Abstract
There is currently extensive discussion and debate in the literature on how, when, and to whom genetic research results should be returned (see Genetics in Medicine, April 2012 issue). Here, we describe our experience in disclosing genetic information on Mendelian disorders discovered during the course of our research in the Hutterites. We first assessed attitudes toward the disclosure of carrier results, which revealed that many individuals wanted carrier information and that many intended to use the information in family planning. Based on this information, we developed a pilot study to test and disclose cystic fibrosis (CF) carrier status. Next, a larger scale project was developed in order to disclose genetic research results for 14 diseases to those interested in receiving the information. We developed brochures, offered a live interactive educational program, conducted a consent process, and disclosed results in letters mailed to the consented individuals. Overall, ∼80% of individuals who participated in the educational program signed consent forms for the release of their results for 14 diseases. We describe our experience with returning individual genetic research results to participants in a population-based research study.
Keywords: disclosure, genetic, results, research, individual, Hutterite
Introduction
The North American Hutterites are one of the best-characterized young founder populations (Boycott et al., 2008; Hostetler, 1974; Ober, Abney, & McPeek, 2001; Steinberg, Bleibtreu, Kurczynski, Martin, & Kurczynski, 1967), with more than 36 autosomal recessive (AR) diseases reported in members of this population (Beaulieu et al., 2013; Bogershausen et al., 2013; Boycott et al., 2010; Boycott et al., 2008; Caliskan et al., 2011; Gerull et al., 2013; Huang et al., 2011; Wiltshire, Hegele, Innes, & Brownell, 2013), such as Joubert syndrome, restrictive dermopathy, and nonsyndromic deafness. The Hutterites are an Anabaptist religious group that originated during the 1500s in the Tyrolean Alps. To escape religious persecution, the Hutterites lived throughout central and eastern Europe for the next 300+ years. In the 1870s they migrated from Russia to the United States and settled on three communal farms (called colonies) in what is now South Dakota. These three colonies gave rise to the three major subdivisions of the Hutterites, referred to as the Schmiedeleut (S-leut), Lehrerleut (L-leut), and Dariusleut (D-leut). The population has since undergone rapid expansion, and today >400 Hutterite colonies of all three ‘leut’ are located in the north central plain states of the U.S. and western provinces of Canada; marriages between leut have been uncommon for at least the past 90 years due to relatively subtle cultural differences between them. Detailed genealogical records that extend back to the early 1700's during their tenure in Russia trace the >40,000 extant members of this founder population to fewer than 90 ancestors (Martin, 1970). The Hutterites utilize outside medical care, but access to genetic testing has been largely limited.
Our studies have focused on the United States S-leut Hutterites who live on colonies primarily in South Dakota, but also in North Dakota and Minnesota. In general, the S-leut Hutterites we have encountered are very interested in participating in research. We had previously obtained broad consent (and assent from children under the age of 18 years) “to study common diseases and conditions that run in families” from participants in our research that spanned nearly 30 years. Because the focus of our studies was primarily on common, complex diseases (e.g., asthma) or phenotypes (e.g., fertility), we also included language in the consent forms indicating that there would be no disclosure of individual genetic research results to study subjects, as had been standard practice at the time (Wolf, 2012). We did not know if study participants would want genetic research results for Mendelian conditions or whether they would understand the implications of genetic results.
There is currently extensive discussion and debate in the literature on how, when, and to whom genetic research results should be returned (see Genetics in Medicine, April 2012 issue). We report here our experience with returning research results to a small, closed population. There is a lack of reports specifically dealing with this type of population, and others considering a similar approach can use the experience shared here.
Motivation for these Studies
The primary investigator for our research team has a long-term relationship with the Hutterites and had often been told by the individuals in the study that they would want to know any results that would be important for them. Based on these informal conversations, we initiated ongoing discussions with our local ethicists and members of our Human Subjects Institutional Review Board (IRB) with the goal of implementing an approach that could eventually allow us to offer individual genetic research results for some Mendelian conditions. The overall design of our approach ultimately included (1) a survey to assess the Hutterites' attitudes towards cystic fibrosis (CF; OMIM 219700) carrier testing, (2) a pilot study on disclosure of CF results, (3) an educational session on genetic diseases in the Hutterites, and (4) disclosure of research results for 15 mutations causing 14 autosomal recessive disorders to all consenting adults primarily via letters sent by mail. Our plan was motivated by and drew upon recommendations from the literature [summarized in (Wolf et al., 2012)] as well as the Hutterites' stated desire to receive results. We obtained IRB approval prior to each of the four parts of the study outlined above.
