Abstract
Background
Serum biomarkers of metabolic syndrome predict abnormal lung function in World Trade Center particulate matter (WTC-PM)-exposed Fire Department of New York (FDNY) rescue workers. In animal models, exposure to ambient PM induces non-alcoholic fatty liver disease (NAFLD), a well-known comorbidity of metabolic syndrome. YKL-40 is an inflammatory biomarker for both liver and lung disease. We tested if YKL-40 is a biomarker for NAFLD in this dust-exposed cohort.
Methods
Using a nested case-control design, we studied 131 FDNY personnel who had Computer Tomography performed within 5 years post 9/11. NAFLD was defined by a liver/spleen attenuation ratio of ≤1. Serum biomarkers, lipid panel and liver function were measured in serum that had been drawn within 6 months of September 11, 2001. YKL-40 and chitotriosidase were assayed by ELISA. We tested biomarker and NAFLD association using logistic regression adjusted for age, BMI, and post-911 lung function.
Results
NAFLD was present in 29/131 (22%) of the cohort. In a multivariable model increasing YKL-40 was protective while increasing triglyceride and alkaline phosphatase were risk factors for NAFLD.
Conclusions
Increased YKL-40 is a protective biomarker in non-alcoholic fatty liver disease. Further studies may reveal a link between PM-induced lung and liver diseases.
Keywords: YKL-40, Non-alcoholic fatty liver disease, Biomarker
Introduction
Non-Alcoholic Fatty Liver Disease (NAFLD) refers to a spectrum of pathologic conditions involving micro- and macrovesicular steatosis in hepatocytes without other causes of chronic liver disease such as hepatitis B virus or hepatitis C virus infection and excess ethanol consumption [1]. NAFLD has emerged as the leading cause of chronic liver disease in the Western world [2,3], and affects about 30% of adults in the United States [4,5]. Its worldwide prevalence has been reported to be as high as 74% in obese individuals [6,7]. Interestingly, the prevalence of obesity/overweight in the World Trade Center (WTC) rescue worker cohort is strikingly higher than that of the general population, affecting 80% of the cohort [8,9]. We therefore investigated the prevalence of NAFLD in this cohort.
WTC-Particulate Matter (PM) exposure from the 9/11 disaster resulted in significant airflow obstruction and reactive airway disease in Fire Department of New York (FDNY) rescue workers [10-13]. We have previously reported biomarkers of metabolic syndrome that predict susceptibility to WTC PM-related lung injury [14]. Studies have also shown that ambient PM exposure to lung is closely associated with pathogenesis of metabolic syndrome [15] affecting non-pulmonary organs including the cardiovascular system [16], adipose tissue [17] and liver [18,19]. Notably, PM exposure has been shown to induce NAFLD, a hepatic manifestation of metabolic syndrome [19]. We extended our biomarker studies by identifying serum biomarkers expressed within 6 months of WTC exposure that predicted NAFLD defined on Computed Tomography (CT) scan performed years later.
The WTC-exposed FDNY cohort is a unique population whose clinical data has been systematically and rigorously captured from 1996 (pre-9/11) until the present time (post-9/11). This firefighter cohort was exposed to extremely high levels of WTC-PM (4000 times as high as the upper limit of safety range) for a brief period during rescue and recovery efforts after the WTC collapse. Firefighters with significant respiratory symptoms were referred to subspecialty pulmonary evaluation and chest CT was obtained in about 50%. Quantitative radiographic attenuation of the liver/spleen ratio seen on these CT scans defined fatty liver disease in our study [20,21].
The molecule of interest in this study is YKL-40, a chitinase-like protein of the glycosyl hydrolase family 18, [22] that is elaborated by macrophages, neutrophils, chondrocytes; fibroblasts, endothelial cells, airway epithelial cells, tumor cells and hepatic stellate cells [23]. It is interesting to note that although YKL-40 contains highly conserved chitin-binding domains, it functionally lacks chitinase activity [24], unlike chitotriosidase which is an enzymatically active chitinase produced in mature monocyte-derived macrophages, lung macrophages and Kupffer cells [25,26]. We recently reported that while increased serum chitotriosidase level is a protective biomarker of airway obstruction after WTC-PM exposure, YKL-40 was not associated with lung dysfunction post-9/11 [27]. In the current study we investigated the relationship between both YKL-40 and chitotriosidase and NAFLD in our dust-exposed firefighter population.
