Abstract
The study examined Hypothalamus Pituitary Adrenal (HPA) axis and inflammatory signaling in 206 youth with histories of prenatal drug exposure and self reported histories of maltreatment. Youth with histories of severe neglect showed elevated levels of cortisol, the end product of the HPA axis, in comparison to youth with lower or minimal levels of neglect. Histories of severe neglect also were associated with increased levels of Macrophage Migration Inhibitory Factor (MIF), a cytokine known to be intricately involved in HPA axis regulation. Salivary MIF levels also were positively associated with youth age and prenatal drug exposure. These MIF and cortisol alterations may signal pathophysiological disruptions in the neuro endocrine and immune systems, which may lead to trajectories of increased disease risk among vulnerable youth. Our findings also provide preliminary support for the validity and reliability of a noninvasive salivary assessment of MIF.
Keywords: parental care, HPA axis, immune system, stress, adolescent
INTRODUCTION
Individuals reared in abusive or neglecting families face increased risk of a variety of long term psychiatric and health problems including depression, cardiovascular disease, obesity, inflammatory illnesses, and cancer (Anda et al., 2009; Dube et al., 2009; Morton et al., 2012; Wegman and Stetler, 2009). Emerging evidence suggests that childhood maltreatment leads to dysregulation in psychobiological stress and immune system responses, which may underlie risk for long-term mental and physical health problems (Miller et al., 2011). Maltreatment-related alterations in cortisol, the end product of the Hypothalamus-Pituitary-Adrenal (HPA) axis, have been demonstrated both at basal levels and in response to stress (Heim et al., 2000; Tarullo and Gunnar, 2006). Evidence for heightened inflammatory signaling among maltreated youth is growing (Danese et al., 2007).
Recent work suggests psychological stress induces alterations in Macrophage Migration Inhibitory Factor (MIF) (Edwards et al., 2010; Katsuura et al., 2010), a pro-inflammatory cytokine and counter-regulator of glucocorticoids such as cortisol (Flaster et al., 2007). However, it is unclear whether alterations in MIF signaling also may be observed among youth with histories of extreme psychological stress involving childhood maltreatment. To further research in this area, the current study examined co-occurring alterations in HPA axis and MIF signaling in a sample of adolescents who vary in their reporting of maltreatment-related experiences and childhood stress.
Dysregulation in the HPA axis is a well-established consequence of childhood maltreatment (Heim et al., 2008; Tarullo and Gunnar, 2006). The HPA axis, a component of the neuroendocrine system, reflects a complex system of direct influences and feedback interactions necessary for adapting to acute and immediate stress. This system also supports the maintenance of circadian rhythms, homeostasis, and metabolic and immune functioning (de Kloet et al., 1996). Persistent activation of this system can promote long-term pathogenesis associated with disease risk (Miller et al., 2009) and is therefore hypothesized to mediate associations between harsh early rearing experiences and long-term mental and physical health problems.
Growing evidence suggests that harsh or neglecting rearing environments also lead to increased inflammatory signaling among youth (Danese et al., 2009; Miller and Chen, 2010; Slopen et al., 2013) and adults (Carpenter et al., 2010; Danese et al., 2007, 2009; Kiecolt-Glaser et al., 2011; Pace et al., 2006; Tarullo and Gunnar, 2006), which may contribute to elevated disease risk later in life. In addition to previously identified inflammatory markers (i.e., Creactive protein, IL-6, TNF-α), MIF may serve as a candidate biomarker for maltreatment-induced dysregulation in inflammatory signaling. MIF was originally discovered for its role in the innate immune system, whereby it mediates macrophage activity at the site of inflammation. It is now well known for having a pleiotropic repertoire of functions (Calandra and Roger, 2003) implicated in a number of pathologies that underlie a variety of chronic diseases (Bifulco et al., 2008; Nishihira and Mitsuyama, 2009; Zernecke et al., 2008).