Survey of Attitudes Towards Cystic Fibrosis Carrier Testing
Our approach for disclosure of genetic results began with a survey that was administered during face-to-face interviews between 2008 and 2009 to 86 adult (≥18 years) Hutterites (62% female; 76% married) (Table I). We asked several questions to assess their attitudes toward CF carrier testing. We focused on CF in this survey because it is common in the Hutterites (carrier frequency is 1 in 11 for either one of the two mutations present in this population), many Hutterites know families who have children with CF and are therefore overall more familiar with CF than most of the other diseases (e.g., 77% of those surveyed knew someone with CF), and many Hutterites had previously inquired about whether we could tell them if they were CF carriers. Forty-one percent of interviewed adults stated that they would want to know their carrier status, 28% were unsure at the time of the interview, and 31% stated they did not want to know. The most common reasons for wanting test results (in an open-ended question) were “just to know” (63%), “to avoid having a child with CF” (11%), and “having a relative with CF” (11%). The most common reason for not wanting test results was the belief that they were not at risk because they “felt fine,” “did not have a family history,” or “did not think it necessary” (40%). Other reasons included “just doesn't want to know” (33%) and “family is completed” (27%). When asked, “If you and your spouse were both carriers, would this affect your family planning,” 65% of all subjects answered yes, 15% answered no, and 20% were unsure. Moreover, the surveyed individuals expressed openness about sharing results of carrier status with spouses or girl/boyfriend (80%), siblings (79%), friends (60%), and their minister (57%). Among 48 individuals with children, 96% said they would want their children to know their carrier status before they got married.
Table I. Summary of individuals who were invited and who participated in each study.
| Number invited to participate/number participating | Percentage of participants requesting results | |
|---|---|---|
| Survey of attitudes towards CF carrier testing | 86/86 | 45% |
| Study offering CF results | 1588/342 | 22% |
| Educational session on genetic diseases in the Hutterites and offer to disclose carrier status | NAa/120 | 80% |
Not applicable: the total number of individuals ‘invited’ to the educational town hall is unknown. Letters and flyers describing the town hall were mailed to the ministers at 17 colonies who previously participated in our studies. The ministers were asked to post the flyers for other colony members to see. Letters were also sent directly to 153 women who were participating in our prospective pregnancy study at the time.
Offering Cystic Fibrosis Results
Based on the results of the survey, we obtained IRB approval to perform a study to offer testing for the two CF mutations present in this population and to return the results. We sent invitational letters, brochures describing CF, and consent forms to 1,588 adult Hutterites (53% female; 83% married) who had previously participated in our studies of fertility (Kosova, Abney, & Ober, 2010; Ober, Hyslop, Elias, Weitkamp, & Hauck, 1998) or asthma (Ober, Tsalenko, Parry, & Cox, 2000; Yao et al., 2013); this sample included the 86 individuals who participated in the CF survey (Table I). Approximately half of the recipients were age ≥45 and we considered them to be beyond their reproductive years (Figure 1A). The letter invited them to participate in a study to learn their carrier status for CF and instructed them to sign and return a consent form to us if they wished to learn their carrier status. The letter described the process involved in participation and the disclosure of results by letters or during in-person visits to their homes. The brochure (Online Resource 1) described clinical characteristics of CF, basic principles of autosomal recessive inheritance, and the distinction between heterozygous carrier status and homozygous affected status.
Figure 1.