Although YKL-40 has been associated with disease states including hepatic fibrosis in alcoholic liver disease [28] and hepatitis C virus infection [29,30], the relationship between YKL-40 and NAFLD is unknown. We hypothesized that NAFLD in our PM-exposed cohort, as assessed by non-contrast CT scan, would be independently associated with specific serum biomarkers including YKL-40 and chitotriosidase [31]. Defining the relationship between fatty liver and YKL-40 would be a critical step in understanding the role of YKL-40 in NAFLD pathophysiology in the setting of PM exposure.
Materials and Methods
Nested case control design
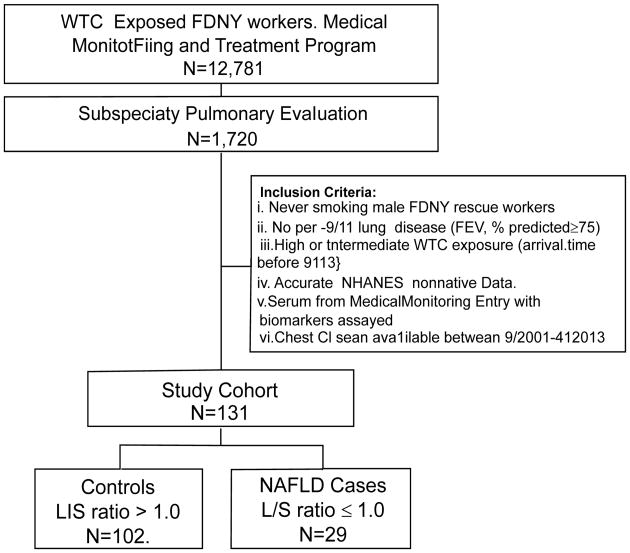
The study cohort was derived from 1,720 exposed workers who presented to subspecialty pulmonary evaluation from October 1, 2001 and March 10, 2002. Serum was stored and available for assay (n=251); 131 of these had a non-contrast chest CT scan that routinely includes sections of the upper abdomen stopping at the adrenal glands. As a result every chest CT contained attenuation data from the liver and spleen. The chest CT was performed on average 5 years after the serum used in biomarkers assays was obtained. The protocol and informed consents were approved by the institutional review boards of Montefiore Medical Center (#07–09–320) and the human subjects review committees of New York University Medical Center Institutional Review Board (#11–00439).
Demographics
Age, race and years of service at the FDNY were obtained from the FDNY-WTC-monitoring database. There was no evidence of alcohol abuse in the medical record of the 131 participants. Degree of exposure was self-reported at the first FDNY-WTC-monitoring and was categorized using the FDNY-WTC Exposure Intensity Index (Arrival Time): i. Presented on the morning of 9/11/2001 ii. Arrived between afternoon on 9/11/2001 and 9/12/2001 (9-11). Those arriving after day three were excluded from analysis as a result of their low numbers in this sample. Body Mass Index (BMI), blood pressure and spirometry were measured at the time of serum collection.
Serum sampling and analysis
Blood drawn at the first post-9/11 FDNY medical monitoring exam was allowed to stand for one hour at room temperature before being centrifuged at 1,800 g for ten minutes. Serum was stored at -80°C (Bio-Reference Laboratories, Inc. Elmwood Park, NJ). Serum was thawed at 4°C and assayed using YKL-40 (MicroVue, USA), chitotriosidase (Quidel, USA) ELISA panel according to manufacturer's instructions. Lipids, glucose and liver function test were obtained from the medical records at the time of serum sampling.
CT imaging-liver fat measurement
Each participant underwent CT scans of the chest without the administration of contrast material. Scans included images of the liver and spleen in all participants. If there were CT scans from different dates, the earliest CT images after 9/11 were examined. Primary scan data was obtained from and re-evaluated. Hepatic and splenic Hounsfield attenuation (HU) were independently measured by two experienced readers. The scan with the greatest coverage of the liver and spleen was selected for liver fat measurement: four Regions of Interest (ROI) were placed in liver and three ROI was placed in the spleen. The liver/spleen attenuation ratio (L/S ratio) was selected as the most stable measure of hepatic fat content, and was calculated by dividing the mean HU measurement of liver by the mean spleen HU measurement. The final L/S ratio was an average of the ratios obtained by the two independent readers.