MIF’s close association with the HPA axis, as well as glucocorticoid regulation more generally, makes it a compelling biomarker for inflammatory activity among maltreated youth. Similar to diurnal cycles of cortisol, MIF levels exhibit a circadian rhythm, with levels fluctuating between 2 and 6 ng/ml, and peak levels evident 2–3 hr in advance, possibly reflecting its role in serving as a glucocorticoid counter-regulator (Petrovsky et al., 2003). Animal studies find that MIF is expressed and released at all levels of the HPA axis including the hypothalamus, the pituitary gland (whereby it is released by the same pituitary cell types that release ACTH; (Bernhagen et al., 1993; Nishino et al., 1995b), and the adrenal glands (Bacher et al., 1997; Tampanaru-Sarmesiu et al., 1997). A body of work supports MIF’s antagonistic relationship with cortisol, in that it renders immune cells less sensitive to the anti-inflammatory actions of glucocorticoids (Calandra and Bucala, 1995). Animal studies show that MIF and cortisol are inversely associated (Calandra et al., 1995; Fingerle-Rowson et al., 2003; Nishino et al., 1995a; Tierney et al., 2005; Waeber et al., 1999), however, this inversion is less consistently observed among humans (Isidori et al., 2002; Petrovsky and Bucala, 2000).
Growing evidence suggests that psychological stress can lead to alterations in MIF levels, via alterations in HPA axis functioning (Calandra et al., 1995; Edwards et al., 2010). MIF elevations are observed in response to psychological stressors (Edwards et al., 2010; Katsuura et al., 2010) and circulating MIF levels have been associated with depressive and anxiety symptoms in a number of human (Christian et al., 2010; Edwards et al., 2010; Musil et al., 2011) and animal (Conboy et al., 2011) studies. It is currently unclear whether extreme childhood stress in the form of maltreatment may be associated with alterations in MIF levels. To address this question, the current study explored associations between youth self-reported histories of maltreatment and dysregulation of peripheral levels of cortisol and MIF during adolescence. We hypothesized that youth exposed to maltreatment would show alterations in MIF and cortisol levels, relative to youth without prior trauma histories. A number of studies suggest that subtypes of childhood maltreatment may differentially influence HPA dysregulation, with neglect often predicting dysregulation above and beyond other types of maltreatment (Carpenter et al., 2007; Flory et al., 2009). Therefore, we explored associations between HPA axis and MIF levels in connection with overall maltreatment and its specific subtypes.
METHODS
Participants
This study examined data from a longitudinal investigation of 206 high risk youth from low income families with and without prenatal drug exposure. Families in this study had been followed biannually since birth. Participants were recruited from a prenatal care/women’s center and a delivery ward in a large urban hospital in the Northeast.
Youth (53% female) ranged in age from 14.4 to 19 years of age (M = 15.3, SD = 1.06). Among them, 22% were not exposed to drugs prenatally, 15% were exposed to alcohol and tobacco, and 61% were exposed to cocaine or other drugs. In terms of race and ethnic distribution of this sample, 12% were Caucasian (non Hispanic), 5% were Hispanic, and 83% African American.
Youth were invited to participate in this follow up study if they had an IQ over 80, did not have an acute psychiatric disorder or chronic medical condition, and, if female were not pregnant. Adolescents also were asked to report on their health status and medication usage on the day of the visit, and girls were asked to report on hormonal birth control use, as these factors may affect HPA axis functioning.
MEASURES
Childhood Trauma Questionnaire (CTQ)
Youth completed the CTQ at a laboratory visit as part of the larger longitudinal study. The CTQ is a 28-item self-report questionnaire of prior maltreatment experiences. This instrument yields a total childhood maltreatment score, representing severity of childhood maltreatment exposure, regardless of subtype.
Subscale scores for five subdomains: physical abuse, physical neglect, emotional abuse, emotional neglect, and sexual abuse are also provided. In the present study, the number of subtypes of maltreatment reported ranged from 0 to 5 (M = 2.6, SD = 1.4), and, as expected, maltreatment subtypes were correlated with each other (with r’s ranging from .18 to .54). Established cutoff scores for each subscale were used to classify abuse histories according to severity (Bernstein et al., 1994). According to these cut offs, subscale scores were divided into three levels of severity: none/minimal, low, and moderate to severe (see Table 1 for descriptive statistics). The CTQ has good reliability and convergent and divergent validity with other measures of maltreatment (Bernstein et al., 1994).
Table 1.
