Abstract
Aims
To determine the perceived risk of type 2 diabetes in a sample of healthy middle-aged adults and examine the association between perceived risk and modelled risk, clinical risk factors, and psychological factors theorised to be antecedents of behaviour change.
Methods
An exploratory, cross-sectional analysis of perceived risk of type 2 diabetes (framed according to time and in comparison with peers) was conducted using baseline data collected from 569 participants of the Diabetes Risk Communication Trial (Cambridgeshire, UK). Type 2 diabetes risk factors were measured during a health assessment and the Framingham Offspring Diabetes Risk Score was used to model risk. Questionnaires assessed psychological factors including anxiety, diabetes-related worry, behavioural intentions, and other theory-based antecedents of behaviour change. Multivariable regression analyses were used to examine associations between perceived risk and potential correlates.
Results
Participants with a high perceived risk were at higher risk according to the Framingham Offspring Diabetes Risk Score (p < 0.001). Higher perceived risk was observed in those with a higher body fat percentage, lower self-rated health, higher diabetes-related worry, and lower self-efficacy for adhering to governmental recommendations for physical activity (all p < 0.001). The framing of perceived risk according to time and in comparison with peers did not influence these results.
Conclusions
High perceived risk of type 2 diabetes is associated with higher risk of developing the disease, and a decreased likelihood of engagement in risk-reducing health behaviours. Risk communication interventions should target high-risk individuals with messages about the effectiveness of prevention strategies.
Keywords: Type 2 diabetes, Perceived risk, Modelled risk, Psychology, Risk communication, Prevention
1. Introduction
The prevalence of type 2 diabetes (T2D) is rising rapidly worldwide, and it is projected that by the year 2035 there will be 592 million individuals with the disease [1]. T2D is associated with costly increases in morbidity and mortality such that the burden attributable to the disease is threatening the health systems and economies of both developed and developing countries alike [2]. Fortunately, research has shown that the development of T2D can be delayed and all together prevented through health behaviour change [3]. Several randomised controlled trials among those at high risk have reported reductions in the incidence of T2D by approximately 50% following interventions resulting in moderate weight loss (defined as 5–10% of body weight) through healthy changes in physical activity and diet [3]. Attempts to translate these research findings into healthcare and community settings have been undertaken [4], and the screening of individuals at high risk has been recommended by prominent health organisations [5].
In order for translation efforts to be effective, they must target individuals at high risk of T2D who have the greatest potential to benefit from prevention or screening programmes. Several valid risk prediction models have been developed to identify such individuals [6]. Models based solely on routinely collected data (e.g., age, sex, family history, height, weight, etc.) have been incorporated into user-friendly risk assessment tools, and diabetes awareness campaigns have promoted their use among both clinicians and members of the general public [7]. Despite the ubiquity of these tools, it is unclear if individuals at high risk for T2D actually perceive themselves to be at increased risk. If high-risk individuals do not believe that they are at increased risk, they may not be responsive to efforts aimed at promoting risk-reducing behaviours (e.g., engagement in physical activity and a healthy diet) or screening.
Perceived risk is a central construct in several health behaviour theories. Each similarly posits that the motivation to engage in risk-reducing health behaviours or to undergo screening is largely dependent upon whether or not an individual is aware of their susceptibility for a disease [8]. A variety of measures have been used to assess perceived risk, however, there is little agreement on which measures provide the best operationalisation of the construct [9]. It is unclear if framing measures (i.e., presenting them in reference to a specific schema) according to time (e.g., 10 years or a lifetime) and/or in comparison with peers influences an individual’s assessment of their risk [10].
The majority of studies that have assessed perceived risk of T2D have focused on differences according to family history and report that individuals determined to be at high risk based on a positive family history are frequently unaware of their increased risk [11]. Although family history is a significant risk factor for T2D, it is only one of several important influences on risk [6]. Very few studies have examined the association between perceived risk and modelled risk calculated using a validated epidemiological model for predicting incident T2D that incorporates multiple risk factors. Those that have report inconsistent findings [12–14], and associations between perceived risk of T2D and cognitive and emotional antecedents to behaviour change that are often the target of interventions have scarcely been studied and remain unclear [14].