Statistical analysis
We tested normality using the Shapiro-Wilk test and Q-Q plots. We used Wilcoxon rank sum test for between group comparisons. Chi-squared test or Fisher's exact test was used for inferences on proportions.
Given the dichotomous outcome of L/S ratio we tested if biomarkers predicted NAFLD using logistic regression. Since variable YKL-40 was severely skewed, we log-transformed it before including it as continuous covariates in the model. Variables identified as potential confounders and those with a P value less than 0.2 in the univariable analysis were included in the multivariable logistic regression model. A backward stepwise approach was used to determine the most parsimonious model for the serum biomarkers, with a pre-specified P value less than 0.10. The Hosmer-Lemeshow goodness-of-fit test was used to assess calibration of the final model. The model discrimination was evaluated using the receiver operating characteristic Area under the Curve (AUC).
Data are expressed as median (interquartile range, IQR) or odds ratio (95% confidence interval), unless otherwise stated. A two-sided P-value less than 0.05 was considered significant. All analyses were performed with STATA/SE version 12.1 (StataCorp LP, College Station, TX).
Results
Demographics
Derivation of cases and controls is described in Figure 1. The prevalence of NAFLD was 22% in this study population of 131 [29 with NAFLD (L/S ratio ≤ 1) and 102 without NAFLD (L/S ratio>1)]. The median L/S ratio was 1.18 (range 0.3-1.48).
Figure 1.
Study design. WTC: World Trade Center; FDNY: Fire Department City of New York; NHANES: National Health and Nutrition Examination Survey; L/S ratio: Liver/Spleen attenuation ratio.
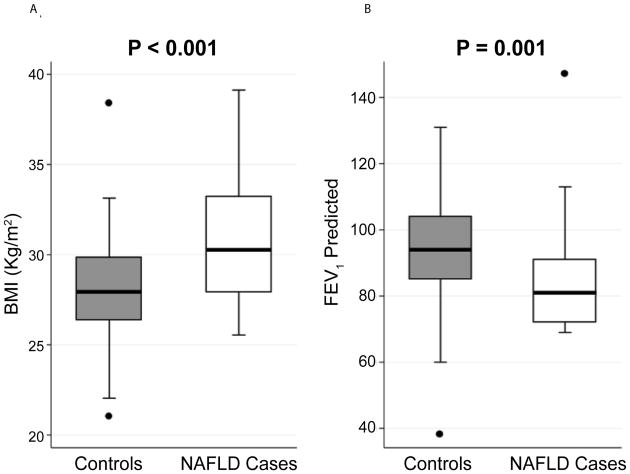
Demographics of cases and controls are shown in Table 1. All had serum biomarkers drawn after 9/11 at an initial medical monitoring exam. The median time to serum draw was 2.7 months post 9/11 and the median time to chest CT scan was 5.4 years. There were no significant differences between cases and controls in time to obtaining of serum biomarkers post-911 whereas time to chest CT scans was 6 months longer in cases (P=0.032). Cases and controls had similar age, year of service, race distribution, WTC exposure intensity, and blood pressure, and forced expiratory volume in 1 second (FEV1) / forced vital capacity (FVC) ratio post-/911. Cases had higher average BMI (P<0.001) and lower FEV1 post-9/11 than controls (P=0.001) (Figure 2).
Table 1.
Demographics.
| Controls | NAFLD Cases | P-value* | ||
|---|---|---|---|---|
| Number of Subjects | 102 | 29 | ||
| Age at 9/11† | 42 (37-46) | 37 (35-45) | 0.030 | |
| Years of Service at 9/11† | 14 (8-19) | 11 (5-16) | 0.050 | |
| Race‡ | Caucasian | 96 (94) | 29 (100) | 0.215 |
| African American | 6 (6) | 0 (0) | ||
| Intensity of WTC Exposure‡ | High§ | 29 (28) | 7 (24) | 0.420 |
| Intermediate¶ | 73 (72) | 22 (76) | ||
| BMI, kg/m2 † | 28 (26-30) | 30 (28-33) | <0.001 | |
| Systolic Blood Pressure, mmHg† | 114 (110-126) | 118 (110-127) | 0.519 | |
| Diastolic Blood Pressure, mmHg† | 72 (70-80) | 72 (70-80) | 0.277 | |
| Time to† | Serum Collection, months | 2.6 (2.1-3.2) | 2.8 (2.0-3.8) | 0.677 |
| Chest CT scan, months | 61 (31-73) | 68 (49-81) | 0.032 | |
| Spirometry† | FEV1, % Predicted | 94 (85-104) | 81 (72-92) | 0.001 |
| FEV1/FVC, % | 85 (81-88) | 83 (81-86) | 0.067 | |
Definition of abbreviations: BMI = body mass index; WTC = World Trade Center; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity
Values Expressed as Median (IQR).
