY, Gai F. Understanding the key factors that control the rate of beta-hairpin folding. Proc Natl Acad Sci USA. 2004;101:15915–15920. doi: 10.1073/pnas.0405904101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markiewicz BN, Jo H, Culik RM, DeGrado WF, Gai F. Assessment of local friction in protein folding dynamics using a helix cross-linker. J Phys Chem B. 2013;117:14688–14696. doi: 10.1021/jp409334h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamm P, Helbing J, Bredenbeck J. Two-dimentional infrared spectrscopy of photoswitchable peptides. Annu Rev Phys Chem. 2008;59:291–317. doi: 10.1146/annurev.physchem.59.032607.093757. [DOI] [PubMed] [Google Scholar]
- 53.Volk M, Kholodenko Y, Lu HSM, Gooding EA, DeGrado WF, Hochstrasser RM. Peptide conformational dynamics and vibrational stark effects following photoinitiated disulfide cleavage. J Phys Chem B. 1997;101:8607–8616. [Google Scholar]
- 54.Kolano C, Helbing J, Bucher G, Sander W, Hamm P. Intramolecular disulfide bridges as a phototrigger to monitor the dynamics of small cyclic peptides. J Phys Chem B. 2007;111:11297–11302. doi: 10.1021/jp074184g. [DOI] [PubMed] [Google Scholar]
- 55.Xie JB, Cao Y, Pan H, Qin M, Yan ZQ, Xiong X, Wang W. Photoinduced fibrils formation of chicken egg white lysozyme under native conditions. Proteins. 2012;80:2501–2513. doi: 10.1002/prot.24132. [DOI] [PubMed] [Google Scholar]
- 56.Waegele MM. Dissertation for the Doctoral Degree. Philadelphia: University of Pennsylvania; 2011. On the folding and conformation of peptides and the development of novel methods for their study. [Google Scholar]
- 57.Vanhooren A, Devreese B, Vanhee K, Van Beeumen J, Hanssens IA. Photoexcitation of tryptophan groups induces reduction of two disufide bonds in goat alpha-lactalbumin. Biochemistry. 2002;41:11035–11043. doi: 10.1021/bi0258851. [DOI] [PubMed] [Google Scholar]
- 58.Kehoe JJ, Remondetto GE, Subirade M, Morris ER, Brodkorb A. Tryptophan-mediated denaturation of beta-lactoglobulin A by UV irradiation. J Agric Food Chem. 2008;56:4720–4725. doi: 10.1021/jf0733158. [DOI] [PubMed] [Google Scholar]
- 59.Wu LZ, Sheng YB, Xie JB, Wang W. Photoexcitation of tryptophan groups induced reduction of disulfide bonds in hen egg white lysozyme. J Mol Struct. 2008;882:101–106. [Google Scholar]
- 60.Neves-Petersen MT, Klitgaard S, Pascher T, Skovsen E, Polivka T, Yartsev A, Sundstrom V, Petersen SB. Flash photolysis of cutinase: Identification and decay kinetics of transient intermediates formed upon UV excitation of aromatic residues. Biophys J. 2009;97:211–226. doi: 10.1016/j.bpj.2009.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hansen KC, Rock RS, Larsen RW, Chan SI. A method for photoinitating protein folding in a nondenaturing environment. J Am Chem Soc. 2000;122:11567–11568. [Google Scholar]
- 62.Tucker MJ, Courier JR, Chen JX, Atasoylu O, Smith AB, Hochstrasser RM. Tetrazine phototriggers: Probes for peptide dynamics. Angew Chem, Int Ed. 2010;49:3612–3616. doi: 10.1002/anie.201000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tadesse L, Nazarbaghi R, Walters L. Isotopically enhanced infrared-spectroscopy - a novel method for examining secondary structure at specific sites in conformationally heterogeneous peptides. J Am Chem Soc. 1991;113:7036–7037. [Google Scholar]
- 64.Tucker MJ, Abdo M, Courier JR, Chen JX, Brown SP, Smith AB, Hochstrasser RM. Nonequilibrium dynamics of helix reorganization observed by transient 2D IR spectroscopy. Proc Natl Acad Sci USA. 2013;110:17314–17319. doi: 10.1073/pnas.1311876110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bochet CG. Photolabile protecting groups and linkers. J Chem Soc. 2002:125–142. [Google Scholar]