Descriptive Statistics for Reports of Childhood Maltreatment
| CTQ Raw Scores |
Classification According to Severity N(%) |
|||||
|---|---|---|---|---|---|---|
| CTQ Domain | Min | Max | M (SD) | None to Minimal | Moderate | Severe |
| Emotional Abuse | 5 | 20 | 7.8 (3.4) | 134 (65.7) | 47 (23) | 23 (11.3) |
| Physical Abuse | 5 | 20 | 6.61 (2.7) | 161 (78.9) | 20 (8.4) | 23 (9.7) |
| Emotional Neglect | 5 | 22 | 9.6 (4.2) | 114 (55.9) | 62 (30.4) | 28 (13.7) |
| Physical Neglect | 5 | 15 | 6.9 (2.6) | 137 (67.5) | 28 (13.8) | 38 (18.7) |
| Sexual Abuse | 5 | 25 | 5.8 (2.9) | 173 (84.8) | 13 (6.4) | 18 (8.8) |
| Total Score | 25 | 79 | 37.0(11.2) | – | – | – |
Prenatal Drug Exposure
Positive histories of prenatal drug exposure were defined through self-report interviews and toxicology screens. The Addiction Severity Interview (ASI) (Mclellan et al., 1980) was administered to mothers at the first prenatal visit or (for those who did not receive prenatal care) at a visit that took place after delivery. During this interview, mothers reported on their prenatal drug use including tobacco, alcohol, marijuana, and other drugs (i.e., sedatives or opiates). Urine samples were obtained for toxicology at several points during pregnancy and at delivery for women who received prenatal care (or just at the time of delivery for women who did not receive prenatal care). The Abbot TDx system (Poklis, 1987) and recommended cut off levels were used for detecting metabolites of cocaine (e.g., benzoylecognine), opioids, benzodiazepines, and marijuana in urine screens. Meconium screening was instituted two years after the initial recruitment. Results from meconium screening did not identify additional users who screened positive for cocaine exposure in interviews or urine screens.
Youth were considered to have significant histories of prenatal drug exposure if their mothers self-reported histories of significant substance use (cocaine, alcohol, marijuana, tobacco) or if urine toxicology results were positive for substance use. Mothers who reported or tested positive for opiate use were not included in this study. For analyses, youth were categorized as being positive or negative for prenatal drug exposure.
Salivary Cortisol and MIF Collection
Cortisol levels are the most stable during the late afternoon making it easier to assess changes in cortisol levels that occur in response to experimental stressors at this time of day (Kirschbaum and Hellhammer, 1989). As cortisol was a primary variable of interest in the larger study, all laboratory visits began at 4:00 p.m. Baseline saliva samples were collected at 4:15 p.m., by asking participants to place a cotton swab between their cheek and tongue for approximately 2 min.
Saliva samples were also collected as part of the Trier Social Stress Test for Children (TSST-C), which was administered according to previously established guidelines (Buske-Kirschbaum et al., 1997) with slight adaptations for adolescents. As part of the TSST-C, two unfamiliar judges entered the testing room and asked youth to write a story for the next 5 min. Youth were asked to make their story “as exciting as possible because they would be competing against other teenagers.” Youth were next asked to recite their story in front of the judges for 5 min while being audio- and video-taped. For the last 5 min, youth were instructed to perform an arithmetic task in front of the judges, who provided them feedback on their performance.
Saliva samples were collected immediately prior to the start of the TSST-C, again during the story preparation, immediately after the arithmetic task, which ended the stressor, and then at 15, 30, 45, and 60 min following the end of the stressor. Following each collection, the swab was placed directly on dry ice and stored at −20° C prior to extraction.
Cortisol Assay
Assays for cortisol were performed in duplicate, following standard procedures for the radioimmunoassay kits (Coat-A-Count Cortisol kit, Diagnostic Products Corporation, Los Angeles, CA) at a University laboratory. Intra-assay coefficients of variation ranged from 3.0 to 1.5%.
MIF Assay
Prior assessments of MIF levels have typically been restricted to blood plasma and serum. In this study, we employed a novel, noninvasive salivary assessment of peripheral MIF levels. The MIF assay was performed after the cortisol assays. Following the completion of the cortisol assay, saliva samples were stored at −20 degrees and rethawed for the MIF assay. Salivary MIF levels were measured with sandwich enzyme-linked immunosorbent assay (ELISA), using a protocol developed by the Bucala Lab at Yale University, which yielded adequate sensitivity and performance criteria (limit of detection: 0.83 ng/ml, CV%: 3.69) over a range of detection of 0–100 ng/ml. A 96-well plate was incubated for 2 hr at room temperature with 100 μL of the capture antibody, anti-human MIF (clone 3H2F mouse IgG1), diluted in 1xPBS. The plate was washed three times with .05% Tween20 in 1xPBS and blocked with 250 μL of 1% BSA and 1% sucrose in 1xPBS, followed by incubation at room temperature for 2 hr. Standard recombinant human MIF and saliva samples (50 μL each) then were added to the wells, along with 50 μL of diluted 1:10 horseradish peroxidase conjugate, followed by overnight incubation at 4° C. The next day, the plate was washed four times with 0.05% Tween20 in 1xPBS and 100 μL of TMB substrate was added to each well to visualize the protein of interest. The plate was then incubated in darkness for 20 min and 100 μL of stop solution (1M H2SO4, 0.5M HCl) was added to each well. The plate was read at 450–590 nm.