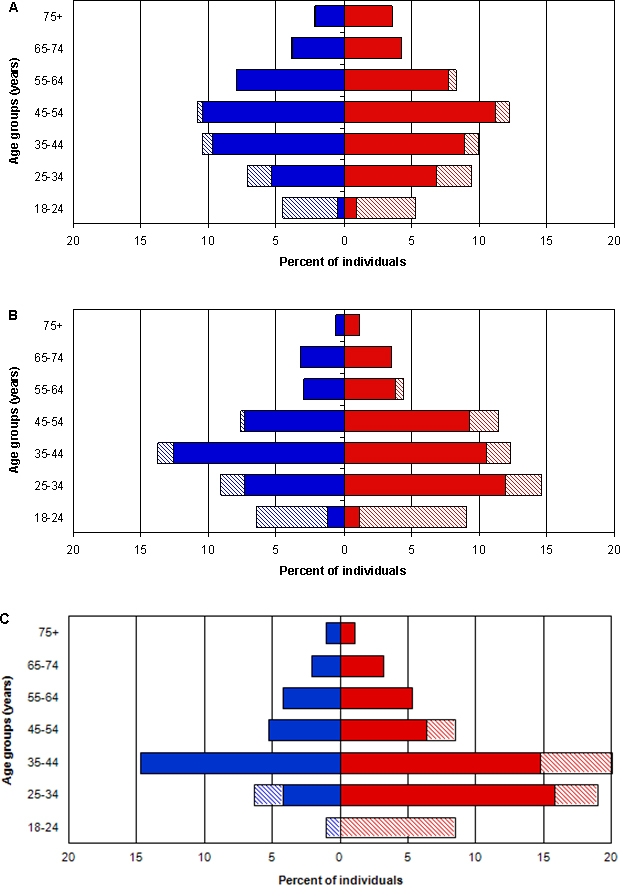
Age and sex distribution of Hutterites A) invited to participate in the CF study (N=1588), B) who consented to participate in the CF study (n=342), and C) who attended the Town Hall and consented to receive results for up to 14 diseases (n=95). Red bars are women, blue bars are men; hatched bars are unmarried individuals.
In response to this invitation, 342 individuals (22%) returned signed consent forms, which is a higher rate of participation than that reported for other similar studies (Henneman, Bramsen, van der Ploeg, & ten Kate, 2002; Tambor et al., 1994). The age and sex composition of the individuals who were invited and those that consented are shown in Figure 1A and Figure 1B, respectively. There were no significant differences in the proportion of respondents who were men vs. women (20% vs. 23%, χ2 = 1.8879, p=0.17), married men vs. married women (19% vs. 20%, χ2 = 0.4342, p=0.51), or single men vs. single women (26% vs. 34%, χ2 = 2.1161, p=0.15). Among the 86 participants in the CF survey, 45% returned signed consent forms to receive their carrier status, which was close to the 41% of the same individuals who stated that they wanted to know their carrier status, and higher than the overall uptake rate of 22%. We attribute the latter to the likely effect of personal contact during the survey with these individuals in the 1-2 years prior to the invitation. However, only 59% of those who stated in the interview that they would want carrier testing consented to these studies when given the opportunity. Among those who stated that they would not want carrier testing, 28% consented to testing, and among those who were unsure about wanting carrier testing, 29% consented to testing. Thus, adult Hutterites who stated a desire to know their carrier status were twice as likely to consent to learn their carrier status as compared to adult Hutterites who stated that they did not want to know their carrier status or to those who were unsure, but their response on the survey was a poor predictor of whether they ultimately consented to learn their carrier status. This is similar to studies in other populations that also reported a low correlation between intent to participate and actual participation (Lakeman et al., 2009; Payne et al., 1997).
Those who expressed an interest in knowing their status on the survey but did not request carrier testing when offered were more likely to be older and beyond their reproductive years. For example, significantly more married or unmarried adults who were still in their reproductive years (defined as under 45 years old) consented to receive information on their carrier status compared to adults beyond their reproductive years (30% vs. 14%; p<0.0001). Thus, consistent with the survey in which the majority of individuals said knowledge of carrier status would affect their reproductive decisions, participation in the study was greatest among individuals or couples who had not yet had children or who had not yet completed their families. Letters were sent to all participants with the results of their CF carrier status beginning in the summer of 2010 (Online Resource 2).
Based on the carrier frequency of CF mutations in the Hutterites, we anticipated that we would identify couples in whom both members were carriers but did not know this prior to our study. Our protocol was to offer genetic counseling in person for any couples where both members of the couple were CF carriers, were still in their childbearing years, and did not already have an affected child (and therefore were likely unaware of their carrier status). We identified 3 carrier couples, 2 of which had at least one child with CF, so we assumed they had knowledge of their carrier status and we sent their results by mail. We did identify one carrier couple that did not have any children with CF (among their five children), but they were beyond their childbearing years and they were informed of their carrier status by telephone and by mail.