- 66.Loudwig S, Bayley H. Light-activated proteins: An overview. In: Goeldner M, Givens R, editors. Dynamic studies in biology: Phototriggers, photoswitches and caged biomolecules. Weinheim, Germany: WILEY-VCH Verlag GmbH & Co. KGaA; 2005. pp. 253–303. [Google Scholar]
- 67.Mayer G, Heckel A. Biologically active molecules with a “light switch”. Angew Chem, Int Ed. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, Pomper MG, Yu SM. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci USA. 2012;109:14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirota S, Fujimoto Y, Choi J, Baden N, Katagiri N, Akiyama M, Hulsker R, Ubbink M, Okajima T, Takabe T, Funasaki N, Watanabe Y, Terazima M. Conformational changes during apoplastocyanin folding observed by photocleavable modification and transient grating. J Am Chem Soc. 2006;128:7551–7558. doi: 10.1021/ja058788e. [DOI] [PubMed] [Google Scholar]
- 70.Chen HL, Hsu JCC, Man HV, Li MS, Hu CK, Liu CH, Luh FY, Chen SSW, Chang ESH, Wang AHJ, Hsu MF, Fann W, Chen RPY. Studying submicrosecond protein folding kinetics using a photolabile caging strategy and time-resolved photoacoustic calorimetry. Proteins. 2010;78:2973–2983. doi: 10.1002/prot.22823. [DOI] [PubMed] [Google Scholar]
- 71.Deeg AA, Rampp MS, Popp A, Pilles BM, Schrader TE, Moroder L, Hauser K, Zinth W. Isomerization- and temperature-jump-induced dynamics of a photoswitchable β-hairpin. Chem-Eur J. 2013;27:387–391. doi: 10.1002/chem.201303189. [DOI] [PubMed] [Google Scholar]
- 72.Guerrero L, Smart OS, Woolley GA, Allemann RK. Photocontrol of DNA binding specificity of a miniature engrailed homeodomain. J Am Chem Soc. 2005;127:15624–15629. doi: 10.1021/ja0550428. [DOI] [PubMed] [Google Scholar]
- 73.Jurt S, Aemissegger A, Guntert P, Zerbe O, Hilvert D. A photoswitchable miniprotein based on the sequence of avian pancreatic polypeptide. Angew Chem, Int Ed. 2006;45:6297–6300. doi: 10.1002/anie.200602084. [DOI] [PubMed] [Google Scholar]
- 74.Pfister R, Ihalainen J, Hamm P, Kolano C. Synthesis, characterization and applicability of three isotope labeled azobenzene photoswitches. Org Biomol Chem. 2008;6:3508–3517. doi: 10.1039/b804568b. [DOI] [PubMed] [Google Scholar]
- 75.Hoppmann C, Schmieder P, Heinrich N, Beyermann M. Photoswitchable click amino acids: Light control of conformation and bioactivity. ChemBioChem. 2011;12:2555–2559. doi: 10.1002/cbic.201100578. [DOI] [PubMed] [Google Scholar]
- 76.Ali AM, Woolley GA. The effect of azobenzene cross-linker position on the degree of helical peptide photo-control. Org Biomol Chem. 2013;11:5325–5331. doi: 10.1039/c3ob40684a. [DOI] [PubMed] [Google Scholar]
- 77.Korbus M, Balasubramanian G, Muller-Plathe F, Kolmar H, Meyer-Almes FJ. Azobenzene switch with a long-lived cis-state to photocontrol the enzyme activity of a histone deacetylase-like amidohydrolase. Biol Chem. 2014;395:401–412. doi: 10.1515/hsz-2013-0246. [DOI] [PubMed] [Google Scholar]
- 78.Sension RJ, Repinec ST, Szarka AZ, Hochstrasser RM. Femtosecond laser studies of the cis-stilbene photoisomerization reactions. J Chem Phys. 1993;98:6291–6315. [Google Scholar]
- 79.Erdelyi M, Karlen A, Gogoll A. A new tool in peptide engineering: A photoswitchable stilbene-type beta-hairpin mimetic. Chem-Eur J. 2006;12:403–412. doi: 10.1002/chem.200500648. [DOI] [PubMed] [Google Scholar]
- 80.Varedian M, Erdelyi M, Persson A, Gogoll A. Interplaying factors for the formation of photoswitchable beta-hairpins: The advantage of a flexible switch. J Pept Sci. 2009;15:107–113. doi: 10.1002/psc.1103. [DOI] [PubMed] [Google Scholar]
- 81.Lednev IK, Ye TQ, Hester RE, Moore JN. Femtosecond time-resolved UV-visible absorption spectroscopy of trans-azobenzene in solution. J Phys Chem. 1996;100:13338–13341. [Google Scholar]