In the present study, we examined perceived risk of T2D in a population-based sample of healthy middle-aged adults. We determined whether or not framing according to time and in comparison with peers influenced the measurement of perceived risk and evaluated if measures were in accordance with modelled risk and other risk factors. We also explored the associations between perceived risk and multiple theory-based antecedents of behaviour change. In order for T2D prevention programmes to successfully reach their intended targets, high-risk individuals must first acknowledge that they are at high risk. A deeper understanding of the factors associated with perceived risk might inform the development of more effective risk communication and prevention efforts.
2. Materials and methods
2.1. Design
This cross-sectional study utilised baseline data collected as part of a randomised controlled trial, the Diabetes Risk Communication Trial (DRCT) [15]. All of the variables in the present study were collected at baseline, prior to randomisation and receipt of any intervention materials. The study obtained full ethical approval from the Cambridgeshire 1 Research Ethics Committee on the 21st of October 2010 (reference number 10/H0304/78).
2.2. Participants and setting
Participants of the DRCT were recruited from the Fenland Study, an ongoing population-based, observational study investigating the influence of lifestyle and genetic factors on the development of diabetes, obesity, and related metabolic disorders [16]. Patients born between 1950 and 1975 and registered with participating general practices in Cambridgeshire, UK were invited to take part. Exclusion criteria assessed by general practitioners included being diagnosed with diabetes, a terminal illness with a prognosis of less than one year, or a psychotic illness. Those who were pregnant or lactating, or unable to walk unaided were also excluded. Between February and September 2011, invitations to take part in the DRCT were sent to those who (1) had agreed to be contacted regarding future studies; (2) had sufficient data to calculate their genetic and phenotypic risk of T2D; (3) wore a combined heart rate monitor and accelerometer to measure free-living physical activity for three or more full days without experiencing a severe skin reaction; and (4) provided at least three days worth of complete physical activity data. Upon response, those who reported being diagnosed with diabetes or actively participating in another study were excluded.
2.3. Measures
During the Fenland Study, participants underwent a health assessment. Anthropometric (e.g., height, weight, waist circumference), body composition (e.g., body fat percentage and distribution using DEXA), clinical (e.g., blood pressure), and physical activity measurements (e.g., heart rate, movement, and oxygen consumption at rest and during a sub-maximal treadmill test) were assessed by trained staff. An oral glucose tolerance test was administered, and two blood samples were taken to assess glucose levels and blood lipids. Demographics, medical history, and general lifestyle were assessed through self-report [16]. Actual risk of T2D was calculated using the continuous version of the previously validated Framingham Offspring Diabetes Risk Score, which has an area under the receiver operating characteristic curve (AUC) equal to 0.881 (an AUC of 1.0 indicates perfect prediction of incident T2D and 0.5 indicates no prediction). The model includes measures of age, sex, parental history of T2D, body mass index (calculated as objectively measured weight in kg divided by height in m2), waist circumference, systolic blood pressure, high-density lipoprotein cholesterol, and triglycerides [6].
After enrolment in the DRCT (median 1.76 years after participation in the Fenland Study), all participants completed an additional questionnaire. We assessed self-rated health by asking participants to rate their overall health as excellent, good, fair, or poor [17]. In order to have a measure of weight that was precisely contemporaneous with the measurement of perceived risk, self-reported weight was assessed by asking participants what their current weight is, without shoes, in either stones and pounds or kilograms [18]. Perceived severity of T2D (belief about how serious the disease and its consequences are) was measured using two Likert items. Physical activity and diet self-efficacy (the individual’s belief that they can adhere to guidelines for physically activity and fruit and vegetable consumption) and response efficacy (belief that adhering to the guidelines for physical activity and fruit and vegetable consumption has beneficial effects) were each assessed with one Likert item. All items included a statement (e.g., “I feel confident in my ability to be active at a moderate intensity for at least 30 minutes per day on at least 5 days a week over the next 8weeks.”), evaluated on a 5-point response scale, ranging from “strongly disagree” to “strongly agree” [19]. We assessed intention to adhere to the physical activity and diet guidelines each with one Likert item. Items included a statement (e.g., “I intend to be active at a moderate intensity for at least 30 minutes per day on at least 5 days a week over the next 8 weeks.”), evaluated on a 5-point response scale, ranging from “extremely unlikely” to “extremely likely” [19]. Anxiety was assessed using the short-form of the state scale of the Spielberger State Trait Anxiety Inventory [20]. Scores range from 20 to 80 and higher scores indicate higher levels of anxiety. Diabetes-related worry was assessed using the Cancer Worry Scale adapted for use in the context of T2D [21]. Scores range from 6 to 24 and higher scores indicate higher levels of worry.