Educational Session & Disclosure of Individual Research Results
As part of our studies of common genetic disorders in the Hutterites, we had characterized the frequency spectrum of 15 causal mutations for 14 autosomal recessive diseases (including CF) in 1,644 Hutterites born between 1901 and 2003 (aged 6-92 years at the time of our studies) (Chong, Ouwenga, Anderson, Waggoner, & Ober, 2012; Gerull et al., 2013). These studies revealed surprisingly high carrier frequencies for some diseases that had not been recognized as common in the Hutterites (e.g., 1 in 8 Hutterites are carriers for the mutation causing spinal muscular atrophy, [SMA; OMIM 253400]; Chong et al., 2011; Chong et al., 2012). The high participation rate in the study offering CF carrier results indicated that many Hutterites were interested in learning about their carrier status for a monogenic disease. Moreover, the higher uptake among men and women in their childbearing years was consistent with the survey responses regarding family planning. Based on these considerations, we sought and obtained IRB approval to expand our disclosure efforts and to offer adult individuals the opportunity to obtain carrier status results for all 14 autosomal recessive diseases. The extent of the Hutterites' familiarity with diseases other than CF was unknown. As part of the informed consent process for the Hutterites to receive genetic research results, we therefore felt it important to review the diseases as well as the implications of autosomal recessive inheritance so that individuals could make an informed decision before signing a consent form to receive their results.
In order to provide education about diseases other than CF and to review autosomal recessive inheritance, we organized an educational Town Hall meeting in November 2011, which was held at one South Dakota colony but to which we invited adult Hutterites from all colonies who previously participated in the PI's (C.O.) IRB-approved studies (Table I). An invitational flyer described the program and included the title of each talk that would be presented. At the Town Hall we distributed a brochure (Online Resource 3) that described the 14 genetic diseases and their carrier frequencies in the Hutterites (Chong et al., 2012; Gerull et al., 2013) and reviewed autosomal recessive inheritance. The format of the Town Hall consisted of three half-hour presentations. The first presentation (by C.O.) provided an overview of our genetic studies in the Hutterites, an introduction to the 14 diseases for which we were offering results, and carrier frequencies for each of those diseases. The provision of such aggregate research results (Beskow, Burke, Fullerton, & Sharp, 2012) has always been an integral part of our protocols in the Hutterites, although we most often communicate summary results of our studies in mailings to study participants or as part of informal discussions during visits to the colonies.
The second presentation (by D.J.W.) was a didactic exercise on Mendelian inheritance that included an interactive game to convey the concept of risks in the range of carrier frequencies for several diseases in the Hutterites and the principles of autosomal recessive inheritance. Colored candies were used to represent normal and mutated alleles, and each participant blindly chose two candies. Each group (those with two normal alleles, carriers, and those with two mutated alleles) was asked to stand up to give a sense of the frequencies of carrier and homozygous (affected) individuals in the population. We used a similar game to illustrate the 1 in 4 risk of disease when both parents are carriers. The final presentation (by K.J.S.) reviewed the genetic basis for and clinical course of SMA in the Hutterites. SMA was chosen because, although the carrier frequency for SMA in the South Dakota Hutterites is the highest ever reported (1 in 8) (Chong et al., 2011), only three children in three South Dakota families had been diagnosed with SMA at the time of the Town Hall, and all three diagnoses had occurred in the previous nine years. It therefore seemed likely that most Hutterites would be unfamiliar with this condition. Furthermore, our genetic studies had revealed additional carrier couples who were still in their child bearing years and unaware of their status because they had not yet had a child diagnosed with SMA.
Finally, we performed a group consent process. Some of the major points made during the consent process included that results may be available to those 18 or older who participated in previous studies, that the results may relate to the individual (or their children's) health or reproductive risks, and that the individual genetic test results are considered research results. Consenting individuals indicated whether they wanted results for all or only a subset of the diseases. Each participant had the opportunity to privately meet with a medical geneticist or genetic counselor and to ask questions prior to a consent being signed and witnessed.
Approximately 120 adult Hutterites from 11 colonies attended the Town Hall. By the end of the evening, we had obtained signed consents from 95 individuals (∼80%)(Figure 1C); an additional 11 consents were sent to us by mail in the weeks following the Town Hall from spouses and adult children of participants at the Town Hall. The much higher consent rate from the Town Hall (80%) compared to the 22% of individuals who consented to receive CF results is likely due to both the ease of “signing up” for results after the Town Hall compared to responding to a letter and the initial self-selection of individuals who elected to attend the Town Hall meeting. Overall, 101 of the consenting individuals (95%) requested results for all 14 diseases and five requested results for one or two diseases only.