- 82.Sporlein S, Carstens H, Satzger H, Renner C, Behrendt R, Moroder L, Tavan P, Zinth W, Wachtveitl J. Ultrafast spectroscopy reveals subnanosecond peptide conformational dynamics and validates molecular dynamics simulation. Proc Natl Acad Sci USA. 2002;99:7998–8002. doi: 10.1073/pnas.122238799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumita JR, Smart OS, Woolley GA. Photo-control of helix content in a short peptide. Proc Natl Acad Sci USA. 2000;97:3803–3808. doi: 10.1073/pnas.97.8.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bredenbeck J, Helbing J, Kumita JR, Woolley GA, Hamm P. Alpha-helix formation in a photoswitchable peptide tracked from picoseconds to microseconds by time-resolved ir spectroscopy. Proc Natl Acad Sci USA. 2005;102:2379–2384. doi: 10.1073/pnas.0406948102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen EF, Kumita JR, Woolley GA, Kliger DS. The kinetics of helix unfolding of an azobenzene cross-linked peptide probed by nanosecond time-resolved optical rotatory dispersion. J Am Chem Soc. 2003;125:12443–12449. doi: 10.1021/ja030277+. [DOI] [PubMed] [Google Scholar]
- 86.Samanta S, Qin CG, Lough AJ, Woolley GA. Bidirectional photocontrol of peptide conformation with a bridged azobenzene derivative. Angew Chem, Int Ed. 2012;51:6452–6455. doi: 10.1002/anie.201202383. [DOI] [PubMed] [Google Scholar]
- 87.Ciardelli F, Fabbri D, Pieroni O, Fissi A. Photomodulation of polypeptide conformation by sunlight in spiropyran-containing poly(l-glutamic acid) J Am Chem Soc. 1989;111:3470–3472. [Google Scholar]
- 88.Frank R, Jakob M, Thunecke F, Fischer G, Schutkowski M. Thioxylation as one-atom-substitution generates a photoswitchable element within the peptide backbone. Angew Chem, Int Ed. 2000;39:1120–1122. [PubMed] [Google Scholar]
- 89.Zhao JZ, Micheau JC, Vargas C, Schiene-Fischer C. Cis/trans photoisomerization of secondary thiopeptide bonds. Chem-Eur J. 2004;10:6093–6101. doi: 10.1002/chem.200400400. [DOI] [PubMed] [Google Scholar]
- 90.Cervetto V, Pfister R, Helbing J. Time-resolved infrared spectroscopy of thiopeptide isomerization and hydrogen-bond breaking. Journal J Phys Chem B. 2008;112:3540–3544. doi: 10.1021/jp710611n. [DOI] [PubMed] [Google Scholar]
- 91.Serrano AL, Waegele MM, Gai F. Spectroscopic studies of protein folding: Linear and nonlinear methods. Protein Sci. 2012;21:157–170. doi: 10.1002/pro.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilmanshin R, Williams S, Callender RH, Woodruff WH, Dyer RB. Fast events in protein folding: Relaxation dynamics of secondary and tertiary structure in native apomyoglobin. Proc Natl Acad Sci USA. 1997;94:3709–3713. doi: 10.1073/pnas.94.8.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gruebele M, Sabelko J, Ballew R, Ervin J. Laser temperature jump induced protein refolding. Acc Chem Res. 1998;31:699–707. [Google Scholar]
- 94.Clark JH, Shapiro SL, Campillo AJ, Winn KR. Picosecond studies of excited-state protonation and deprotonation kinetics - laser pH jump. J Am Chem Soc. 1979;101:746–748. [Google Scholar]
- 95.Pines E, Huppert D, Gutman M, Nachliel N, Fishman M. The pOH jump - determination of deprotonation rates of water by 6-methoxyquinoline and acridine. J Phys Chem. 1986;90:6366–6370. [Google Scholar]
- 96.Abbruzzetti S, Crema E, Masino L, Vecli A, Viappiani C, Small JR, Libertini LJ, Small EW. Fast events in protein folding: Structural volume changes accompanying the early events in the n -> i transition of apomyoglobin induced by ultrafast pH jump. Biophys J. 2000;78:405–415. doi: 10.1016/S0006-3495(00)76603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jones CM, Henry ER, Hu Y, Chan CK, Luck SD, Bhuyan A, Roder H, Hofrichter J, Eaton WA. Fast events in protein-folding initiated by nanosecond laser photolysis. Proc Natl Acad Sci USA. 1993;90:11860–11864. doi: 10.1073/pnas.90.24.11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pascher T, Chesick JP, Winkler JR, Gray HB. Protein folding triggered by electron transfer. Science. 1996;271:1558–1560. doi: 10.1126/science.271.5255.1558. [DOI] [PubMed] [Google Scholar]