Values Expressed as N (%).
Arrival at WTC 9/11 Morning,
Arrival at WTC between noon of 9/11 and midnight of 9/12.
Significance assessed by Wilkoxon Rank Sum test or Chi-squared test.
Figure 2.
BMI (Panel A) and FEV1 post-911 (Panel B) are significantly different between cases (N=29) and control subjects (N=102). Results represented as box (median and interquartile range) and whiskers (10th–90th percentile). Significance determined by Wilcoxon Rank Sum test. NAFLD=Non-alcoholic fatty liver disease.
Serum biomarkers
Serum YKL-40, chitotriosidase, glucose, lipid panel and liver function tests were measured an average of 3 months after September 11, 2001 (Table 2). Compared to controls, cases had significantly lower YKL-40 and High-Density Lipoprotein (HDL) (P=0.028, P=0.023 respectively) and significantly higher glucose, triglyceride, Alanine Aminotransferase (ALT) Alkaline Phosphatase (ALP) and gamma-glutamyl transferase (G-GTP) levels (P=0.042, P=0.020, P=0.001 and P=0.012, respectively). There was no difference in chitotriosidase levels between cases and controls (31 ng/mL vs. 28 ng/mL, P=0.708).
Table 2.
Serum Levels of Analytes.
| Predictor | Controls† N=102 | NAFLD Cases† N=29 | P-value‡ |
|---|---|---|---|
| YKL-40, log10 ng/mL | 1.64 (1.5-1.8) | 1.55 (1.5-1.7) | 0.028 |
| Chitotriosidase, ng/mL | 31 (17-48) | 28 (16-47) | 0.513 |
| Glucose, mg/dL | 90 (84-95) | 93 (88-100) | 0.042 |
| Triglyceride, mg/dL | 135 (105-207) | 226 (103-441) | 0.020 |
| HDL, mg/dL | 47 (41-54) | 42 (35-50) | 0.027 |
| LDL, mg/dL | 131 (106-157) | 130 (110-157) | 0.725 |
| ALT, U/L | 30 (22-39) | 39 (33-54) | 0.001 |
| AST, U/L | 24 (21-28) | 25 (23-32) | 0.069 |
| ALP, U/L | 71 (60-82) | 80 (73-85) | 0.012 |
| G-GTP, U/L | 24 (19-41) | 29 (21-46) | 0.189 |
| Albumin, g/L | 43 (41-45) | 45 (42-46) | 0.168 |
Definition of abbreviations: OR = odds ratio; 95% CI = 95% confidence interval; HDL = high-density lipoprotein; LDL = low-density lipoprotein; ALT = alanine aminotransferase; AST = aspartate transaminase; ALP = alkaline phosphatase; G-GTP = gamma-glutamyl transpeptidase
Values Expressed as Median (IQR).
Significance assessed by Wilkoxon Rank Sum test
Univariable logistic model predicting L/S ratio ≤ 1
Univariable analysis was initially used to identify potential biomarkers predicting NAFLD. YKL-40, triglyceride, HDL, ALT, ALP and albumin were found to have P-values of less the 0.2 (Table 3) and were selected for multivariable analysis. There was no association between chitotriosidase and NAFLD after WTC exposure (95% CI: 0.976-1.013). Increasing YKL-40 and HDL reduced the odds of NAFLD while increasing triglyceride, ALT, ALP and albumin increased the odds of disease. In this small cohort there was no association between L/S ratio and either Low-Density Lipoprotein (LDL), Aspartate Transaminase (AST) or G-GTP.
Table 3.