Of the 106 individuals who signed consents to obtain results, 45% were carriers for one mutation, 25% were carriers for two mutations, and 8% were carriers for three mutations. In eight couples both the husband and wife carried the same mutation: one couple each for oculocutaneous albinism type 1A (OCA1A; OMIM 203100) and limb girdle muscular dystrophy 2H (LGMD2H; OMIM 254110), two for CF, and four for SMA. None of the participant couples in this study carried more than one mutation in common. Of these eight carrier couples, three were unaware of their status prior to our studies (i.e., had not yet had a child diagnosed with the corresponding disease: SMA, OCA1A, or LGMD2H) and we conducted in person visits to them during a trip to the colonies. None of the participants were homozygous for any mutations (or compound heterozygotes for the two CF-causing mutations). We have disclosed these results in letters to each adult who provided consent (Online Resource 4). The letter emphasizes that these are research results and should be clinically confirmed before being used to guide medical care or decision making. The letter also provides interpretation of all results, reviews autosomal recessive inheritance and risks, and emphasizes that the participant could still be a carrier for mutations causing any of the remaining autosomal recessive diseases that are segregating in this population (Armistead et al., 2009; Beaulieu et al., 2013; Bogershausen et al., 2013; Boycott et al., 2010; Boycott et al., 2008; Chong et al., 2012; Wiltshire, Hegele, Innes, & Brownell, 2013). The letter invites recipients to contact us by mail or phone with further questions and offers our assistance in arranging clinical confirmation of results. For all couples in whom both partners were carriers of mutations for the same disease, we either disclosed results during an in-person meeting or over the telephone, during which times we provided genetic counseling.
Discussion
Our studies of carrier frequencies in the Hutterite population revealed a surprisingly high carrier frequency for 14 disorders. Most of the Hutterites were not familiar with the conditions or their carrier risk for any except CF. Our experience with the survey, consent process, and Town Hall meeting showed that many individuals were interested in knowing their carrier status and that many indicated they would use this information in family planning. In fact, the participants in the Town Hall expressed much appreciation for our taking the time to provide this information to them and that the knowledge provided was both useful and desired. In-depth questions were asked by the participants at the Town Hall meeting regarding treatment options and evaluations for CF and SMA, which indicated an appropriate understanding of the severity of these diseases and their implications.
We also note that approximately 20% of individuals at the Town Hall did not return a consent form and will not therefore receive their carrier results. We believe this indicates that there were people who did not want results and were comfortable in making that decision. Interestingly, 47 individuals who did not respond to our invitation to participate in the CF carrier testing study did attend the Town Hall and signed a consent form at the Town Hall or sent their consent by mail following the Town Hall, and 45 (96%) of these individuals consented to receive results for all 14 diseases. This is consistent with studies in other populations that have found that individuals who decline testing when offered are often still interested in receiving results in the future (Lakeman et al., 2009; Payne et al., 1997).
The brochure and Town Hall meeting included education about autosomal recessive inheritance, which is critical in understanding the results of the carrier testing. One individual privately discussed with a member of the team his recognition that primary care doctors in the local community were uncomfortable addressing the relatedness among Hutterite individuals and discussing their overall increased risk for autosomal recessive conditions. This individual felt it was important to have these conversations and to increase general awareness of genetic disease risk among the Hutterites and the local medical community. It is difficult to predict how the Hutterites will use this information and how the knowledge of risk will impact, if at all, reproductive decisions in the future.
Conclusion
The process described here is valuable in developing experience with reporting individual research results, a task which researchers will face more frequently as technologies such as exome and whole genome sequencing become more routine as research tools (Wolf, 2012). Current discussion in the literature on study participants' views and reactions to the return of research results as well as proposed approaches to results disclosure have typically targeted participants in larger, general populations (Bollinger, Scott, Dvoskin, & Kaufman, 2012; Heshka, Palleschi, Howley, Wilson, & Wells, 2008; Knoppers, Joly, Simard, & Durocher, 2006; Murphy et al., 2008; Trinidad et al., 2010). However, as emphasized in the NHLBI Working Group recommendations (Bookman et al., 2006; Fabsitz et al., 2010), special consideration should be given to returning results to identifiable communities. These recommendations helped inform our disclosure process from early conversations with community members and leaders to assess their desire for results, to integrating experience gained through the investigator's extensive history of population-based research with the Hutterites (Beskow & Burke, 2010), to designing the Town Hall activities and other correspondence with the participants.
Prior to our surveying attitudes toward CF carrier testing and our subsequent pilot study offering CF results, we were unsure how widespread the Hutterite's interest would be in receiving individual genetic results. Through these studies, we learned that a significant number of individuals in the Hutterite community were interested in getting back individual genetic results for a monogenic disease. This interest then prompted our expanded efforts to offer results for all 14 autosomal recessive diseases that were part of our studies.