- 99.Huang CY, He S, DeGrado WF, McCafferty DG, Gai F. Light-induced helix formation. J Am Chem Soc. 2002;124:12674–12675. doi: 10.1021/ja028084u. [DOI] [PubMed] [Google Scholar]
- 100.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 101.Dobson CM. Protein misfolding, evolution and disease. Trends Biochem Sci. 1999;24:329–332. doi: 10.1016/s0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 102.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 103.Knowles TPJ, Buehler MJ. Nanomechanics of functional and pathological amyloid materials. Nat Nanotechnol. 2011;6:469–479. doi: 10.1038/nnano.2011.102. [DOI] [PubMed] [Google Scholar]
- 104.Loo Y, Zhang S, Hauser CA. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol Adv. 2012;30:593–603. doi: 10.1016/j.biotechadv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 105.Waldauer SA, Hassan S, Paoli B, Donaldson PM, Pfister R, Hamm P, Caflisch A, Pellarin R. Photocontrol of reversible amyloid formation with a minimal-design peptide. J Phys Chem B. 2012;116:8961–8973. doi: 10.1021/jp305311z. [DOI] [PubMed] [Google Scholar]
- 106.Deeg AA, Schrader TE, Pfizer J, Moroder L, Zinth W. Amyloid-like structures formed by azobenzene-peptides: Light-triggered disassembly. Spectrosc Int J. 2013;7:209–213. [Google Scholar]
- 107.Doran TM, Anderson EA, Latchney SE, Opanashuk LA, Nilsson BL. An azobenzene photoswitch sheds light on turn nucleation in amyloid-β self-assembly. ACS Chem Neurosci. 2012;3:211–220. doi: 10.1021/cn2001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamill AC, Lee CT. Photocontrol of beta-amyloid peptide (1-40) fibril growth in the presence of a photosurfactant. J Phys Chem B. 2009;113:6164–6172. doi: 10.1021/jp8080113. [DOI] [PubMed] [Google Scholar]
- 109.Taniguchi A, Skwarczynski M, Sohma Y, Okada T, Ikeda K, Prakash H, Mukai H, Hayashi Y, Kimura T, Hirota S, Matsuzaki K, Kiso Y. Controlled production of amyloid beta peptide from a photo-triggered, water-soluble precursor “click peptide”. ChemBioChem. 2008;9:3055–3065. doi: 10.1002/cbic.200800503. [DOI] [PubMed] [Google Scholar]
- 110.Measey TJ, Gai F. Light-triggered disassembly of amyloid fibrils. Langmuir. 2012;28:12588–12592. doi: 10.1021/la302626d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meijer JT, Henckens M, Minten IJ, Lowik D, van Hest JCM. Disassembling peptide-based fibres by switching the hydrophobic-hydrophilic balance. Soft Matter. 2007;3:1135–1137. doi: 10.1039/b708847g. [DOI] [PubMed] [Google Scholar]
- 112.Loewik D, Meijer JT, Minten IJ, Van Kalkeren H, Heckenmuller L, Schulten I, Sliepen K, Smittenaar P, Van Hest JCM. Controlled disassembly of peptide amphiphile fibres. J Pept Sci. 2008;14:127–133. doi: 10.1002/psc.969. [DOI] [PubMed] [Google Scholar]
- 113.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 114.Matsuzawa Y, Ueki K, Yoshida M, Tamaoki N, Nakamura T, Sakai H, Abe M. Assembly and photoinduced organization of mono- and oligopeptide molecules containing an azobenzene moiety. Adv Funct Mater. 2007;17:1507–1514. [Google Scholar]
- 115.Huang YC, Qiu ZJ, Xu YM, Shi JF, Lin HK, Zhang Y. Supramolecular hydrogels based on short peptides linked with conformational switch. Org Biomol Chem. 2011;9:2149–2155. doi: 10.1039/c0ob01057j. [DOI] [PubMed] [Google Scholar]