To assess validity of the assay, salivary MIF values from the current sample were compared with serum MIF levels from a separate population of 71 healthy adults ranging in age from 21 to 87 years (M = 44.9 years, SD = 15.4 years), 70% female, varied in ethnicity (15% African-American, 8% Hispanic, 1% Asian, 1% other non-Caucasian, and 75% Caucasian). Results from an independent t-test indicated that the distributional properties from each sample were not significantly different in terms of the mean values, F (1,275) = 1.7, p = .08, and variances t(1,275) = 1.7, p = .06 of MIF levels. This suggests that inter-individual variation of MIF in saliva may mirror that which is typically found in blood plasma.
RESULTS
Preliminary Analyses
Of the 206 youth recruited for the original study, one participant was missing CTQ data. Prior to analyses, data were examined for normality and outlying values. Cortisol and MIF values were considered outliers if they fell three standard deviations beyond the mean. This only excluded outlying high values, as the lowest possible value (0) was within three standard deviations of the mean. Three cortisol values and one MIF value were identified as outliers and were excluded from the data analysis.
Visual inspection of the distribution of the MIF data revealed significant positive skew. A square root transformation was applied to salivary MIF values for analyses. Data remained significantly positively skewed even after applying the transformation (Shapiro–Wilkes = .74, df = 205, p < .001). For this reason, raw values were examined in subsequent analyses. Cortisol data in the current study were normally distributed. Therefore, raw values were analyzed. MIF levels were not significantly associated baseline cortisol values, r = −.03, p = .35 or any of the cortisol values collected during the TSST-C, all p values > .54.
Associations between child demographic characteristics and prenatal drug exposure and MIF and cortisol values were explored next. Child gender was not associated with MIF or cortisol levels. Child age was inversely associated with MIF levels (r = −.18, p = .018), but not cortisol levels. Youth with significant histories of prenatal drug exposure had significantly higher MIF levels relative to youth without prenatal drug exposure, F(1, 203) = 4.85, p = .028. Race/ethnicity was marginally significantly associated with MIF levels (p = .075), with African-American youth showing significantly higher levels of MIF than Caucasian youth and no differences in MIF levels in Hispanic youth compared with Caucasian and African-American youth.
A multivariate model was used to examine associations between childhood maltreatment and MIF and cortisol values, with MIF and cortisol values entered as dependent variables. We first examined whether reports of childhood maltreatment (the total CTQ score) were associated with MIF and cortisol values, while controlling for child age, race/ethnicity, and prenatal drug exposure. Results indicated that total childhood maltreatment score was not significantly associated with MIF and cortisol values F(2,183) = 2.29, p = .10. Next, we examined whether the subtype of reported maltreatment may differentiate youth in terms of their baseline cortisol and MIF levels. In this model, childhood physical neglect and abuse, emotional neglect and abuse, and sexual abuse were entered as independent variables with child age, prenatal drug exposure, and race/ethic background included as covariates. Results indicated that reports of physical neglect, F(2,178) = 4.42, p = .01, but no other subtypes of childhood maltreatment (all p values ≥ .64), were significantly associated with cortisol and MIF values. Examination of separate univariate ANOVAs on the dependent variables revealed that childhood neglect was significantly associated with both cortisol F(1,179) = 4.61, p = .03 and MIF values F(1,179) = 3.77, p = .05. Post hoc comparisons revealed that youth with moderate to severe levels of physical neglect showed marginally higher MIF levels in comparison to youth with low levels of neglect (p = .062) and significantly higher levels in comparison to youth with no to minimal levels of neglect (p = .032; see Fig. 1). Further, youth with moderate to severe physical neglect also showed significantly higher levels of cortisol in comparison to youth with no to minimal levels of physical neglect (p = .032; see Fig. 2).
FIGURE 1.