Although some overall guidance related to the reporting of genetic research results was provided by the NHLBI Working Group recommendations (Bookman et al., 2006; Fabsitz et al., 2010), we still faced uncertainties and challenges about the logistics of the process. One challenge in particular was how to provide appropriate education and obtain informed consent from a large number of individuals. Although a group consent process may not be typical, we thought it was a good option for the Hutterites because they are familiar with receiving information as a group because of their communal lifestyle. We speculated that most would feel comfortable asking questions in a group setting, although we also provided each individual with the opportunity to ask questions privately. The typical counseling and consent process through individual meetings would be prohibitive from reaching a large number of participants and the large educational meeting was useful in allowing many individuals to access the information if they wanted. A group setting for education and consent may not work for other research studies, but in this situation it allowed more individuals the opportunity than if we had taken a more traditional approach. Overall, we felt that the education we provided allowed individuals to make informed decisions about participation.
Another challenge we faced was the logistics of delivering results on a large number of tests to many individuals by mail. In this process, bioinformatic support was helpful in automating the process of generating the personalized letters. Particular care was taken to ensure results were returned only for the disease(s) that had been indicated on the consent form of each individual.
We ultimately were able to provide individual genetic research results for >10 monogenic diseases to more than 100 adult Hutterites. Personal communication with some participants after they received their results has indicated that they felt it useful to have received these results. Perhaps further evidence of this is that we have since been contacted by other Hutterites who have heard about the study and who are also interested in participating. Because the Hutterites are located in remote communities and their access to researchers is limited, the approach we chose was to go to their community to provide education and explain the consent process in a group setting, offer the opportunity for individual meetings with research staff as needed, and then return the results by mail with the opportunity to contact us with questions. Our approach to providing genetic test results has been particularly tailored towards the Hutterite community at all stages, but the lessons learned and experiences reported may be beneficial to other researchers, genetic counselors, or clinicians who are returning results to small, isolated communities or other populations who may also benefit from receiving individual genetic research results.
Supplementary Material
1. Brochure on Cystic Fibrosis (CF) in the Hutterites.
2. CF results letter sent to CF carriers who were married.
3. Brochure distributed at the Town Hall describing 14 genetic diseases in the Hutterites.
4. Disclosure letter for 14 conditions.
Acknowledgments
The authors acknowledge Dr. Elizabeth McNally and Dr. Amy Lemke for guidance and helpful discussions; Dr. Cheryl Rockman-Greenberg for advising us on the Town Hall format; Ms. Amanda Schleif for designing the Cystic Fibrosis informational brochure; Dr. Preeti Sharma, Ms. Kathleen Shanovich, Ms. Michelle Stein, Ms. Donata Russell, and Ms. Tara Newcomb for field trip assistance, Mr. William Wentworth-Sheilds for computational support, and the Hutterites for their continued enthusiasm and support for our studies. This research was supported in part by grants R01 HD21244 and R01 HL085197.
Kathryn J. Swoboda has grant funding from NINDS U10 NS077305, NICHD R01 HD69045 and Families of SMA for SMA-related studies; she has contracts for SMA clinical trials and studies with F. Hoffmann-La Roche Genetech and ISIS.