Logistic Regression Model Predicting Liver Spleen Attenuation Ratio ≤ 1.
| Predictor | OR (95%CI)† | P-value | |
|---|---|---|---|
| Univariable Analysis | YKL-40, log10 ng/mL | 0.105 (0.016-0.694) | 0.105 |
| Chitotriosidase, ng/mL | 0.992 (0.974-1.010) | 0.392 | |
| Glucose, mg/dL | 1.014 (0.991-1.037) | 0.238 | |
| Triglyceride, mg/dL | 1.005 (1.002-1.008) | 0.003 | |
| HDL, mg/dL | 0.963 (0.922-1.006) | 0.093 | |
| LDL, mg/dL | 0.996 (0.985-1.008) | 0.996 | |
| ALT, U/L | 1.016 (0.997-1.034) | 0.094 | |
| AST, U/L | 1.012 (0.972-1.054) | 0.548 | |
| ALP, U/L | 1.026 (1.000-1.052) | 0.051 | |
| G-GTP, U/L | 1.004 (0.990-1.019) | 0.553 | |
| Albumin, g/L | 1.161 (0.929-1.451) | 0.188 | |
| Multivariable Analysis | YKL-40, log10 ng/mL | 0.036 (0.003-0.427) | 0.009 |
| Triglyceride, mg/dL | 1.006 (1.002-1.011) | 0.006 | |
| ALP, U/L | 1.032 (1.001-1.065) | 0.046 |
Definition of abbreviations: OR = odds ratio; 95% CI = 95% confidence interval; HDL = high-density lipoprotein; LDL = low-density lipoprotein; ALT = alanine aminotransferase; AST = aspartate transaminase; ALP = alkaline phosphatase; G-GTP = gamma-glutamyl transpeptidase
Adjusted for age, BMI and FEV1, % Predicted. Omnibus test X2 (6) = 44.349, P = <0.001. Hosmer and Lemeshow's goodness-of-fit test P=0.089. Area under ROC curve=0.866.
Multivariable logistic regression model
After adjustment for age at 9/11, BMI and FEV1 at the time of serum draw, the final model retained YKL-40, triglyceride and ALP as significant predictors of fatty liver (Table 3). Increasing YKL-40 reduced the odds of having NAFLD while increasing triglyceride and ALP increased the odds of having a L/S ratio ≤ 1. With every 1 log10 ng/mL increase in serum YKL-40, the probability of having NAFLD decreased by 42%. To determine the clinical significance of this association, we added the range of YKL-40 to the adjusted logistic model. When holding all other variables in the model constant the probability of NAFLD declined from 57% to 2% as the concentration of log10 YKL-40 increased from 1 to 2.33 ng/mL (Figure 3 panel A). For each 1 mg/ dL increase of triglyceride, the probability of having NAFLD decreased by 0.1%. The probability of NAFLD increased from 6% to 83% as the concentration of triglyceride increased from 53 to 748 mg/dL (Figure 3 panel B). With every 1U/L increase of ALP, the probability of having NAFLD decreased by 0.6%. The probability of NAFLD increased from 5% to 46% as the concentration of ALP increased from 35 to 126U/L (Figure 3 panel C). Area under the Receiver Operating Characteristic (ROC) of the final model to predict NAFLD was 0.866 (Figure 4).
Figure 3.
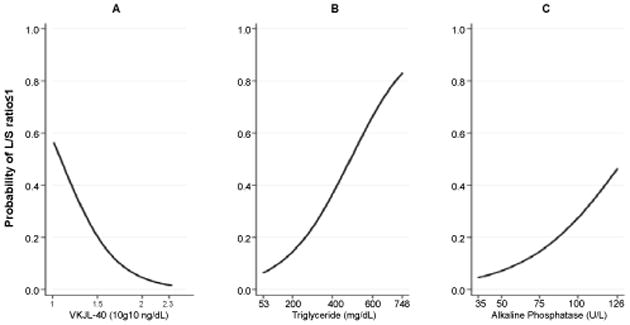
Final logistic model prediction A. Calculated probability of L/S ratio ≤ 1.0 as the concentration of YKL-40 increased over the observed biomarker range with all other covariates held constant. B. Calculated probability of L/S ratio ≤ 1.0 as triglyceride increases C. Calculated probability of L/S ratio ≤ 1.0 as ALP increases. L/S ratio: Liver/Spleen attenuation ratio; ALP: Alkaline phosphatase.