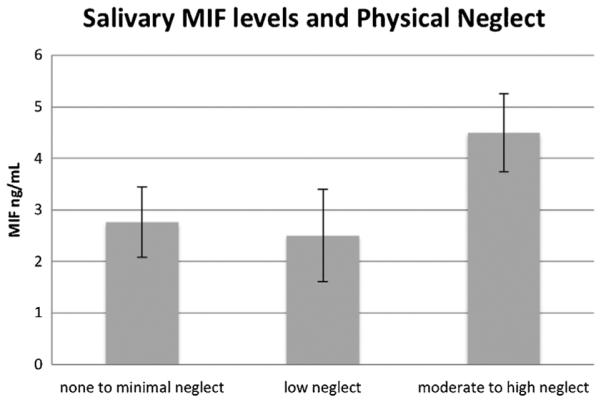
Associations between physical neglect severity and MIF levels controlling for child age, race/ethnicity, and prenatal drug exposure.
FIGURE 2.
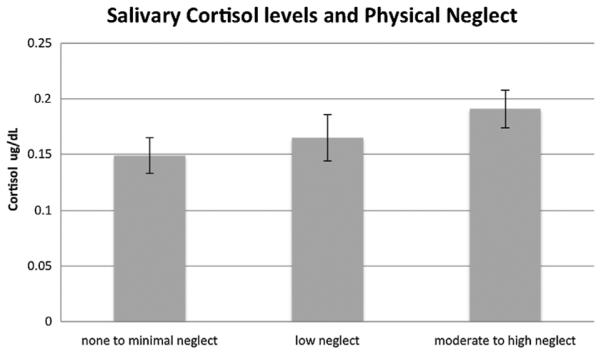
Associations between physical neglect severity and cortisol levels controlling for child age, race/ethnicity, and prenatal drug exposure.
In the last step of analyses, we explored whether histories of maltreatment were associated with cortisol reactivity across the TSST-C. MIF was not assayed from saliva samples collected during the TSST-C and was therefore not available for analyses. Repeated measures ANOVA revealed no significant associations between total CTQ scores (p =.877) and cortisol reactivity across the TSST-C. Histories of sexual abuse were associated with cortisol reactivity at a trend level, with significant histories of sexual abuse associated with marginally greater cortisol reactivity, F (5,900) = 2.58, p =.06, when compared with no reported history of sexual abuse. No other forms of childhood maltreatment were associated with cortisol reactivity (all p values > .33).
DISCUSSION
To our knowledge, this is the first study to demonstrate an association between two markers of HPA axis and glucocorticoid activity, MIF and cortisol, and self-reports of childhood maltreatment. In this investigation, adolescents who reported histories of severe neglect showed elevated levels of both MIF and cortisol at a baseline laboratory assessment, in comparison to youth without self-reported histories of neglect. Furthermore, youth exposed to teratogens (marijuana, nicotine, alcohol, cocaine, or other drugs) in utero showed higher levels of MIF than those without prenatal drug exposure. Associations persisted even after controlling for significant prenatal risk factors and youth demographic characteristics.
Our findings add to the broader body of work documenting alterations in HPA axis function and inflammatory signaling associated with a specific form of extreme childhood stress. First, they are consistent with prior investigations showing over-activity and/or dysregulation of the HPA axis in neglected youth (Carlson and Earls, 1997). Second, they demonstrate elevations of an HPA-axis associated pro-inflammatory cytokine, MIF, among youth with histories of severe neglect. Reports of physical neglect were significantly associated with both MIF and cortisol values. Follow-up results from univariate ANOVAs revealed that MIF and cortisol were elevated in youth with higher levels of physical neglect when compared with youth without histories of neglect, with youth reporting lower levels of neglect falling in between the two groups. Interestingly, histories of childhood maltreatment were only associated with baseline assessments of cortisol, but not cortisol reactivity in response to a stressor; with the exception of a marginal association between histories of sexual abuse and cortisol reactivity.
Although more research is needed, results from this study point to disruptions in signaling of two biologically inter-related systems, which may underlie risk for long-term chronic diseases. For example, long-term and/or prolonged elevations in cortisol have long been theorized to increase susceptibility to chronic disease. This has been attributed to both increased cortisol-mediated anti-inflammatory activity that may lead to prolonged immunosuppression (Coutinho and Chapman, 2011) as well as a cortisol-induced “glucocorticoid insensitivity”, in which immune cells become less sensitive to the anti-inflammatory actions of glucocorticoids (Miller and Chen, 2010), leading to persistent state of low-grade inflammation (Cohen et al., 2012). Elevations in MIF, especially if prolonged, may signal increased risk for inflammatory associated diseases, known to afflict individuals with histories of extreme childhood stress.