- 116.Lin YY, Qiao Y, Tang PF, Li ZB, Huang JB. Controllable self-assembled laminated nanoribbons from dipeptide-amphiphile bearing azobenzene moiety. Soft Matter. 2011;7:2762–2769. [Google Scholar]
- 117.Qiu ZJ, Yu HT, Li JB, Wang Y, Zhang Y. Spiropyran-linked dipeptide forms supramolecular hydrogel with dual responses to light and to ligand-receptor interaction. Chem Commun. 2009;23:3342–3344. doi: 10.1039/b822840j. [DOI] [PubMed] [Google Scholar]
- 118.Doran TM, Ryan DM, Nilsson BL. Reversible photocontrol of self-assembled peptide hydrogel viscoelasticity. Polym Chem. 2014;5:241–248. [Google Scholar]
- 119.He MT, Li JB, Tan S, Wang RZ, Zhang Y. Photodegradable supramolecular hydrogels with fluorescence turn-on reporter for photomodulation of cellular microenvironments. J Am Chem Soc. 2013;135:18718–18721. doi: 10.1021/ja409000b. [DOI] [PubMed] [Google Scholar]
- 120.Haines LA, Rajagopal K, Ozbas B, Salick DA, Pochan DJ, Schneider JP. Light-activated hydrogel formation via the triggered folding and self-assembly of a designed peptide. J Am Chem Soc. 2005;127:17025–17029. doi: 10.1021/ja054719o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Measey TJ, Markiewicz BN, Gai F. Amide I band and photoinduced disassembly of a peptide hydrogel. Chem Phys Lett. 2013;580:135–140. doi: 10.1016/j.cplett.2013.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Griffin DR, Schlosser JL, Lam SF, Nguyen TH, Maynard HD, Kasko AM. Synthesis of photodegradable macromers for conjugation and release of bioactive molecules. Biomacromolecules. 2013;14:1199–1207. doi: 10.1021/bm400169d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater. 2004;3:249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 124.Muraoka T, Koh CY, Cui H, Stupp SI. Light-triggered bioactivity in three dimensions. Angew Chem, Int Ed. 2009;48:5946–5949. doi: 10.1002/anie.200901524. [DOI] [PubMed] [Google Scholar]
- 125.Goubko CA, Majumdar S, Basak A, Cao XD. Hydrogel cell patterning incorporating photocaged RGDS peptides. Biomed Microdevices. 2010;12:555–568. doi: 10.1007/s10544-010-9412-7. [DOI] [PubMed] [Google Scholar]
- 126.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 2011;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raeburn J, McDonald TO, Adams DJ. Dipeptide hydrogelation triggered via ultraviolet light. Chem Commun. 2012;48:9355–9357. doi: 10.1039/c2cc34677j. [DOI] [PubMed] [Google Scholar]
- 128.Collier JH, Hu BH, Ruberti JW, Zhang J, Shum P, Thompson DH, Messersmith PB. Thermally and photochemically triggered self-assembly of peptide hydrogels. J Am Chem Soc. 2001;123:9463–9464. doi: 10.1021/ja011535a. [DOI] [PubMed] [Google Scholar]
- 129.Ellis-Davies GCR. Caged compounds: Photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee HM, Larson DR, Lawrence DS. Illuminating the chemistry of life: Design, synthesis, and applications of “caged” and related photoresponsive compounds. ACS Chem Bio. 2009;4:409–427. doi: 10.1021/cb900036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goguen BN, Aemissegger A, Imperiali B. Sequential activation and deactivation of protein function using spectrally differentiated caged phosphoamino acids. Journal of the American Chemical Society. 2011;133:11038–11041. doi: 10.1021/ja2028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Goguen BN, Imperiali B. Chemical tools for studying directed cell migration. ACS Chem Bio. 2011;6:1164–1174. doi: 10.1021/cb200299k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rusiecki VK, Warne SA. Synthesis of Nα-Fmoc-Nε-Nvoc-lysine and use in the preparation of selectively functionalized peptides. Bioorg Med Chem Lett. 1993;3:707–710. [Google Scholar]