Figure 4.
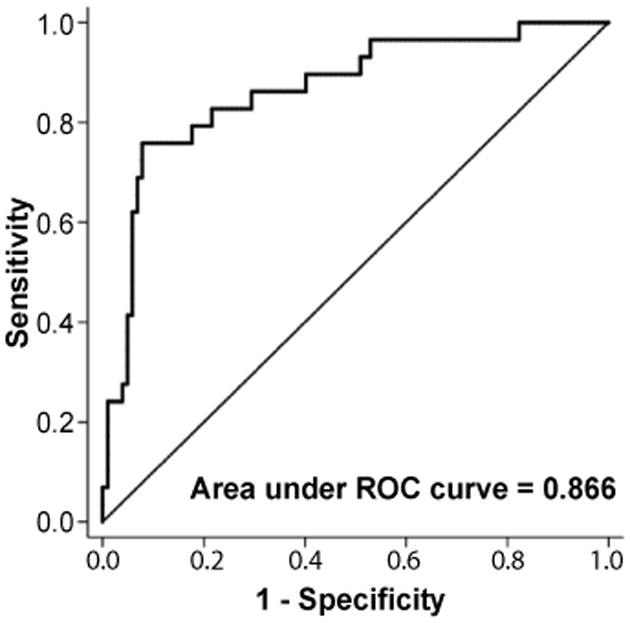
Receiver operating characteristic (ROC) of the final model to predict L/S ratio ≤ 1.0. Area under the curve (AUC) = 0.866. L/S ratio=Liver/Spleen attenuation ratio.
Discussion
The WTC collapse produced a massive acute exposure to PM causing a high incidence of respiratory dysfunction that has persisted years after 9/11 [10-13]. We have previously reported that serum biomarkers of metabolic syndrome expressed within 6 months of September 11, 2011 were associated with increased risk of abnormal FEV1 years later [14,32,33]. In the current study, we report that early elevation of serum YKL-40 reduces the risk of having NAFLD, the hepatic expression of metabolic syndrome, as assessed by the L/S ratio.
While 38% of firefighters in our cohort with low lung function (FEV1 < lower limit of normal, LLN) had NAFLD, only 12% of those with normal lung function (FEV1 ≥ LLN) were afflicted by fatty liver. BMI, a well-known risk factor for NAFLD, was also significantly higher in NAFLD cases. We therefore adjusted our final model for both FEV1 % predicted at the time of serum draw and BMI. YKL-40, triglyceride and albumin were predictive biomarkers for NAFLD after these adjustments.
It is interesting that two members of the chitinase protein family are protective biomarkers in this population. YKL-40 is a protective biomarker for NAFLD in WTC-exposed firefighters, while chitotriosidase is not. Conversely, we have previously reported that chitotriosidase, is a protective biomarker of lung dysfunction whereas YKL-40 is not [27]. Having differential associations with lung dysfunction and NAFLD may reflect distinct but overlapping pathophysiologic mechanisms of lung and liver disease after PM exposure. Such overlap is supported by our finding that elevated serum triglyceride is strongly associated with NAFLD in our cohort, just as triglyceride was found to predict abnormal FEV1 in our prior study [14]. Importantly, YKL-40 is enzymatically inactive and reduces risk for toxicity in an organ distant from the lung while chitotriosidase is enzymatically active and reduces risk in the organ most affected by dust inhalation. In a more general sense, ambient PM is known to induce systemic inflammation, oxidative stress response and vascular dysfunction, ultimately affecting multiple organs including liver and lungs [17,34]. The chitinase protein family may reflect counter-regulatory responses induced by PM.
Chitinases and chitinase-like proteins are active immune modulators, with chitotriosidase partaking predominantly in the innate immune system [35] while YKL-40 plays an important role in the Th2 adaptive immune response [36,37]. The effects of YKL-40 in the lung have been well described. YKL-40 over expression in a genetically modified mouse model is strongly associated with a Th2 response [38]. As Th2 cytokines are known to have an anti-inflammatory and would healing function [39], it is conceivable that YKL-40 is protective at the point of metabolic injury in the liver by curbing inflammatory processes leading to NAFLD. However, there have been no direct functional studies describing YKL-40's role in liver metabolism.