Our results suggest that alterations in cortisol and MIF were specific to reports of physical neglect, when compared with other forms of childhood maltreatment. This finding is somewhat surprising given that self-reported experiences of physical neglect were correlated with other forms of maltreatment. However these results are consistent with several other studies showing associations between childhood neglect and abnormal cortisol activity. Prior research indicates that children with histories of neglect are more likely to show blunted cortisol awakening responses (Bruce et al., 2009; Bernard et al., 2010; Carlson and Earls, 1997) and either atypically low or high cortisol rhythms across the day (Bernard et al., 2010; Dozier et al., 2006). Similar to the results of our study, a prior examination revealed that physical neglect, but not other forms of maltreatment, was associated with elevations in basal cortisol levels, assessed prior to a neuroendocrine challenge (Bruce et al., 2009; Carlson and Earls, 1997; Flory et al., 2009). It is currently unclear whether abnormalities observed in our study represent dysregulation in diurnal rhythms or acute responses to stress. Elevations at this baseline assessment could also result from transitioning from the home setting to the laboratory. Anticipatory stress associated with arrival at the laboratory visit may also have led to elevations in MIF and cortisol values. However, the youth in this longitudinal study have visited the same laboratory more than 10 times, typically under generally benign circumstances, which we expect would mitigate effects of anticipatory stress.
The findings from this study provide preliminary validation of a non-invasive measurement of salivary MIF. Whereas prior work typically assessed MIF levels in serum, this is one of the first studies to assess salivary levels of MIF in association with psychosocial variables. In this study, comparisons between distributional properties of MIF levels extracted from saliva did not differ significantly from those of MIF levels extracted from serum in a sample of healthy adults. To our knowledge, no studies have examined intra-individual associations between salivary and serum or plasma levels of MIF—a potentially important direction for future research. For instance, it is possible that increased MIF levels in saliva may reflect systemic processes of interest that pertain to abnormal immune system functioning, however, this possibility needs further investigation. Finally, it has been theorized that stress-induced disruptions in HPA axis and inflammatory activity place individual youth at risk for chronic mental and physical problems (Miller et al., 2011). Thus, it will be useful to examine whether or not salivary levels of MIF and cortisol may serve as biomarkers for later mental health problems and chronic illnesses.
Our results should be considered in light of the study limitations. First, salivary MIF levels were examined in a high-risk population of youth from disadvantaged backgrounds. Future work would benefit from comparison with a normative developmental frame. Second, for this sub-study, MIF samples were examined at a single assessment at the start of a laboratory visit. Consequently, it will be important to examine potential fluctuations that occur diurnally or in response to acute or chronic stress. Third, maltreatment histories were assessed using a retrospective self-report measure. Information about abuse severity and timing was not available through this assessment. Fourth, the salivary MIF assay occurred after the cortisol assay, leading to potential degradation in protein levels as a result of long-term storage. Finally, associations were examined on an exploratory basis and were considered significant at p < .05, without correction for multiple comparisons. Hence, replication of this work is needed.
Not withstanding these limitations, our results hold important implications for public health, intervention, and prevention efforts, as childhood neglect is the most prevalent form of maltreatment, representing approximately 80% of all cases reported to child welfare authorities (DHHS, 2012). Findings also provide preliminary support for a non-invasive salivary assessment of MIF as a biomarker for increased inflammatory-mediated disease risk among vulnerable youth. Moreover, our findings suggest that assessments of salivary MIF could be useful for early detection and prevention of long-term health problems among maltreated individuals.
Acknowledgments
Support for this project was provided by the National Institutes of Health (NIH) through grants P50-DA-16556, R01-DA-06025, R01-DA-017863, and KO5-DA-020091 (Mayes), K01-DA034125 (Crowley), RO1-AI042310 (Bucala), T32MH18268 (PI: Leckman, T32 fellow: Bick) and a grant from the Gustavus and Louise Pfeiffer Research Foundation (Mayes). The study sponsors had no involvement in the study design; collection, analysis, and interpretation of data; the writing of the manuscript or the decision to submit the manuscript for publication.
National Institutes of Health (NIH)
P50 DA 16556, R01 DA 06025, R01-DA 017863, KO5 DA 020091, K01 DA034125, RO1 AI042310, T32MH18268
Gustavus and Louise Pfeiffer Research Foundation
Footnotes
Conflicts of interest: none.
Dr. Elena L. Grigorenko and Dr. Linda C. Mayes jointly served as senior authors for this manuscript.