We assessed absolute and comparative perceived risk of T2D within the time span of 10 years and a lifetime. Absolute risk was measured using both a continuous and categorical measure. Participants were asked, “How likely are you to get type 2 diabetes in the next 10 years/in your lifetime?” They answered on a 5-point response scale, ranging from “very unlikely” to “very likely”. Participants were also asked, “On a scale from 0 to 100, where 0 = certain not to happen, and 100 = certain to happen, how likely are you to get type 2 diabetes in the next 10 years/in your lifetime?” Comparative risk was measured categorically, by asking participants, “Compared with other people your sex and age, how likely are you to get type 2 diabetes in the next 10 years/in your lifetime?” They answered on a 5-point response scale, ranging from “much less likely” to “much more likely”. These items were adapted according to recommendations provided by Diefenbach et al. [9], and have been used in previous research [22].
2.4. Statistical analysis
All statistical analyses were conducted using STATA software [23] and two-tailed p-values with the predefined cut-off for statistical significance set at 0.05. Descriptive statistics (proportions, means, and standard deviations (SD)) were used to describe key demographic and health characteristics. We examined to what extent framing influenced the relation between measures of perceived risk using Spearman’s rank correlation coefficient. 95% confidence intervals were calculated using the bootstrap method [24] and correlations greater than 0.70 were interpreted as high [25]. Univariable associations with absolute lifetime perceived risk were assessed with linear regression. Ordered logistic regression was used to examine associations with comparative lifetime perceived risk. Statistically significant variables were selected for inclusion in multivariable regression models, adjusted for age and sex. Multicollinearity was checked and variance inflation factor statistics for each variable were less than 2.5. Variables that did not retain their significance were manually removed, one at a time, starting with the variable with the highest p-value, until a model containing only variables with significant associations was achieved. To distinguish associations between proximal and distal factors, and to avoid overadjustment (i.e., the inclusion of variables hypothesised to be on the causal pathway), the psychological factors were modelled separately from modelled risk and clinical risk factors. Those with missing data were not included in the analyses.
3. Results
Invitations to take part in the DRCT were mailed to 1150 Fenland Study participants, 635 (55%) replied positively and were assessed for eligibility, and 569 (49%) completed baseline. Table 1 shows the participants’ characteristics. The majority were middle-aged (ranging from 35 to 61 years) and female. Most obtained at least a secondary school level of education and were employed full-time. Participants were overweight (mean (SD) body mass index 26.10 (4.23) kg/m2), 111 (19.9%) had a parental history of diabetes, andon average HbA1c levels were in the normal range. The vast majority rated their health as either good or excellent.
Table 1.
Sample characteristics.
| n | ||
|---|---|---|
| Demographic factors | ||
| Age (years) | 558 | 48.8 (7.3) |
| Male, n (%) | 558 | 265 (47.5%) |
| Age ending full-time education (years) | 549 | 19.5 (4.5) |
| Employed full-time, n (%) | 548 | 373 (68.1%) |
| Modelled risk and risk factors | ||
| Framingham Offspring Study Diabetes Risk Score (0–100) | 552 | 11.7 (19.0) |
| HbA1c (% and mmol/mol) | 556 | 5.5 (0.7) (36.4 (4.4)) |
| VO2 max (ml/kg/min) | 549 | 36.0 (7.2) |
| Body fat percentage (%) | 555 | 31.8 (9.2) |
| Self-reported weight (kg) | 551 | 75.7 (14.6) |
| Self-rated health good or excellent, n (%) | 555 | 470 (84.7%) |
| Psychological factors | ||
| Worry (6–24) | 558 | 7.5 (1.9) |
| Anxiety (20–80) | 523 | 33.0 (10.9) |
| Intention (1–5) | ||
| Physical activity | 557 | 3.8 (1.0) |
| Diet | 557 | 3.8 (1.0) |
| Response efficacy (1–5) | ||
| Physical activity | 556 | 3.9 (0.7) |
| Diet | 557 | 3.9 (0.7) |
| Self efficacy (1–5) | ||
| Physical activity | 557 | 3.9 (0.9) |
| Diet | 557 | 3.9 (1.0) |
| Perceived severity (1–5) | 555 | 3.6 (0.8) |
Values are means (standard deviations) unless otherwise specified.