That our WTC-PM exposed patients with NAFLD had lower YLK-40 levels than controls may seem inconsistent with the observation that YKL-40 levels are usually increased in other liver diseases [28-30]. These studies were performed after liver disease was established while our investigation measured biomarker expression prior to diagnosis of NAFLD. This suggests potentially differential roles of YKL-40 in patients with a pre- or early NAFLD state versus an advanced fibrotic state of which none of our patients were diagnosed.
Our case control biomarker study has advantages over standard cross-sectional analysis in that serum biomarkers were measured on average within 3 months after acute WTC-PM exposure and CT scans were performed 5 years after serum analysis. It is therefore unlikely that our biomarkers are a consequence of the outcome. It is important to note that we have identified early biomarkers that are predictive of developing NAFLD at a later time point. However, a limitation of our study is that CT scans were not performed at the time of serum draw, precluding the assessment of disease progression or regression over time.
Our study has other limitations. Liver biopsy to pathologically confirm NAFLD was not performed due to excess risk to benefit ratio. The ratio of liver and spleen attenuation in non-contrast CT was therefore used as a noninvasive alternative to assess for fatty infiltration of the liver. We adopted the CT method for our study because it has a good correlation with histological samples [21]. Secondly, our FDNY firefighter cohort incurred massive acute exposure to WTC-PM which limits the generalizability of our findings to other study populations with less or no PM exposure. Finally, we did not have an unexposed control group to compare and therefore were unable to determine the direct effect of WTC-PM exposure on serum biomarkers. Replication of these findings in other longitudinally followed populations with and without PM exposure will ultimately be important to demonstrate the generalizability of our findings.
Increased serum YKL-40 levels were found to reduce the risk of developing NAFLD after WTC-PM exposure in a firefighter cohort, suggesting a protective role for YKL-40 in NAFLD. Further investigation is required to define if YKL-40 has a mechanistic role in mediating the protection against NAFLD after PM exposure.
Abbreviations
- NAFLD
Non-alcoholic Fatty Liver Disease
- FDNY
Fire Department City of New York
- WTC
World Trade Center
- PM
Particulate Matter
- FEV1
Forced Expiratory Volume in 1 Second
- LLN
Lower Limit of Normal
- MME
Medical Monitoring Entry
- SPE
Subspecialty Pulmonary Evaluation
- NHANES
National Health and Nutrition Examination Survey
- PFT
Pulmonary Function Test
- BMI
Body Mass Index
- L/S ratio
Liver/Spleen Attenuation Ratio
- HDL
High-density Lipoprotein
- LDL
Low-density Lipoprotein
- ALT
Alanine Aminotransferase
- AST
Aspartate Transaminase
- ALP
Alkaline Phosphatase
- G-GTP
Gamma-glutamyl Transpeptidase
References
- 1.Day CP. Clinical spectrum and therapy of non-alcoholic steatohepatitis. Dig Dis. 2012;30:69–73. doi: 10.1159/000341128. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity-associated liver disease. J Clin Endocrinol Metab. 2008;93:S74–80. doi: 10.1210/jc.2008-1399. [DOI] [PubMed] [Google Scholar]
- 3.Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38. doi: 10.1161/ATVBAHA.107.147538. [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 7.Luyckx FH, Desaive C, Thiry A, Dewe W, Scheen AJ, et al. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22:222–226. doi: 10.1038/sj.ijo.0800571. [DOI] [PubMed] [Google Scholar]
- 8.de la Hoz RE. Occupational asthma and lower airway disease among World Trade Center workers and volunteers. Curr Allergy Asthma Rep. 2010;10:287–294. doi: 10.1007/s11882-010-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skloot GS, Schechter CB, Herbert R, Moline JM, Levin SM, et al. Longitudinal assessment of spirometry in the World Trade Center medical monitoring program. Chest. 2009;135:492–498. doi: 10.1378/chest.08-1391. [DOI] [PubMed] [Google Scholar]
- 10.Banauch GI, Alleyne D, Sanchez R, Olender K, Cohen HW, et al. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. Am J Respir Crit Care Med. 2003;168:54–62. doi: 10.1164/rccm.200211-1329OC. [DOI] [PubMed] [Google Scholar]