Footnotes
Authors Rebecca L. Anderson, Kathleen Murray, Jessica X. Chong, Rebecca Ouwenga, Marina Antillon, Peixian Chen, Lorena Diaz de Leon, Lucille A. Lester, Soma Das, Carole Ober, and Darrel J. Waggoner declare that they have no conflict of interest.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
References
- Armistead J, Khatkar S, Meyer B, Mark BL, Patel N, Coghlan G, et al. Triggs-RaineB B. Mutation of a gene essential for ribosome biogenesis, EMG1, causes Bowen-Conradi syndrome. Am J Hum Genet. 2009;84(6):728–739. doi: 10.1016/j.ajhg.2009.04.017. Comparative Study Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu CL, Huang L, Innes AM, Akimenko MA, Puffenberger EG, Schwartz C, et al. Boycott KM. Intellectual disability associated with a homozygous missense mutation in THOC6. Orphanet J Rare Dis. 2013;8(1):62. doi: 10.1186/1750-1172-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow LM, Burke W. Offering individual genetic research results: context matters. Sci Transl Med. 2010;2(38):38cm20. doi: 10.1126/scitranslmed.3000952. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow LM, Burke W, Fullerton SM, Sharp RR. Offering aggregate results to participants in genomic research: opportunities and challenges. Genet Med. 2012;14(4):490–496. doi: 10.1038/gim.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogershausen N, Shahrzad N, Chong JX, von Kleist-Retzow JC, Stanga D, Li Y, et al. Lamont RE. Recessive TRAPPC11 Mutations Cause a Disease Spectrum of Limb Girdle Muscular Dystrophy and Myopathy with Movement Disorder and Intellectual Disability. Am J Hum Genet. 2013;93(1):181–190. doi: 10.1016/j.ajhg.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JM, Scott J, Dvoskin R, Kaufman D. Public preferences regarding the return of individual genetic research results: findings from a qualitative focus group study. Genet Med. 2012;14(4):451–457. doi: 10.1038/gim.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookman EB, Langehorne AA, Eckfeldt JH, Glass KC, Jarvik GP, Klag M, et al. Luepker RV. Reporting genetic results in research studies: summary and recommendations of an NHLBI working group. Am J Med Genet A. 2006;140(10):1033–1040. doi: 10.1002/ajmg.a.31195. Congresses Guideline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott KM, Beaulieu C, Puffenberger EG, McLeod DR, Parboosingh JS, Innes AM. A novel autosomal recessive malformation syndrome associated with developmental delay and distinctive facies maps to 16ptel in the Hutterite population. Am J Med Genet A. 2010;152A(6):1349–1356. doi: 10.1002/ajmg.a.33379. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Boycott KM, Parboosingh JS, Chodirker BN, Lowry RB, McLeod DR, Morris J, et al. Innes AM. Clinical genetics and the Hutterite population: a review of Mendelian disorders. Am J Med Genet A. 2008;146A(8):1088–1098. doi: 10.1002/ajmg.a.32245. [DOI] [PubMed] [Google Scholar]
- Caliskan M, Chong JX, Uricchio L, Anderson R, Chen P, Sougnez C, et al. Ober C. Exome sequencing reveals a novel mutation for autosomal recessive non-syndromic mental retardation in the TECR gene on chromosome 19p13. Hum Mol Genet. 2011;20(7):1285–1289. doi: 10.1093/hmg/ddq569. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JX, Oktay AA, Dai Z, Swoboda KJ, Prior TW, Ober C. A common spinal muscular atrophy deletion mutation is present on a single founder haplotype in the US Hutterites. Eur J Hum Genet. 2011;19(10):1045–1051. doi: 10.1038/ejhg.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JX, Ouwenga R, Anderson RL, Waggoner DJ, Ober C. A population-based study of autosomal-recessive disease-causing mutations in a founder population. Am J Hum Genet. 2012;91(4):608–620. doi: 10.1016/j.ajhg.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabsitz RR, McGuire A, Sharp RR, Puggal M, Beskow LM, Biesecker LG, et al. Burke GL. Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute working group. Circ Cardiovasc Genet. 2010;3(6):574–580. doi: 10.1161/CIRCGENETICS.110.958827. Practice Guideline Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerull B, Kirchner F, Chong J, Tagoe J, Chandrasekharan K, Strohm O, et al. Duff HJ. A Homozygous Founder Mutation in Desmocollin-2 (DSC2) Causes Arrhythmogenic Cardiomyopathy in the Hutterite Population. Circ Cardiovasc Genet. 2013 doi: 10.1161/CIRCGENETICS.113.000097. [DOI] [PubMed] [Google Scholar]
- Henneman L, Bramsen I, van der Ploeg HM, ten Kate LP. Preconception cystic fibrosis carrier couple screening: impact, understanding, and satisfaction. Genet Test. 2002;6(3):195–202. doi: 10.1089/109065702761403351. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Heshka JT, Palleschi C, Howley H, Wilson B, Wells PS. A systematic review of perceived risks, psychological and behavioral impacts of genetic testing. Genet Med. 2008;10(1):19–32. doi: 10.1097/GIM.0b013e31815f524f. Research Support, Non-U.S. Gov't Review. [DOI] [PubMed] [Google Scholar]