- 134.Marriott G. Caged protein conjugates and light-directed generation of protein activity: Preparation, photoactivation, and spectroscopic characterization of caged G-actin conjugates. Biochemistry. 1994;33:9092–9097. doi: 10.1021/bi00197a010. [DOI] [PubMed] [Google Scholar]
- 135.Lin Y, Wang Q. Caged peptides to control enzymatic activity within hydrogel scaffolds. ChemBioChem. 2014;15:787–788. doi: 10.1002/cbic.201400071. [DOI] [PubMed] [Google Scholar]
- 136.Umezawa N, Noro Y, Ukai K, Kato N, Higuchi T. Photocontrol of peptide function: Backbone cyclization strategy with photocleavable amino acid. ChemBioChem. 2011;12:1694–1698. doi: 10.1002/cbic.201100212. [DOI] [PubMed] [Google Scholar]
- 137.Zou K, Miller WT, Givens RS, Bayley H. Caged thiophosphotyrosine peptides. Angew Chem, Int Ed. 2001;40:3049–3051. doi: 10.1002/1521-3773(20010817)40:16<3049::AID-ANIE3049>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 138.Kohse S, Neubauer A, Pazidis A, Lochbrunner S, Kragl U. Photoswitching of enzyme activity by laser-induced ph-jump. J Am Chem Soc. 2013;135:9407–9411. doi: 10.1021/ja400700x. [DOI] [PubMed] [Google Scholar]
- 139.Watai Y, Sase I, Shiono H, Nakano Y. Regulation of nuclear import by light-induced activation of caged nuclear localization signal in living cells. FEES Lett. 2001;488:39–44. doi: 10.1016/s0014-5793(00)02399-1. [DOI] [PubMed] [Google Scholar]
- 140.Pirrung MC, Drabik SJ, Ahamed J, Ali H. Caged chemotactic peptides. Bioconjugate Chem. 2000;11:679–681. doi: 10.1021/bc000001g. [DOI] [PubMed] [Google Scholar]
- 141.Mizukami S, Hosoda M, Satake T, Okada S, Hori Y, Furuta T, Kikuchi K. Photocontrolled compound release system using caged antimicrobial peptide. J Am Chem Soc. 2010;132:9524–9525. doi: 10.1021/ja102167m. [DOI] [PubMed] [Google Scholar]
- 142.Vázquez ME, Nitz M, Stehn J, Yaffe MB, Imperiali B. Fluorescent caged phosphoserine peptides as probes to investigate phosphorylation-dependent protein associations. J Am Chem Soc. 2003;125:10150–10151. doi: 10.1021/ja0351847. [DOI] [PubMed] [Google Scholar]
- 143.Nguyen A, Rothman DM, Stehn J, Imperiali B, Yaffe MB. Caged phosphopeptides reveal a temporal role for 14-3-3 in G1 arrest and S-phase checkpoint function. Nat Biotechnol. 2004;22:993–1000. doi: 10.1038/nbt997. [DOI] [PubMed] [Google Scholar]
- 144.Tang X, Dmochowski IJ. Regulating gene expression with light-activated oligonucleotides. Mol Biosyst. 2007;3:100–110. doi: 10.1039/b614349k. [DOI] [PubMed] [Google Scholar]
- 145.Liu QY, Deiters A. Optochemical control of deoxyoligonucleotide function via a nucleobase-caging approach. Acc Chem Res. 2014;47:45–55. doi: 10.1021/ar400036a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Callaway EM, Yuste R. Stimulating neurons with light. Curr Opin Neurobiol. 2002;12:587–592. doi: 10.1016/s0959-4388(02)00364-1. [DOI] [PubMed] [Google Scholar]
- 147.Rea AC, Vandenberg LN, Ball RE, Snouffer AA, Hudson AG, Zhu Y, McLain DE, Johnston LL, Lauderdale JD, Levin M, Dore TM. Light-activated serotonin for exploring its action in biological systems. Chemistry & biology. 2013;20:1536–1546. doi: 10.1016/j.chembiol.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin W, Albanese C, Pestell RG, Lawrence DS. Spatially discrete, light-driven protein expression. Chem Biol. 2002;9:1347–1353. doi: 10.1016/s1074-5521(02)00288-0. [DOI] [PubMed] [Google Scholar]
- 149.Pelliccioli AP, Wirz J. Photoremovable protecting groups: Reaction mechanisms and applications. Photochem Photobiol Sci. 2002;1:441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 150.Olson JP, Banghart MR, Sabatini BL, Ellis-Davies GCR. Spectral evolution of a photochemical protecting group for orthogonal two-color uncaging with visible light. J Am Chem Soc. 2013;135:15948–15954. doi: 10.1021/ja408225k. [DOI] [PMC free article] [PubMed] [Google Scholar]