3.1. Framing of perceived risk
Of all 569 participants, 11 (1.9%) did not have complete data for each measure of perceived risk of T2D and were excluded from the analyses. The mean of each measure of perceived risk framed within the next 10 years was less than the mean of the same measure framed within a lifetime, but the correlations between 10-year and lifetime measures were high (Fig. 1). The continuous measure of absolute lifetime risk was highly correlated with the categorical measure of absolute lifetime risk, but each was only moderately correlated with comparative lifetime risk. Based on these findings, only the continuous measure of absolute lifetime risk and the comparative lifetime risk measure were examined in subsequent analyses.
Fig. 1.
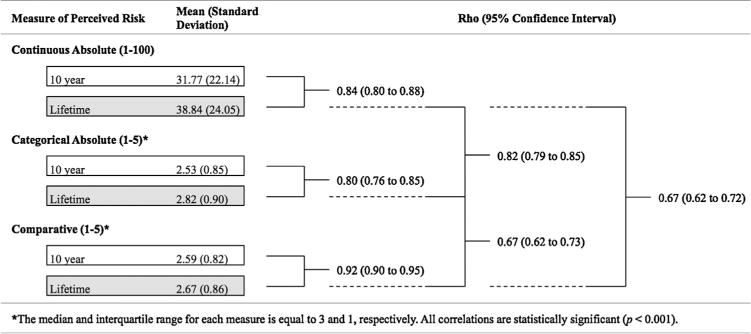
Summary statistics and Spearman’s rank correlations between 10 year and lifetime measures of continuous absolute, categorical absolute, and comparative perceived risk of type 2 diabetes (n = 588).
3.2. Univariable associations
Table 2 shows the univariable associations between perceived risk of T2D, modelled risk and risk factors, and the theory based antecedents of behaviour change. None of the sociodemographic factors were associated with perceived risk of T2D (all p > 0.05), so they were not used as covariates in subsequent analyses. Participants with high perceived risk were at higher risk according to the Framingham Offspring Diabetes Risk Score. Additionally, HbA1c level, body fat percentage, and self-reported weight were positively associated with perceived risk, whereas VO2 max, and self-rated health were negatively associate with perceived risk.
Table 2.
Univariable associations of lifetime measures of continuous absolute and comparative perceived risk of type 2 diabetes with modelled risk, risk factors, and theory-based antecedents of behaviour change.