- Hostetler JA. Hutterite Society. Baltimore: Johns Hopkins University Press; 1974. [Google Scholar]
- Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, et al. Boycott KM. TMEM237 is mutated in individuals with a Joubert syndrome related disorder and expands the role of the TMEM family at the ciliary transition zone. Am J Hum Genet. 2011;89(6):713–730. doi: 10.1016/j.ajhg.2011.11.005. Research Support, American Recovery and Reinvestment Act Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoppers BM, Joly Y, Simard J, Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006;14(11):1170–1178. doi: 10.1038/sj.ejhg.5201690. Historical Article Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Kosova G, Abney M, Ober C. Colloquium papers: Heritability of reproductive fitness traits in a human population. Proc Natl Acad Sci U S A. 2010;107 Suppl 1:1772–1778. doi: 10.1073/pnas.0906196106. doi:0906196106 [pii] 10.1073/pnas.0906196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakeman P, Plass AM, Henneman L, Bezemer PD, Cornel MC, ten Kate LP. Preconceptional ancestry-based carrier couple screening for cystic fibrosis and haemoglobinopathies: what determines the intention to participate or not and actual participation? Eur J Hum Genet. 2009;17(8):999–1009. doi: 10.1038/ejhg.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AO. The founder effect in a human isolate: Evolutionary implications. Am J Phys Anthropology. 1970;32:351–368. doi: 10.1002/ajpa.1330320305. [DOI] [PubMed] [Google Scholar]
- Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K. Public expectations for return of results from large-cohort genetic research. Am J Bioeth. 2008;8(11):36–43. doi: 10.1080/15265160802513093. Multicenter Study Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C, Abney M, McPeek MS. The genetic dissection of complex traits in a founder population. Am J Hum Genet. 2001;69(5):1068–1079. doi: 10.1086/324025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ober C, Hyslop T, Elias S, Weitkamp LR, Hauck WW. Human leukocyte antigen matching and fetal loss: Results of a 10-year prospective study. Hum Reprod. 1998;13:33–38. doi: 10.1093/humrep/13.1.33. [DOI] [PubMed] [Google Scholar]
- Ober C, Tsalenko A, Parry R, Cox NJ. A second-generation genomewide screen for asthma-susceptibility alleles in a founder population. Am J Hum Genet. 2000;67(5):1154–1162. doi: 10.1016/s0002-9297(07)62946-2. doi:S0002-9297(07)62946-2 [pii] 10.1016/S0002-9297(07)62946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne Y, Williams M, Cheadle J, Stott NC, Rowlands M, Shickle D, et al. Clarke A. Carrier screening for cystic fibrosis in primary care: evaluation of a project in South Wales. The South Wales Cystic Fibrosis Carrier Screening Research Team. Clin Genet. 1997;51(3):153–163. doi: 10.1111/j.1399-0004.1997.tb02445.x. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Steinberg AG, Bleibtreu HK, Kurczynski TW, Martin AO, Kurczynski EM. Genetic studies in an inbred human isolate. In: Crow JF, Neel JV, editors. Proceedings of the Third International Congress of Human Genetics. Baltimore: Johns Hopkins University Press; 1967. pp. 267–290. [Google Scholar]
- Tambor ES, Bernhardt BA, Chase GA, Faden RR, Geller G, Hofman KJ, Holtzman NA. Offering cystic fibrosis carrier screening to an HMO population: factors associated with utilization. Am J Hum Genet. 1994;55(4):626–637. Research Support, U.S. Gov't, P.H.S. [PMC free article] [PubMed] [Google Scholar]
- Trinidad SB, Fullerton SM, Bares JM, Jarvik GP, Larson EB, Burke W. Genomic research and wide data sharing: views of prospective participants. Genet Med. 2010;12(8):486–495. doi: 10.1097/GIM.0b013e3181e38f9e. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltshire KM, Hegele RA, Innes AM, Brownell AK. Homozygous lamin A/C familial lipodystrophy R482Q mutation in autosomal recessive Emery Dreifuss muscular dystrophy. Neuromuscul Disord. 2013;23(3):265–268. doi: 10.1016/j.nmd.2012.11.011. Research Support, Non-U.S. Gov't. [DOI] [PubMed] [Google Scholar]
- Wolf SM. The past, present, and future of the debate over return of research results and incidental findings. Genet Med. 2012;14(4):355–357. doi: 10.1038/gim.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, et al. Wolf WA. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med. 2012;14(4):361–384. doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao TC, Du G, Han L, Sun Y, Hu D, Yang JJ, et al. Ober C. Genome-wide association study of lung function phenotypes in a founder population. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. Brochure on Cystic Fibrosis (CF) in the Hutterites.
2. CF results letter sent to CF carriers who were married.
3. Brochure distributed at the Town Hall describing 14 genetic diseases in the Hutterites.
4. Disclosure letter for 14 conditions.