- 11.Banauch GI, Hall C, Weiden M, Cohen HW, Aldrich TK, et al. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department. Am J Respir Crit Care Med. 2006;174:312–319. doi: 10.1164/rccm.200511-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiden MD, Ferrier N, Nolan A, Rom WN, Comfort A, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137:566–574. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldrich TK, Gustave J, Hall CB, Cohen HW, Webber MP, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362:1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naveed B, Weiden MD, Kwon S, Gracely EJ, Comfort AL, et al. Metabolic syndrome biomarkers predict lung function impairment: a nested case-control study. Am J Respir Crit Care Med. 2012;185:392–399. doi: 10.1164/rccm.201109-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JC, Schwartz J. Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environ Health Perspect. 2008;116:612–617. doi: 10.1289/ehp.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laing S, Wang G, Briazova T, Zhang C, Wang A, et al. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol. 2010;299:C736–749. doi: 10.1152/ajpcell.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Z, Xu X, Zhang X, Wang A, Zhang C, et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol. 2013;58:148–154. doi: 10.1016/j.jhep.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawata R, Sakata K, Kunieda T, Saji S, Doi H, et al. Quantitative evaluation of fatty liver by computed tomography in rabbits. AJR Am J Roentgenol. 1984;142:741–746. doi: 10.2214/ajr.142.4.741. [DOI] [PubMed] [Google Scholar]
- 21.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 22.Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007;177:959–970. doi: 10.1534/genetics.107.075846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 24.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- 25.Di Rosa M, Musumeci M, Scuto A, Musumeci S, Malaguarnera L. Effect of interferon-gamma, interleukin-10, lipopolysaccharide and tumor necrosis factor-alpha on chitotriosidase synthesis in human macrophages. Clin Chem Lab Med. 2005;43:499–502. doi: 10.1515/CCLM.2005.088. [DOI] [PubMed] [Google Scholar]
- 26.Malaguarnera L, Musumeci M, Di Rosa M, Scuto A, Musumeci S. Interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. J Clin Lab Anal. 2005;19:128–132. doi: 10.1002/jcla.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho SJ, Nolan A, Echevarria GC, Kwon S, Naveed B, et al. Chitotriosidase is a biomarker for the resistance to World Trade Center lung injury in New York City firefighters. J Clin Immunol. 2013;33:1134–1142. doi: 10.1007/s10875-013-9913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran A, Benzaken S, Saint-Paul MC, Guzman-Granier E, Hastier P, et al. Chondrex (YKL-40), a potential new serum fibrosis marker in patients with alcoholic liver disease. Eur J Gastroenterol Hepatol. 2000;12:989–993. doi: 10.1097/00042737-200012090-00004. [DOI] [PubMed] [Google Scholar]
- 29.Berres ML, Papen S, Pauels K, Schmitz P, Zaldivar MM, et al. A functional variation in CHI3L1 is associated with severity of liver fibrosis and YKL-40 serum levels in chronic hepatitis C infection. J Hepatol. 2009;50:370–376. doi: 10.1016/j.jhep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Fontana RJ, Litman HJ, Dienstag JL, Bonkovsky HL, Su G, et al. YKL-40 genetic polymorphisms and the risk of liver disease progression in patients with advanced fibrosis due to chronic hepatitis C. Liver Int. 2012;4:665–674. doi: 10.1111/j.1478-3231.2011.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, et al. YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. Am J Respir Crit Care Med. 2012;185:692–694. doi: 10.1164/rccm.201202-0203ED. [DOI] [PubMed] [Google Scholar]
- 32.Nolan A, Naveed B, Comfort AL, Ferrier N, Hall CB, et al. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest. 2012;142:412–418. doi: 10.1378/chest.11-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiden MD, Naveed B, Kwon S, Cho SJ, Comfort AL, et al. Cardiovascular biomarkers predict susceptibility to lung injury in World Trade Center dust-exposed firefighters. Eur Respir J. 2013;41:1023–1030. doi: 10.1183/09031936.00077012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mills NL, Törnqvist H, Gonzalez MC, Vink E, Robinson SD, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 35.van Eijk M, van Roomen CP, Renkema GH, Bussink AP, Andrews L, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005;17:1505–1512. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 36.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 37.Ober C, Tan Z, Sun Y, Possick JD, Pan L, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358:1682–1691. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]