| n | Continuous absolute (1–100)a
|
Comparative (1–5)b
|
|||||
|---|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | ||
| Modelled risk and risk factors | |||||||
| Framingham Offspring Study Diabetes Risk Score (0–100) | 552 | 0.27 | 0.17–0.38 | <0.001 | 0.03 | 0.02–0.04 | <0.001 |
| HbA1c (mmol/mol) | 556 | 0.52 | 0.07–0.97 | 0.03 | 0.04 | 0.004–0.07 | 0.03 |
| VO2 max (ml/kg/min) | 549 | −0.34 | −0.62 to −0.06 | 0.02 | −0.04 | −0.06 to −0.02 | <0.001 |
| Body fat percentage (%) | 555 | 0.61 | 0.39–0.82 | <0.001 | 0.07 | 0.05–0.08 | <0.001 |
| Self-reported weight (kg) | 551 | 0.27 | 0.14–0.41 | <0.001 | 0.03 | 0.02–0.04 | <0.001 |
| Self-rated health good or excellent (yes) | 555 | −13.64 | −19.07 to −8.20 | <0.001 | −1.36 | −1.80 to −0.93 | <0.001 |
| Psychological factors | |||||||
| Worry (6–24) | 558 | 6.15 | 5.22–7.07 | <0.001 | 0.50 | 0.40–0.59 | <0.001 |
| Anxiety (20–80) | 523 | 0.29 | 0.10–0.48 | <0.01 | 0.04 | 0.02–0.05 | <0.001 |
| Intention (1–5) | |||||||
| Physical activity | 557 | −3.61 | −5.54 to −1.69 | <0.001 | −0.41 | −0.57 to −0.26 | <0.001 |
| Diet | 557 | 0.09 | −1.96 to 2.14 | 0.93 | −0.16 | −0.32 to 0.002 | 0.05 |
| Response efficacy (1–5) | |||||||
| Physical activity | 556 | −5.66 | −8.72 to −2.61 | <0.001 | −0.60 | −0.84 to −0.35 | <0.001 |
| Diet | 557 | −4.84 | −7.71 to −1.97 | <0.01 | −0.59 | −0.82 to −0.36 | <0.001 |
| Self efficacy (1–5) | |||||||
| Physical activity | 557 | −6.11 | −8.23 to −3.99 | <0.001 | −0.67 | −0.85 to −0.49 | <0.001 |
| Diet | 557 | −1.44 | −3.53 to 0.65 | 0.18 | −0.31 | −0.47 to −0.15 | <0.001 |
| Perceived severity (1–5) | 555 | 0.79 | −1.78 to 3.36 | 0.55 | −0.003 | −0.21 to 0.20 | 0.98 |
CI: confidence interval.
Values were calculated using linear regression.
Values were calculated using ordinal regression.
Participants with high perceived risk had higher diabetes-related worry and anxiety. Physical activity intentions, response efficacy, and self-efficacy were each negatively associated with perceived risk. Diet intentions were unrelated to perceived risk. Diet response efficacy was negatively associated with perceived risk. Diet self-efficacy was negatively associated with comparative lifetime risk, but was unrelated to the continuous measure of absolute lifetime risk. Perceived severity was unrelated to perceived risk.
3.3. Multivariable associations
Table 3 shows the multivariable associations. The results of model 1 show that after mutually adjusting for all of the measured risk factors, high perceived risk was associated with higher modelled risk and body fat percentage. Also, self-rated health was negatively associated with perceived risk. Model 2 shows that high perceived risk was associated with higher diabetes-related worry and low self-efficacy for physical activity. Physical activity response efficacy was negatively associated with the continuous measure of absolute lifetime risk, but not the comparative measure. Anxiety was positively associated with comparative lifetime risk, but not the continuous measure of absolute lifetime risk. Similarly, diet response efficacy and physical activity response efficacy were negatively associated with only comparative lifetime risk.
Table 3.
Multivariable regression models showing the associations of lifetime measures of continuous absolute and comparative perceived risk of type 2 diabetes with modelled risk, risk factors (n = 530), and theory-based antecedents of behaviour change (n = 521).
| Continuous absolute (1–100)a
|
Comparative (1–5)b
|
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | |
| Modelled risk and risk factors (Model 1) | ||||||
| Framingham Offspring Study Diabetes Risk Score (%) | 0.19 | 0.09–0.30 | <0.001 | 0.02 | 0.01–0.03 | <0.001 |
| HbA1c (mmol/mol) | Removed from model | Removed from model | ||||
| VO2 max (ml/kg/min) | Removed from model | Removed from model | ||||
| Body fat percentage (%) | 0.44 | 0.22–0.66 | <0.001 | 0.05 | 0.03–0.07 | <0.001 |
| Self-reported weight (kg) | Removed from model | Removed from model | ||||
| Self-rated health good or excellent (yes) | −9.55 | −15.04 to −4.05 | <0.01 | −1.01 | −1.46 to −0.55 | <0.001 |
| Psychological factors (Model 2)c | ||||||
| Worry | 5.98 | 5.04–6.92 | <0.001 | 0.48 | 0.37–0.58 | <0.001 |
| Anxiety | Removed | from model | 0.02 | 0.00–0.03 | <0.05 | |
| Intention | ||||||
| Physical activity | Removed from model | Removed from model | ||||
| Diet | – | – | – | – | – | – |
| Response efficacy | ||||||
| Physical activity | −3.52 | −6.31 to −0.72 | <0.05 | Removed from model | ||
| Diet | Removed from model | −0.51 | −0.76 to −0.25 | <0.001 | ||
| Self efficacy | ||||||
| Physical activity | −4.54 | −6.47 to −2.60 | <0.001 | −0.61 | −0.80 to −0.42 | <0.001 |
| Diet | – | – | – | Removed from model | ||
CI: confidence interval.