REFERENCES
- Anda RF, Dong M, Brown DW, Felitti VJ, Giles WH, Perry GS, Dube SR. The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health. 2009;9:106. doi: 10.1186/1471-2458-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher M, Meinhardt A, Lan HY, Mu W, Metz CN, Chesney JA, Bucala R. Migration inhibitory factor expression in experimentally induced endotoxemia. American Journal of Pathology. 1997;150(1):235–246. [PMC free article] [PubMed] [Google Scholar]
- Bernard K, Butzin Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs. children placed in foster care following involvement of Child Protective Services. Archives of Pediatrics and Adolescent Medicine. 2010;164:438–443. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Bucala R. MIF is a pituitary derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365(6448):756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bifulco C, Mcdaniel K, Leng L, Bucala R. Tumor growth promoting properties of macrophage migration inhibitory factor. Current Pharmaceutical Design. 2008;14(36):3790–3801. doi: 10.2174/138161208786898608. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool aged foster children: Differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59(4):419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Bucala R. MIF as a glucocorticoid induced modulator of cytokine production. Nature. 1995;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bucala R. Macrophage migration inhibitory factor: A counter regulator of glucocorticoid action and critical mediator of septic shock. Journal of Inflammation. 1995;47(1–2):39–51. [PubMed] [Google Scholar]
- Calandra T, Roger T. Macrophage migration inhibitory factor: A regulator of innate immunity. Nat Rev Immunol. 2003;3(10):791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuro-endocrinological sequelae of early social deprivation in institutionalized children in Romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL 6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35(13):2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain, Behavior, and Immunity. 2010;24(1):49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(16):5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L, Varea E, Castro JE, Sakouhi Ouertatani H, Calandra T, Lashuel HA, Sandi C. Macrophage migration inhibitory factor is critically involved in basal and fluoxetine stimulated adult hippocampal cell proliferation and in anxiety, depression, and memory-related behaviors. Molecular Psychiatry. 2011;16(5):533–547. doi: 10.1038/mp.2010.15. [DOI] [PubMed] [Google Scholar]
- Coutinho AE, Chapman KE. The anti inflammatory and immunosupressive effects of glucocorti coids, recent developments, and mechanistic insights. Molecular Cell Endocrinology. 2011;335(1):2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Caspi A. Adverse childhood experiences and adult risk factors for age related disease: Depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics and Adolescent Medicine. 2009;163(12):1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Rots NY, Cools AR. Brain corticosteroid hormone dialogue: Slow and persistent. Cellular and Molecular Neurobiology. 1996;16(3):345–356. doi: 10.1007/BF02088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS Administration for Children and Families, Administration on Children, Youth and Families, Children’s Bureau. 2012. Child Maltreatment, 2011. U.S. Department of Health and Human Services.
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall McClough KC, Levine S. Foster children’s diurnal production of cortisol: An exploratory study. Child Maltreat. 2006;11(2):189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71(2):243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KM, Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Elevated Macrophage Migration Inhibitory Factor (MIF) is associated with depressive symptoms, blunted cortisol reactivity to acute stress, and lowered morning cortisol. Brain, Behavior, and Immunity. 2010;24(7):1202–1208. doi: 10.1016/j.bbi.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Fingerle Rowson G, Koch P, Bikoff R, Lin X, Metz CN, Dhabhar FS, Bucala R. Regulation of macrophage migration inhibitory factor expression by glucocorticoids in vivo. American Journal of Pathology. 2003;162(1):47–56. doi: 10.1016/S0002-9440(10)63797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor glucocorticoid dyad: Regulation of inflammation and immunity. Molecular Endocrinology. 2007;21(6):1267–1280. doi: 10.1210/me.2007-0065. [DOI] [PubMed] [Google Scholar]
- Flory JD, Yehuda R, Grossman R, New AS, Mitropoulou V, Siever LJ. Childhood trauma and basal cortisol in people with personality disorders. Comprehensive Psychiatry. 2009;50(1):34–37. doi: 10.1016/j.comppsych.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Miller AH, Nemeroff CB. Long-term neuroendocrine effects of childhood maltreatment. JAMA. 2000;284(18):2321. [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Isidori AM, Kaltsas GA, Korbonits M, Pyle M, Gueorguiev M, Meinhardt A, Grossman AB. Response of serum macrophage migration inhibitory factor levels to stimulation or suppression of the hypothalamo pituitary adrenal axis in normal subjects and patients with Cushing’s disease. Journal of Clinical Endocrinology and Metabolism. 2002;87(4):1834–1840. doi: 10.1210/jcem.87.4.8382. [DOI] [PubMed] [Google Scholar]
- Katsuura S, Kamezaki Y, Tominaga K, Masuda K, Nishida K, Yamamoto Y, Rokutan K. High-throughput screening of brief naturalistic stress responsive cytokines in university students taking examinations. International Journal of Psychophysiology. 2010;77(2):135–140. doi: 10.1016/j.ijpsycho.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Kiecolt Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later life caregiving stress on telomere length and inflammation. Psychosomatic Medicine. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An overview. Neuro-psychobiology. 1989;22(3):150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Mclellan AT, Luborsky L, Woody GE, O’brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science. 2010;21(6):848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin. 2011;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton PM, Schafer MH, Ferraro KF. Does childhood misfortune increase cancer risk in adulthood. Journal of Aging and Health. 2012;24(6):948–984. doi: 10.1177/0898264312449184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil R, Schwarz MJ, Riedel M, Dehning S, Cerovecki A, Spellmann I, Muller N. Elevated macrophage migration inhibitory factor and decreased transform ing growth factor beta levels in major depression no influence of celecoxib treatment. Journal of Affective Disorders. 2011;134(1–3):217–225. doi: 10.1016/j.jad.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Nishihira J, Mitsuyama K. Overview of the role of macrophage migration inhibitory factor (MIF) in inflammatory bowel disease. Current Pharmaceutical Design. 2009;15(18):2104–2109. doi: 10.2174/138161209788489113. [DOI] [PubMed] [Google Scholar]
- Nishino T, Bernhagen J, Shiiki H, Calandra T, Dohi K, Bucala R. Localization of macrophage migration inhibitory factor (MIF) to secretory granules within the corticotrophic and thyrotrophic cells of the pituitary gland. Molecular Medicine. 1995a;1(7):781–788. [PMC free article] [PubMed] [Google Scholar]
- Nishino T, Bernhagen J, Shiiki H, Calandra T, Dohi K, Bucala R, Bucala R. Localization of macrophage migration inhibitory factor (MIF) to secretory granules within the corticotrophic and thyrotrophic cells of the pituitary gland MIF as a glucocorticoid induced modulator of cytokine production. Molecular Medicine. 1995;1(7):781–788. [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Bucala R. Macrophage migration inhibitory factor (MIF). A critical neurohumoral mediator. Annals of the New York Academy of Sciences. 2000;917:665–671. doi: 10.1111/j.1749-6632.2000.tb05432.x. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Socha L, Silva D, Grossman AB, Metz C, Bucala R. Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter regulator. Immunology and Cell Biology. 2003;81(2):137–143. doi: 10.1046/j.0818-9641.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- Poklis A. Evaluation of TDx cocaine metabolite assay. Journal of Analytical Toxicology. 1987;11(5):228–230. doi: 10.1093/jat/11.5.228. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, Mclaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: A prospective study. Psychoneuroendocrinology. 2013;38(2):188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampanaru Sarmesiu A, Stefaneanu L, Thapar K, Kovacs K, Donnelly T, Metz CN, Bucala R. Immunocytochemical localization of macrophage migration inhibitory factor in human hypophysis and pituitary adenomas. Archives of Pathology and Laboratory Medicine. 1997;121(4):404. [PubMed] [Google Scholar]
- Tarullo A, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Tierney T, Patel R, Stead CA, Leng L, Bucala R, Buckingham JC. Macrophage migration inhibitory factor is released from pituitary folliculo stellate like cells by endotoxin and dexamethasone and attenuates the steroid induced inhibition of interleukin 6 release. Endocrinology. 2005;146(1):35–43. doi: 10.1210/en.2004-0946. [DOI] [PubMed] [Google Scholar]
- Waeber G, Calandra T, Bonny C, Bucala R. A role for the endocrine and pro inflammatory mediator MIF in the control of insulin secretion during stress. Diabetes/Metabolism Research and Reviews. 1999;15(1):47–54. doi: 10.1002/(sici)1520-7560(199901/02)15:1<47::aid-dmrr9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wegman HL, Stetler C. A meta analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosomatic Medicine. 2009;71(8):805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bernhagen J, Weber C. Macrophage migration inhibitory factor in cardiovascular disease. Circulation. 2008;117(12):1594–1602. doi: 10.1161/CIRCULATIONAHA.107.729125. [DOI] [PubMed] [Google Scholar]