Values were calculated using linear regression.
Values were calculated using ordered logistic regression.
Values are adjusted for age and sex.
4. Discussion
This study showed that healthy middle-aged adults who perceived themselves to be at high risk of developing T2D were actually at higher risk according to the Framingham Offspring Diabetes Risk Score. This finding was bolstered by the observation that additional T2D risk factors, such as body fat percentage and self-rated health, were similarly associated with perceived risk. Our findings are in line with those of Hivert et al., who showed that primary care patients with higher perceived risk were at higher risk based on the same epidemiological model used in the present study [14]. However, studies that have assessed risk using family history alone [11] or with epidemiological models that include only self-report measures [12,13] have contradictory results that suggest individuals are unaware of their risk. This discrepancy may be due to other studies relying on imprecise assessments of risk, as well as frequent dichotomisation of individuals into categories of high and low risk, which is likely to cause misclassification.
Based upon previous research, individuals with higher perceived risk of T2D were expected to also have higher levels of worry about the disease, and this was the case in our sample [11]. Contrary to expectations, high perceived risk was not consistently associated with anxiety or intention to comply with physical activity and diet guidelines. Those with a high perceived risk did express lower physical activity self-efficacy, which implies that they are unlikely to engage in healthy levels of physical activity. Although the associations between perceived risk and physical activity and diet response efficacy and diet self-efficacy were mixed, when associations did exist, they were consistently negative. This again implies that those with a high perceived risk are also unlikely to engage in healthy levels of physical activity and a healthy diet. These findings reinforce the need for risk communication efforts to target individuals at high risk with clear explanations of the health benefits of complying with recommendations about physical activity and diet, and they should provide individuals with information about how they might achieve meeting the guidelines.
Those with a high perceived risk of T2D do not appear to believe that the disease is more or less severe than those with a low perceived risk. Risk communication efforts should be explicit regarding the severity of the disease. Although those at high risk are likely to already be worried about their risk, such negative emotions are unlikely to have significant psychological consequences [26].
It is widely recognised that perceived risk is a multidimensional construct and that individuals often do not assess their risk in ways that are entirely rational [27]. Subsequently, there is no consensus regarding which measure of perceived risk researchers should use [9], and it is unclear what influence framing has on measurement [10]. An important strength of the present study is the use of multiple measures of perceived risk with varied framing. We found that 10-year and lifetime measures were highly related, suggesting that individuals did not differentiate greatly between short-term and long-term risk. Additionally, measures of absolute risk were moderately related to comparative risk. With few exceptions, the associations between the different measures of perceived risk, actual risk, and the theory-based antecedents of behaviour change were consistent. This provides much needed evidence that the different measures represent a similar construct, and it contributes to the sparse literature addressing the best approach to measuring perceived risk [9].
It is important to highlight that, on average, participants’ perceived risk according to the continuous absolute measure was higher than their corresponding modelled risk. In this study, we did not attempt to describe the magnitude of the discrepancy between perceived risk and modelled risk, nor did we examine whether or not the individual’s degree of accuracy in assessing their risk might also be related to the cognitive and emotional antecedents of behaviour change. This represents a potential area for future research, as it might have implications for the impact of communicating T2D risk information. More specifically, if a high perceived risk suggests a greater likelihood of engagement in risk reducing behaviours, then the provision of a risk estimate that results in perceived risk becoming more accurate (i.e., substantially lower) might provide a false sense of reassurance and result in a lack of motivation or intention to change behaviour.
The results of this study should be considered within its limitations. With the exceptions of anxiety, worry, and perceived severity, we relied on single-item measures. A more comprehensive assessment of the cognitive and emotional antecedents of behaviour change is necessary, and future research should evaluate whether or not measures of perceived risk that include multiple items or a qualitative component may be preferable to those used here. Qualitative data may also be helpful in explaining why some of the observed associations were inconsistent across the different measures of perceived risk, and it may shed light on whether or not the inconsistencies were due to chance, bias, or confounding. In addition, previous research shows that numeracy can influence understanding of risk [28], however, we did not evaluate its impact on perceived risk. Participants were from one location in the United Kingdom and were physically and psychologically healthy. Therefore, the results might not generalise to other settings or to those who are less healthy. Finally, the cross-sectional study design prohibits the establishment of any temporal associations.
5. Conclusions
In this population-based sample of middle-aged adults, we found that high perceived risk of T2D was associated with higher risk of developing the disease. Only one other study that we know of has examined this association using a robust epidemiological model and other well-established risk factors for T2D [14]. Thus, more research is needed to determine the extent to which perceived risk is related to modelled risk. It is also important for future studies to examine the discrepancy between perceived risk and modelled risk, and determine if it might be a barrier to the uptake of preventive measures. This is particularly important as diabetes prevention efforts become more widely implemented. Our study also showed that high perceived risk of T2D was associated with higher diabetes-related worry and less strong beliefs in the ability to adhere to governmental recommendations for physical activity. Furthermore, the framing of perceived risk did not influence these results. These findings point to a need for risk communication interventions that focus on the effectiveness of preventive measures and address the individual’s ability to engage in risk-reducing behaviours.
Acknowledgments
We thank all staff from the MRC Epidemiology Functional Group Team in particular for study coordination, data collection, physical activity data processing, anthropometry data processing, data management, associated laboratory work, business operations, IT, and research governance. We thank Stephen J. Sharp for providing advice on the statistical analyses. We also thank those who participated in the study.
Funding: This study was funded by the Medical Research Council (MC_U106179474) and was conducted at the MRC Epidemiology Unit in Cambridge, UK. SJG receives support from the Department of Health NIHR Programme Grant funding scheme (RP-PG-0606-1259).
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
Authors contributions
JGG, EMFvS, and SJG defined the research question. JGG wrote the statistical analysis plan, conducted the statistical analyses, and drafted the manuscript. All authors have contributed to the interpretation of the results, were involved in critical revisions, and have read and approved the final manuscript. All authors contributed to the development of the DRCT and the measures utilised. JGG created the study materials and coordinated the study throughout.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence in adults for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, Shaw J, et al. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ. 2007;334:299. doi: 10.1136/bmj.39063.689375.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson M, Jones R, Freeman C, Woods HB, Gillett M, Goyder E, et al. Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence. Diabetes Med. 2013;30:3–15. doi: 10.1111/dme.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diabetes UK. Early Identification of people with and at high risk of Type 2 diabetes and interventions for those at high risk. London: 2012. [Google Scholar]
- 6.Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev. 2011;33:46–62. doi: 10.1093/epirev/mxq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes UK. Diabetes risk score. https://www.diabetes.org.uk/Riskscore/ [accessed 17.03.13]
- 8.Brewer NT, Weinstein ND, Cuite CL, Herrington JE. Risk perceptions and their relation to risk behavior. Ann Behav Med. 2004;27:125–30. doi: 10.1207/s15324796abm2702_7. [DOI] [PubMed] [Google Scholar]
- 9.Diefenbach M, Weinstein N, O’Reilly J. Scales for assessing perceptions of health hazard susceptibility. Health Educ Res. 1993;8:181–92. doi: 10.1093/her/8.2.181. [DOI] [PubMed] [Google Scholar]
- 10.Edwards A, Elwyn G. Presenting risk information—a review of the effects of ‘framing’ and other manipulations on patient outcomes. J Health Commun. 2001;6:61–82. doi: 10.1080/10810730150501413. [DOI] [PubMed] [Google Scholar]
- 11.Dorman JS, Valdez R, Liu T, Wang C, Rubinstein WS, O’Neill SM, et al. Health beliefs among individuals at increased familial risk for type 2 diabetes: implications for prevention. Diabetes Res Clin Pract. 2012;96:156–62. doi: 10.1016/j.diabres.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham GN, Leath B, Payne K, Guendelman M, Reynolds G, Kim S, et al. Perceived versus actual risk for hypertension and diabetes in the African American community. Health Promot Pract. 2006;7:34–46. doi: 10.1177/1524839905283891. [DOI] [PubMed] [Google Scholar]
- 13.Adriaanse MC, Twisk JWR, Dekker JM, Spijkerman AMW, Nijpels G, Heine RJ, et al. Perceptions of risk in adults with a low or high risk profile of developing type 2 diabetes; a cross-sectional population-based study. Patient Educ Couns. 2008;73:307–12. doi: 10.1016/j.pec.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Hivert M, Warner A, Shrader P, Grant R, Meigs J. Diabetes risk perception and intention to adopt healthy lifestyles among primary care patients. Diabetes Care. 2009;32:1820–1822. doi: 10.2337/dc09-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godino JG, van Sluijs EMF, Marteau TM, Sutton S, Sharp SJ, Griffin SJ. Effect of communicating genetic and phenotypic risk for type 2 diabetes in combination with lifestyle advice on objectively measured physical activity: protocol of a randomised controlled trial. BMC Public Health. 2012;12:444. doi: 10.1186/1471-2458-12-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rolfe E, Loos R, Druet C, Stolk R, Ekelund U, Griffin S, et al. Association between birth weight and visceral fat in adults. Am J Clin Nutr. 2010;92:347–52. doi: 10.3945/ajcn.2010.29247. [DOI] [PubMed] [Google Scholar]
- 17.Fayers P, Sprangers M. Understanding self-rated health. Lancet. 2002;359:3–4. doi: 10.1016/S0140-6736(02)07466-4. [DOI] [PubMed] [Google Scholar]
- 18.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–26. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 19.Blue CL. Does the theory of planned behavior identify diabetes-related cognitions for intention to be physically active and eat a healthy diet? Public Health Nurs. 2007;24:141–50. doi: 10.1111/j.1525-1446.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 20.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31:301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 21.Rees G, Fry A, Cull A, Sutton S. Illness perceptions and distress in women at increased risk of breast cancer. Psychol Health. 2004;19:749–65. [Google Scholar]
- 22.Lipkus I, Kuchibhatla M, McBride C, Bosworth H, Pollak K, Siegler I, et al. Relationships among breast cancer perceived absolute risk, comparative risk, and worries. Cancer Epidemiol Biomark Prev. 2000;9:973–5. [PubMed] [Google Scholar]
- 23.StataCorp. Stata Statistical Software: Release. 12:2011. [Google Scholar]
- 24.Haukoos JS, Lewis RJ. Advanced statistics: bootstrapping confidence intervals for statistics with difficult distributions. Acad Emerg Med. 2005;12:360–5. doi: 10.1197/j.aem.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Kraemer H, Morgan G, Leech N, Gliner J, Vaske J, Harmon R. Measures of clinical significance. J Am Acad Child Adolesc Psychiatry. 2003;42:1524–9. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
- 26.Park P, Simmons RK, Prevost AT, Griffin SJ. Screening for type 2 diabetes is feasible, acceptable, but associated with increased short-term anxiety: a randomised controlled trial in British general practice. BMC Public Health. 2008;8:350. doi: 10.1186/1471-2458-8-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein N. What does it mean to understand a risk? Evaluating risk comprehension. J Natl Cancer Inst Monogr. 1999;25:15–20. doi: 10.1093/oxfordjournals.jncimonographs.a024192. [DOI] [PubMed] [Google Scholar]
- 28.Gigerenzer G, Edwards A. Simple tools for understanding risks: from innumeracy to insight. BMJ. 2003;327:741–4. doi: 10.1136/bmj.327.7417.741. [DOI] [PMC free article] [PubMed] [Google Scholar]


